The Gum–Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Periodontitis and Gut Diseases: Where Is the Link?
2.1. What Is Periodontitis
2.2. The Emerging “Gum–Gut Axis”
3. The Oro-Intestinal Microbiome as a Carcinogen
3.1. Gut Microbiota in Health and Disease
3.2. Oral–Gut Dysbiosis in the Pathogenesis of Gastrointestinal Cancers
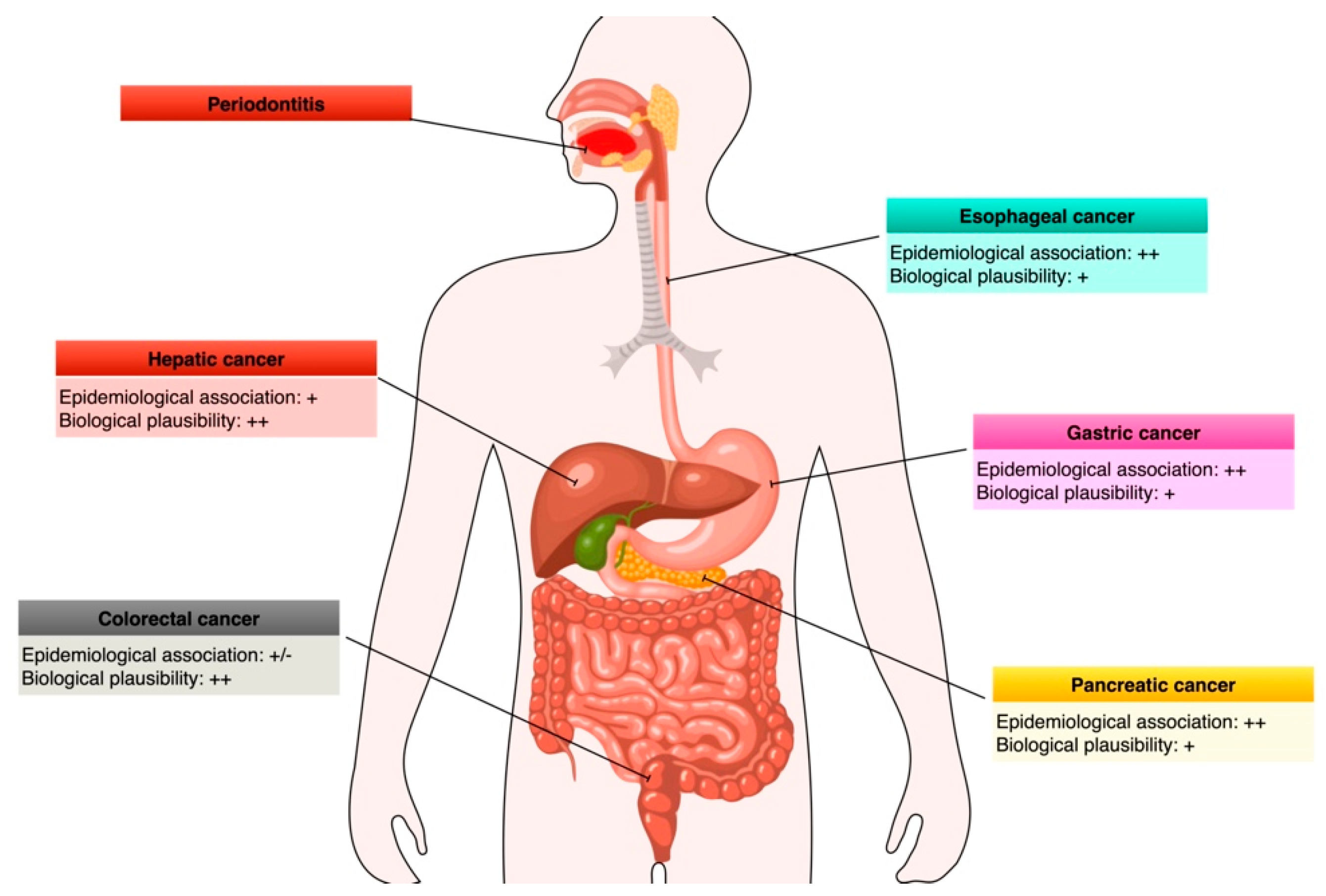
4. Link between Periodontitis/Oral Bacteria and Esophageal Cancers
4.1. Epidemiology and Risk Factors of Esophageal Cancer
4.2. Mechanistic Insights into the Perio-Esophageal Cancer Link
5. Link between Periodontitis/Oral Bacteria and Gastric Cancers
5.1. Epidemiology and Risk Factors for Gastric Cancer
5.2. Mechanistic Insights into the Periodontitis–Gastric Cancer Link
6. Link between Periodontitis/Oral Bacteria and Pancreatic Cancers
6.1. Epidemiology and Risk Factors for Pancreatic Cancer
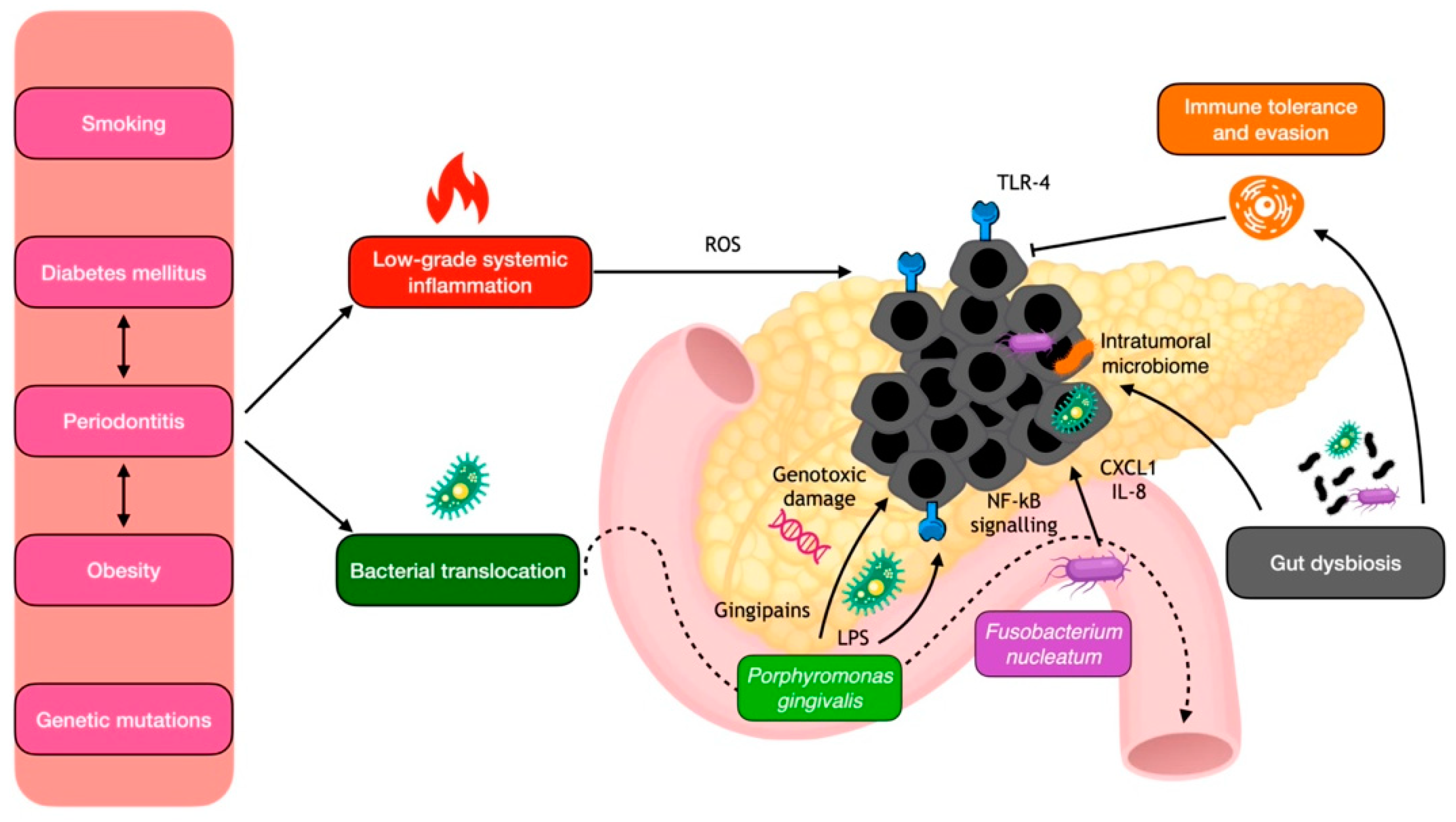
6.2. Mechanistic Insights into the Periodontitis–Pancreatic Cancer Link
7. Link between Periodontitis/Oral Bacteria and Colorectal Cancers
7.1. Epidemiology and Risk Factors for Colorectal Cancer
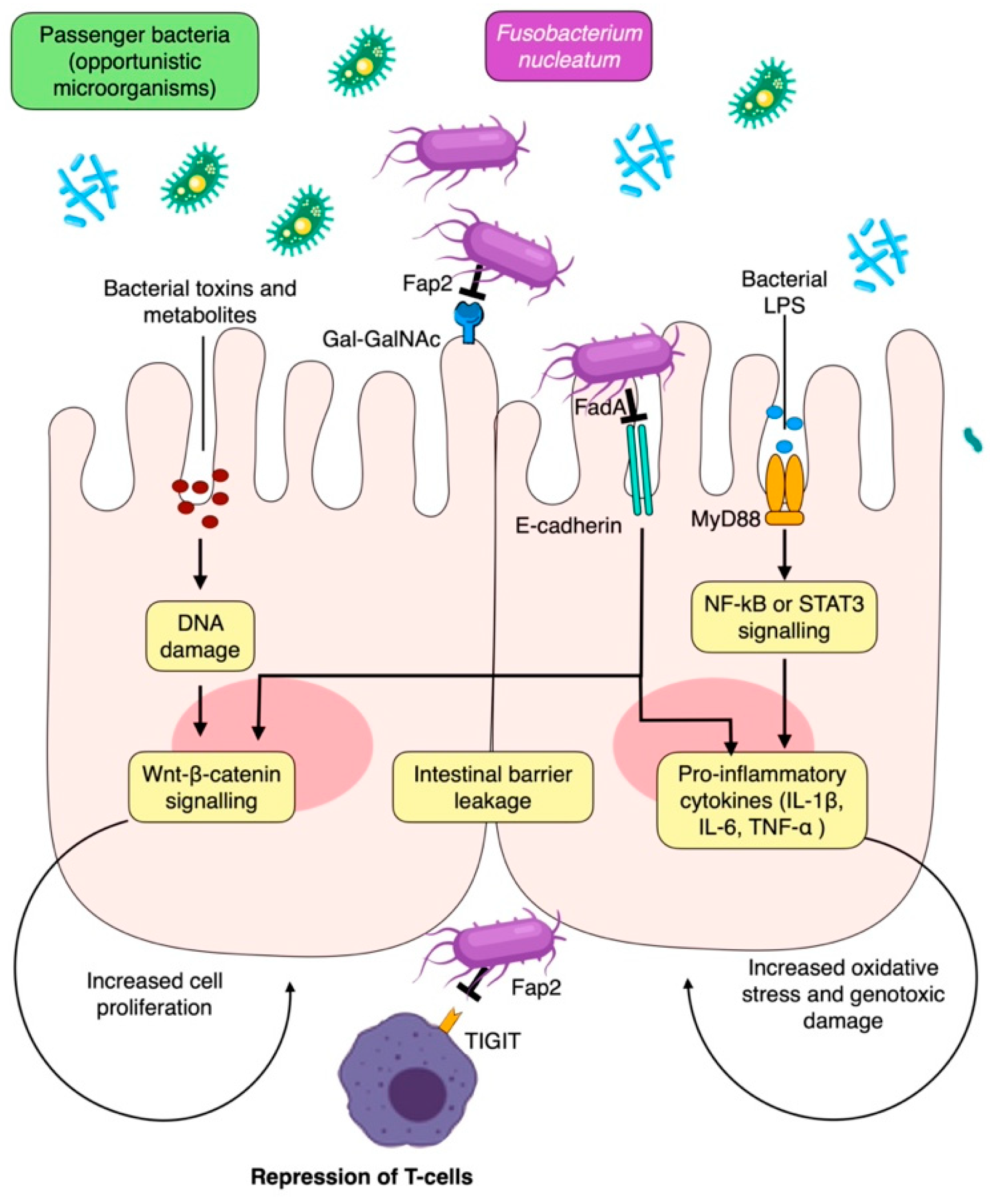
7.2. Mechanistic Insights into the Periodontitis–CRC Link
8. Link between Periodontitis/Oral Bacteria and Liver Cancers
8.1. Epidemiology and Risk Factors for Liver Cancer
8.2. Mechanistic Insights into the Periodontitis–Liver Cancer Link
9. Future Research Priorities
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Carlo, V.D.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating Intrinsic and Non-Intrinsic Cancer Risk Factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Teles, F.R.F.; Alawi, F.; Castilho, R.M.; Wang, Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020, 99, 1411–1424. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal Disease and Cancer: Epidemiologic Studies and Possible Mechanisms. Periodontology 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Fitzsimonds, Z.R.; Rodriguez-Hernandez, C.J.; Bagaitkar, J.; Lamont, R.J. From Beyond the Pale to the Pale Riders: The Emerging Association of Bacteria with Oral Cancer. J. Dent. Res. 2020, 99, 604–612. [Google Scholar] [CrossRef]
- Michaud, D.S.; Lu, J.; Peacock-Villada, A.Y.; Barber, J.R.; Joshu, C.E.; Prizment, A.E.; Beck, J.D.; Offenbacher, S.; Platz, E.A. Periodontal Disease Assessed Using Clinical Dental Measurements and Cancer Risk in the ARIC Study. J. Natl. Cancer. Inst. 2018, 110, 843–854. [Google Scholar] [CrossRef]
- Sung, C.-E.; Lin, F.-G.; Huang, R.-Y.; Fang, W.-H.; Cheng, W.-C.; Tsai, Y.-W.C.; Chen, W.-L. Periodontitis, Helicobacter Pylori Infection, and Gastrointestinal Tract Cancer Mortality. J. Clin. Periodontol. 2022, 49, 210–220. [Google Scholar] [CrossRef]
- Lo, C.-H.; Kwon, S.; Wang, L.; Polychronidis, G.; Knudsen, M.D.; Zhong, R.; Cao, Y.; Wu, K.; Ogino, S.; Giovannucci, E.L.; et al. Periodontal Disease, Tooth Loss, and Risk of Oesophageal and Gastric Adenocarcinoma: A Prospective Study. Gut 2021, 70, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Strauss, F.J.; Hämmerle, C.H.F.; Romandini, M.; Cavalla, F.; Baeza, M.; Sanz, M.; Gamonal, J. Performance of the 2017 AAP/EFP Case Definition Compared with the CDC/AAP Definition in Population-Based Studies. J. Periodontol. 2022, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Perotto, S.; Castiglione, A.; Mariani, G.M.; Ferrarotti, F.; Romano, F. Prevalence of Periodontitis in an Adult Population from an Urban Area in North Italy: Findings from a Cross-Sectional Population-Based Epidemiological Survey. J. Clin. Periodontol. 2015, 42, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of Periodontitis in Dentate People between 2011 and 2020: A Systematic Review and Meta-Analysis of Epidemiological Studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the Global Burden of Periodontal Diseases on Health, Nutrition and Wellbeing of Mankind: A Call for Global Action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk Factors for Periodontal Disease. Periodontology 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current Understanding of Periodontal Disease Pathogenesis and Targets for Host-Modulation Therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific Evidence on the Links between Periodontal Diseases and Diabetes: Consensus Report and Guidelines of the Joint Workshop on Periodontal Diseases and Diabetes by the International Diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Marruganti, C.; Baima, G.; Aimetti, M.; Grandini, S.; Sanz, M.; Romandini, M. Periodontitis and Low Cognitive Performance: A Population-Based Study. J. Clin. Periodontol. 2023, 50, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Perotto, S.; Mohamed, S.E.O.; Bernardi, S.; Giraudi, M.; Caropreso, P.; Mengozzi, G.; Baima, G.; Citterio, F.; Berta, G.N.; et al. Bidirectional Association between Metabolic Control in Type-2 Diabetes Mellitus and Periodontitis Inflammatory Burden: A Cross-Sectional Study in an Italian Population. J. Clin. Med. 2021, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Marruganti, C.; Sanz, M.; Aimetti, M.; Romandini, M. Periodontitis and COVID-19: Biological Mechanisms and Meta-Analyses of Epidemiological Evidence. J. Dent. Res. 2022, 101, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, G.N.; Romandini, M.; Meurman, J.H.; Surakka, M.; Janket, S.-J.; Sanz, M. Periodontitis and Edentulism as Risk Indicators for Mortality: Results from a Prospective Cohort Study with 20 Years of Follow-Up. J. Periodontal Res. 2023, 58, 12–21. [Google Scholar] [CrossRef]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-Analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proença, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An Umbrella Review of the Evidence Linking Oral Health and Systemic Noncommunicable Diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Van Dyke, T.E.; Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and Atherosclerotic Cardiovascular Disease: Consensus Report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S24–S29. [Google Scholar] [CrossRef]
- Kitamoto; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive Transmission of Microbes along the Gastrointestinal Tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Baima, G.; Ribaldone, D.G.; Muwalla, M.; Romano, F.; Citterio, F.; Armandi, A.; Aimetti, M. Can Periodontitis Affect the Health and Disease of the Digestive System? A Comprehensive Review of Epidemiological Evidence and Biological Mechanisms. Curr. Oral Health Rep. 2021, 8, 96–106. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.M.; Gulati, A.S. The “Gum–Gut” Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances. Front. Immunol. 2021, 12, 620124. [Google Scholar] [CrossRef] [PubMed]
- Lourenςo, T.G.B.; Spencer, S.J.; Alm, E.J.; Colombo, A.P.V. Defining the Gut Microbiota in Individuals with Periodontal Diseases: An Exploratory Study. J. Oral Microbiol. 2018, 10, 1487741. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.G.B.; de Oliveira, A.M.; Tsute Chen, G.; Colombo, A.P.V. Oral-gut Bacterial Profiles Discriminate between Periodontal Health and Diseases. J. Periodontal Res. 2022, 57, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Nagao, J.-I.; Kishikawa, S.; Tanaka, H.; Toyonaga, K.; Narita, Y.; Negoro-Yasumatsu, K.; Tasaki, S.; Arita-Morioka, K.-I.; Nakayama, J.; Tanaka, Y. Pathobiont-Responsive Th17 Cells in Gut-Mouth Axis Provoke Inflammatory Oral Disease and Are Modulated by Intestinal Microbiome. Cell Rep. 2022, 40, 111314. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Leviatan, S.; Shoer, S.; Rothschild, D.; Gorodetski, M.; Segal, E. An Expanded Reference Map of the Human Gut Microbiome Reveals Hundreds of Previously Unknown Species. Nat. Commun. 2022, 13, 3863. [Google Scholar] [CrossRef]
- Bourdeau-Julien, I.; Castonguay-Paradis, S.; Rochefort, G.; Perron, J.; Lamarche, B.; Flamand, N.; Di Marzo, V.; Veilleux, A.; Raymond, F. The Diet Rapidly and Differentially Affects the Gut Microbiota and Host Lipid Mediators in a Healthy Population. Microbiome 2023, 11, 26. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K. Amino Acids and Immune Response: A Role for Cysteine, Glutamine, Phenylalanine, Tryptophan and Arginine in T-Cell Function and Cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mitev, K.; Taleski, V. Association between the Gut Microbiota and Obesity. Open Access Maced. J. Med. Sci. 2019, 7, 2050–2056. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial Control of Astrocytes in Response to Microbial Metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Greten, T.F. Gut Microbiome in HCC—Mechanisms, Diagnosis and Therapy. J. Hepatol. 2020, 72, 230–238. [Google Scholar] [CrossRef]
- Zakerska-Banaszak, O.; Tomczak, H.; Gabryel, M.; Baturo, A.; Wolko, L.; Michalak, M.; Malinska, N.; Mankowska-Wierzbicka, D.; Eder, P.; Dobrowolska, A.; et al. Dysbiosis of Gut Microbiota in Polish Patients with Ulcerative Colitis: A Pilot Study. Sci. Rep. 2021, 11, 2166. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut Microbiota and Inflammation in Chronic Kidney Disease and Their Roles in the Development of Cardiovascular Disease. Hypertens. Res. 2019, 42, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter Pylori Infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef]
- Song, C.; Lv, J.; Liu, Y.; Chen, J.G.; Ge, Z.; Zhu, J.; Dai, J.; Du, L.-B.; Yu, C.; Guo, Y.; et al. Associations between Hepatitis B Virus Infection and Risk of All Cancer Types. JAMA Netw. Open 2019, 2, e195718. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human Papillomavirus Types from Infection to Cancer in the Anus, According to Sex and HIV Status: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef]
- Boleij, A.; van Gelder, M.M.H.J.; Swinkels, D.W.; Tjalsma, H. Clinical Importance of Streptococcus Gallolyticus Infection Among Colorectal Cancer Patients: Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2011, 53, 870–878. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.-Y. Fusobacterium nucleatum, a Key Pathogenic Factor and Microbial Biomarker for Colorectal Cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Rychter, A.M.; Łykowska-Szuber, L.; Zawada, A.; Szymczak-Tomczak, A.; Ratajczak, A.E.; Skoracka, K.; Kolan, M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Why Does Obesity as an Inflammatory Condition Predispose to Colorectal Cancer? J. Clin. Med. 2023, 12, 2451. [Google Scholar] [CrossRef]
- Ait-Zenati, F.; Djoudi, F.; Mehelleb, D.; Madaoui, M. Involvement of the Human Microbiome in Frequent Cancers, Current Knowledge and Carcinogenesis Mechanisms. Bull. Cancer 2023, 110, 776–789. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.S.; Azmy, A.F.; Dishisha, T.; Mohamed, W.R.; Ahmed, K.A.; Hassan, A.; Aidy, S.E.; El-Gendy, A.O. Irinotecan-Gut Microbiota Interactions and the Capability of Probiotics to Mitigate Irinotecan-Associated Toxicity. BMC Microbiol. 2023, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Li, L.; Zhang, Y.; Wang, M.; Chen, F.; Ge, S.; Chen, B.; Yan, F. Periodontitis May Induce Gut Microbiota Dysbiosis via Salivary Microbiota. Int. J. Oral Sci. 2022, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yilmaz, Ö. Possible Role of Porphyromonas gingivalis in Orodigestive Cancers. J. Oral Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human Oral Microbiome and Prospective Risk for Pancreatic Cancer: A Population-Based Nested Case-Control Study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef]
- Mu, W.; Jia, Y.; Chen, X.; Li, H.; Wang, Z.; Cheng, B. Intracellular Porphyromonas gingivalis Promotes the Proliferation of Colorectal Cancer Cells via the MAPK/ERK Signaling Pathway. Front. Cell. Infect. Microbiol. 2020, 10, 584798. [Google Scholar] [CrossRef]
- Ge, Z.; Rogers, A.B.; Feng, Y.; Lee, A.; Xu, S.; Taylor, N.S.; Fox, J.G. Bacterial Cytolethal Distending Toxin Promotes the Development of Dysplasia in a Model of Microbially Induced Hepatocarcinogenesis. Cell. Microbiol. 2007, 9, 2070–2080. [Google Scholar] [CrossRef]
- Graillot, V.; Dormoy, I.; Dupuy, J.; Shay, J.W.; Huc, L.; Mirey, G.; Vignard, J. Genotoxicity of Cytolethal Distending Toxin (CDT) on Isogenic Human Colorectal Cell Lines: Potential Promoting Effects for Colorectal Carcinogenesis. Front. Cell. Infect. Microbiol. 2016, 6, 34. [Google Scholar] [CrossRef]
- Abed, J.; Emgård, J.E.M.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Narikiyo, M.; Tanabe, C.; Yamada, Y.; Igaki, H.; Tachimori, Y.; Kato, H.; Muto, M.; Montesano, R.; Sakamoto, H.; Nakajima, Y.; et al. Frequent and Preferential Infection of Treponema denticola, Streptococcus Mitis, and Streptococcus anginosus in Esophageal Cancers. Cancer Sci. 2004, 95, 569–574. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.-Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.S.; Raza, S.A.; El-Serag, H.B.; Thrift, A.P. Trends in Esophageal Adenocarcinoma and Esophageal Squamous Cell Carcinoma Incidence in the United States from 1992 to 2019. Cancers 2022, 14, 6049. [Google Scholar] [CrossRef] [PubMed]
- Tarazi, M.; Chidambaram, S.; Markar, S.R. Risk Factors of Esophageal Squamous Cell Carcinoma beyond Alcohol and Smoking. Cancers 2021, 13, 1009. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Liu, X.; Zhang, X.; He, J.; Feng, Q.; Zhou, Z.; Wang, L.; Yin, W.; Xiao, Z. Prognosis of Esophageal Squamous Cell Carcinoma Patients with Preoperative Radiotherapy: Comparison of Different Cancer Staging Systems. Thorac. Cancer 2014, 5, 204–210. [Google Scholar] [CrossRef]
- Chen, X.; Winckler, B.; Lu, M.; Cheng, H.; Yuan, Z.; Yang, Y.; Jin, L.; Ye, W. Oral Microbiota and Risk for Esophageal Squamous Cell Carcinoma in a High-Risk Area of China. PLoS ONE 2015, 10, e0143603. [Google Scholar] [CrossRef]
- Deshpande, N.P.; Riordan, S.M.; Castaño-Rodríguez, N.; Wilkins, M.R.; Kaakoush, N.O. Signatures within the Esophageal Microbiome Are Associated with Host Genetics, Age, and Disease. Microbiome 2018, 6, 227. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Ikeda, Y.; Ikeda, E.; Takahashi, M.; Tanaka, D.; Nakajima, Y.; Arakawa, S.; Izumi, Y.; Miyake, S. Oral Infectious Bacteria in Dental Plaque and Saliva as Risk Factors in Patients with Esophageal Cancer. Cancer 2021, 127, 512–519. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Ma, Z.; Liang, S.; Shan, T.; Zhang, M.; Zhu, X.; Zhang, P.; Liu, G.; Zhou, F.; et al. Presence of Porphyromonas gingivalis in Esophagus and Its Association with the Clinicopathological Characteristics and Survival in Patients with Esophageal Cancer. Infect. Agents Cancer 2016, 11, 3. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef]
- Li, Z.; Shi, C.; Zheng, J.; Guo, Y.; Fan, T.; Zhao, H.; Jian, D.; Cheng, X.; Tang, H.; Ma, J. Fusobacterium nucleatum Predicts a High Risk of Metastasis for Esophageal Squamous Cell Carcinoma. BMC Microbiol. 2021, 21, 301. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Zicola, E.; Munaron, L.; Rivolo, P.; Mandracci, P.; Carossa, S. Early Response of Fibroblasts and Epithelial Cells to Pink-Shaded Anodized Dental Implant Abutments: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Baba, Y.; Miyake, K.; Nakamura, K.; Shigaki, H.; Mima, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Sakamoto, Y.; et al. Fusobacterium nucleatum in Gastroenterological Cancer: Evaluation of Measurement Methods Using Quantitative Polymerase Chain Reaction and a Literature Review. Oncol. Lett. 2017, 14, 6373–6378. [Google Scholar] [CrossRef] [PubMed]
- Fenno, J.C. Treponema denticola Interactions with Host Proteins. J. Oral Microbiol. 2012, 4, 9929. [Google Scholar] [CrossRef]
- Jo, A.; Baek, K.J.; Shin, J.E.; Choi, Y. Mechanisms of IL-8 Suppression by Treponema denticola in Gingival Epithelial Cells. Immunol. Cell Biol. 2014, 92, 139–147. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Thrift, A.P.; Wenker, T.N.; El-Serag, H.B. Global Burden of Gastric Cancer: Epidemiological Trends, Risk Factors, Screening and Prevention. Nat. Rev. Clin. Oncol. 2023, 20, 338–349. [Google Scholar] [CrossRef]
- Shah, D.; Bentrem, D. Environmental and Genetic Risk Factors for Gastric Cancer. J. Surg. Oncol. 2022, 125, 1096–1103. [Google Scholar] [CrossRef]
- Ortigão, R.; Brito, M.; Pinto, C.; Sá, I.; Libânio, D.; Dinis-Ribeiro, M.; Brandão, C. Risk Factors for Gastric Cancer in Patients with Lynch Syndrome. Eur. J. Gastroenterol. Hepatol. 2022, 34, 912–918. [Google Scholar] [CrossRef]
- Polk, D.B.; Peek, R.M. Helicobacter Pylori: Gastric Cancer and Beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Pellicano, R.; Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Saracco, G.M.; Mégraud, F. A 2016 Panorama of Helicobacter Pylori Infection: Key Messages for Clinicians. Panminerva Med. 2016, 58, 304–317. [Google Scholar] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.; Sheh, A.; Shen, Z.; Dzink-Fox, J.; Piazuelo, M.B.; Wilson, K.T.; Peek, R.; Fox, J.G. Shotgun Metagenomics of Gastric Biopsies Reveals Compositional and Functional Microbiome Shifts in High- and Low-Gastric-Cancer-Risk Populations from Colombia, South America. Gut Microbes 2023, 15, 2186677. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lee, W.J.J.; Chua, S.J.; Zhu, F.; Yeoh, K.G.; Zhang, Y. Mucosal Microbiome Associates with Progression to Gastric Cancer. Theranostics 2022, 12, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Nam, S.; Park, C.H.; Kim, Y.; Lee, M.; Ahn, J.B.; Shin, S.J.; Park, Y.R.; Jung, H.I.; Kim, B.-I.; et al. Periodontal Disease and Cancer Risk: A Nationwide Population-Based Cohort Study. Front. Oncol. 2022, 12, 901098. [Google Scholar] [CrossRef]
- Ida, S.; Watanabe, M.; Baba, H. Chronic Inflammation and Gastrointestinal Cancer. J. Cancer Metastasis Treat. 2015, 1, 138–143. [Google Scholar] [CrossRef]
- Shao, W.; Yang, Z.; Fu, Y.; Zheng, L.; Liu, F.; Chai, L.; Jia, J. The Pyroptosis-Related Signature Predicts Prognosis and Indicates Immune Microenvironment Infiltration in Gastric Cancer. Front. Cell Dev. Biol. 2021, 9, 676485. [Google Scholar] [CrossRef]
- Bockerstett, K.A.; DiPaolo, R.J. Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 47–53. [Google Scholar] [CrossRef]
- Lee, B.L.; Lee, H.S.; Jung, J.; Cho, S.J.; Chung, H.-Y.; Kim, W.H.; Jin, Y.-W.; Kim, C.S.; Nam, S.Y. Nuclear Factor-ΚB Activation Correlates with Better Prognosis and Akt Activation in Human Gastric Cancer. Clin. Cancer Res. 2005, 11, 2518–2525. [Google Scholar] [CrossRef]
- Jaroenlapnopparat, A.; Bhatia, K.; Coban, S. Inflammation and Gastric Cancer. Diseases 2022, 10, 35. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.; Chen, L.; Zhou, T.; Huang, W.; Zhou, X.; Shao, L. The Role of Toll-like Receptors in Periodontitis. Oral Dis. 2017, 23, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, L.; Hao, Y.; Zhou, B.; Hu, J.; Yang, Y.; Bedi, S.; Sanichar, N.G.; Cheng, C.; Perez-Perez, G.; et al. Oral and Gastric Microbiome in Relation to Gastric Intestinal Metaplasia. Int. J. Cancer 2022, 150, 928–940. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, M.A.; Duval, M.X. The Presence of Periodontal Pathogens in Gastric Cancer. Explor. Res. Hypothes. Med. 2020, 5, 87–96. [Google Scholar] [CrossRef]
- Zhou, C.-B.; Pan, S.-Y.; Jin, P.; Deng, J.-W.; Xue, J.-H.; Ma, X.-Y.; Xie, Y.-H.; Cao, H.; Liu, Q.; Xie, W.-F.; et al. Fecal Signatures of Streptococcus anginosus and Streptococcus constellatus for Noninvasive Screening and Early Warning of Gastric Cancer. Gastroenterology 2022, 162, 1933–1947.e18. [Google Scholar] [CrossRef]
- Usui, G.; Matsusaka, K.; Mano, Y.; Urabe, M.; Funata, S.; Fukayama, M.; Ushiku, T.; Kaneda, A. DNA Methylation and Genetic Aberrations in Gastric Cancer. Digestion 2021, 102, 25–32. [Google Scholar] [CrossRef]
- Palioto, D.B.; Finoti, L.S.; Kinane, D.F.; Benakanakere, M. Epigenetic and Inflammatory Events in Experimental Periodontitis Following Systemic Microbial Challenge. J. Clin. Periodontol. 2019, 46, 819–829. [Google Scholar] [CrossRef]
- Dye, B.A.; Kruszon-Moran, D.; McQuillan, G. The Relationship between Periodontal Disease Attributes and Helicobacter Pylori Infection Among Adults in the United States. Am. J. Public Health 2002, 92, 1809–1815. [Google Scholar] [CrossRef]
- Veisani, Y.; Jenabi, E.; Khazaei, S.; Nematollahi, S. Global Incidence and Mortality Rates in Pancreatic Cancer and the Association with the Human Development Index: Decomposition Approach. Public Health 2018, 156, 87–91. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Zhou, B.-G.; Mei, Y.-Z.; Wang, J.-S.; Xia, J.-L.; Jiang, X.; Ju, S.-Y.; Ding, Y.-B. Is Helicobacter Pylori Infection Associated with Pancreatic Cancer? A Systematic Review and Meta-Analysis of Observational Studies. Ther. Adv. Chronic. Dis. 2023, 14, 20406223231155120. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal Disease, Edentulism, and Pancreatic Cancer: A Meta-Analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Swidnicka-Siergiejko, A.K.; Gomez-Chou, S.B.; Cruz-Monserrate, Z.; Deng, D.; Liu, Y.; Huang, H.; Ji, B.; Azizian, N.; Daniluk, J.; Lu, W.; et al. Chronic Inflammation Initiates Multiple Forms of K-Ras-Independent Mouse Pancreatic Cancer in the Absence of TP53. Oncogene 2017, 36, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Sollie, S.; Michaud, D.S.; Sarker, D.; Karagiannis, S.N.; Josephs, D.H.; Hammar, N.; Santaolalla, A.; Walldius, G.; Garmo, H.; Holmberg, L.; et al. Chronic Inflammation Markers Are Associated with Risk of Pancreatic Cancer in the Swedish AMORIS Cohort Study. BMC Cancer 2019, 19, 858. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.J.; Glen, P.; McMillan, D.C. Chronic Inflammation and Pancreatic Cancer. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.; Guo, S.; Mei, Z.; Liao, H.; Dong, H.; Wu, K.; Ye, H.; Zhang, Y.; Zhu, Y.; et al. Tumor Microbiome Contributes to an Aggressive Phenotype in the Basal-like Subtype of Pancreatic Cancer. Commun. Biol. 2021, 4, 1019. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in Pancreatic Health and Disease: The next Frontier in Microbiome Research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 53–64. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.-H.; Grote, V.A.; Tjønneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma Antibodies to Oral Bacteria and Risk of Pancreatic Cancer in a Large European Prospective Cohort Study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Alkharaan, H.; Lu, L.; Gabarrini, G.; Halimi, A.; Ateeb, Z.; Sobkowiak, M.J.; Davanian, H.; Fernández Moro, C.; Jansson, L.; Del Chiaro, M.; et al. Circulating and Salivary Antibodies to Fusobacterium nucleatum Are Associated with Cystic Pancreatic Neoplasm Malignancy. Front. Immunol. 2020, 11, 2003. [Google Scholar] [CrossRef]
- Chung, M.; Zhao, N.; Meier, R.; Koestler, D.C.; Wu, G.; de Castillo, E.; Paster, B.J.; Charpentier, K.; Izard, J.; Kelsey, K.T.; et al. Comparisons of Oral, Intestinal, and Pancreatic Bacterial Microbiomes in Patients with Pancreatic Cancer and Other Gastrointestinal Diseases. J. Oral Microbiol. 2021, 13, 1887680. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via Its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis Promotes Invasion of Oral Squamous Cell Carcinoma through Induction of ProMMP9 and Its Activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Andersson, R. Intervention on Toll-like Receptors in Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 5808–5817. [Google Scholar] [CrossRef] [PubMed]
- Udayasuryan, B.; Ahmad, R.N.; Nguyen, T.T.D.; Umaña, A.; Monét Roberts, L.; Sobol, P.; Jones, S.D.; Munson, J.M.; Slade, D.J.; Verbridge, S.S. Fusobacterium nucleatum Induces Proliferation and Migration in Pancreatic Cancer Cells through Host Autocrine and Paracrine Signaling. Sci. Signal. 2022, 15, eabn4948. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Chen, F.; Chen, S.; Luo, Y.; Si, A.; Yang, Y.; Li, Y.; Hu, W.; Zhang, Y. Long-Time Trend of Colorectal Cancer Mortality Attributable to High Processed Meat Intake in China and a Bayesian Projection from 2020 to 2030: A Model-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 10603. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Zhang, R.; Li, Y.; Wang, J.; Zhang, X.; Lin, L. Is Periodontal Disease a Risk Indicator for Colorectal Cancer? A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2021, 48, 336–347. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and Cancer. Periodontology 2000 2022, 89, 166–180. [Google Scholar] [CrossRef]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.K.; Zhao, L.; Chen, Z.; Chan, F.K.L.; Kristiansen, K.; Sung, J.J.Y.; Wong, S.H.; et al. Multi-Cohort Analysis of Colorectal Cancer Metagenome Identified Altered Bacteria across Populations and Universal Bacterial Markers. Microbiome 2018, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Watanabe, H.; Masuda, K.; Nishimoto, Y.; Kubo, M.; et al. Metagenomic and Metabolomic Analyses Reveal Distinct Stage-Specific Phenotypes of the Gut Microbiota in Colorectal Cancer. Nat. Med. 2019, 25, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Yamamoto, E.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.; Sugai, T.; An, B.; et al. Fusobacterium in Colonic Flora and Molecular Features of Colorectal Carcinoma. Cancer Res. 2014, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue and Patient Prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating TLR4 Signaling to NFκB, Upregulating Expression of MicroRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Armandi, A.; Caviglia, G.P.; Abdulle, A.; Rosso, C.; Gjini, K.; Castelnuovo, G.; Guariglia, M.; Perez Diaz Del Campo, N.; D’Amato, D.; Ribaldone, D.G.; et al. Prognostic Value of Simple Non-Invasive Tests for the Risk Stratification of Incident Hepatocellular carcinoma in Cirrhotic Individuals with Non-Alcoholic Fatty Liver Disease. Cancers 2023, 15, 1659. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Zhu, B.; Wu, C.; Lin, R.; Zhang, X. Association between Periodontal Disease, Tooth Loss and Liver Diseases Risk. J. Clin. Periodontol. 2020, 47, 1053–1063. [Google Scholar] [CrossRef]
- Sanghera, C.; Teh, J.J.; Pinato, D.J. The Systemic Inflammatory Response as a Source of Biomarkers and Therapeutic Targets in Hepatocellular carcinoma. Liver Int. 2019, 39, 2008–2023. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, Diabetes Mellitus, Atherosclerosis and Chronic Periodontitis: A Shared Pathology via Oxidative Stress and Mitochondrial Dysfunction? Periodontology 2000 2014, 64, 139–153. [Google Scholar] [CrossRef]
- Tomofuji, T.; Sanbe, T.; Ekuni, D.; Azuma, T.; Irie, K.; Maruyama, T.; Tamaki, N.; Yamamoto, T. Oxidative Damage of Rat Liver Induced by Ligature-Induced Periodontitis and Chronic Ethanol Consumption. Arch Oral Biol. 2008, 53, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Takaki, A.; Tomofuji, T.; Endo, Y.; Kasuyama, K.; Ekuni, D.; Yasunaka, T.; Yamamoto, K.; Morita, M. Stage of Hepatocellular carcinoma Is Associated with Periodontitis. J. Clin. Periodontol. 2011, 38, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Hara, E. Gut-Liver Axis-Mediated Mechanism of Liver Cancer: A Special Focus on the Role of Gut Microbiota. Cancer Sci. 2021, 112, 4433–4443. [Google Scholar] [CrossRef]
- Li, D.; Xi, W.; Zhang, Z.; Ren, L.; Deng, C.; Chen, J.; Sun, C.; Zhang, N.; Xu, J. Oral Microbial Community Analysis of the Patients in the Progression of Liver Cancer. Microb. Pathog. 2020, 149, 104479. [Google Scholar] [CrossRef]
- Kageyama, S.; Sakata, S.; Ma, J.; Asakawa, M.; Takeshita, T.; Furuta, M.; Ninomiya, T.; Yamashita, Y. High-Resolution Detection of Translocation of Oral Bacteria to the Gut. J. Dent. Res. 2023, 102, 752–758. [Google Scholar] [CrossRef]
- Ni, J.; Huang, R.; Zhou, H.; Xu, X.; Li, Y.; Cao, P.; Zhong, K.; Ge, M.; Chen, X.; Hou, B.; et al. Analysis of the Relationship between the Degree of Dysbiosis in Gut Microbiota and Prognosis at Different Stages of Primary Hepatocellular carcinoma. Front. Microbiol. 2019, 10, 1458. [Google Scholar] [CrossRef]
- Yang, X.; Lu, D.; Zhuo, J.; Lin, Z.; Yang, M.; Xu, X. The Gut-Liver Axis in Immune Remodeling: New Insight into Liver Diseases. Int. J. Biol. Sci. 2020, 16, 2357–2366. [Google Scholar] [CrossRef]
- Yin, H.; Miao, Z.; Wang, L.; Su, B.; Liu, C.; Jin, Y.; Wu, B.; Han, H.; Yuan, X. Fusobacterium nucleatum Promotes Liver Metastasis in Colorectal Cancer by Regulating the Hepatic Immune Niche and Altering Gut Microbiota. Aging 2022, 14, 1941–1958. [Google Scholar] [CrossRef]
- Marruganti, C.; Romandini, M.; Gaeta, C.; Cagidiaco, E.F.; Discepoli, N.; Parrini, S.; Graziani, F.; Grandini, S. Healthy Lifestyles Are Associated with a Better Response to Periodontal Therapy: A Prospective Cohort Study. J. Clin. Periodontol. 2023, 50, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Baima, G.; Grandini, S.; Graziani, F.; Aimetti, M.; Sanz, M.; Romandini, M. Leisure-Time and Occupational Physical Activity Demonstrate Divergent Associations with Periodontitis: A Population-Based Study. J. Clin. Periodontol. 2023, 50, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Shin, H.-S.; Sim, S.-J.; Grandini, S.; Laforí, A.; Romandini, M. Air Pollution as a Risk Indicator for Periodontitis. Biomedicines 2023, 11, 443. [Google Scholar] [CrossRef]
- Romandini, M.; Shin, H.-S.; Romandini, P.; Laforí, A.; Cordaro, M. Hormone-Related Events and Periodontitis in Women. J. Clin. Periodontol. 2020, 47, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Fitch, M.D.; Fleming, S.E. Diet Induced Obesity Increases the Risk of Colonic Tumorigenesis in Mice. Pathol. Oncol. Res. 2013, 19, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Ferrocino, I.; Gavrilova, N.; Genova, T.; Dell’Acqua, A.; Cocolin, L.; Carossa, S. Apical Periodontitis: Preliminary Assessment of Microbiota by 16S RRNA High Throughput Amplicon Target Sequencing. BMC Oral Health 2018, 18, 55. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Radaic, A.; Ganther, S.; Kamarajan, P.; Grandis, J.; Yom, S.S.; Kapila, Y.L. Paradigm Shift in the Pathogenesis and Treatment of Oral Cancer and Other Cancers Focused on the Oralome and Antimicrobial-Based Therapeutics. Periodontology 2000 2021, 87, 76–93. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]


| Periodontal Pathogens | Main Mechanisms | Tumor Location | References |
|---|---|---|---|
| Adhesion to keratinocytes, invasion and induction of NF-kB pathway | Esophageal | [64] | |
| Porphyromonas gingivalis | Gingipain-mediated activation of the MAPK/ERK signaling pathway | Colorectal | [65,66] |
| Endotoxins (LPS) induction of higher TLR4 expression | Pancreatic | ||
| Aggregatibacter actinomycetemcomitans | Cytolethal distending toxin genotoxicity and activation of NF-kB pathway | Liver and colorectal | [67,68] |
| Fusobacterium nucleatum | FadA–E-cadherin interaction inducing activation of Wnt–β-catenin signaling and CRC cell proliferation Fap2–TIGIT interaction on T and NK cells inducing immune repression Fap2–Gal-GalNac interaction inducing pro-metastatic cytokines Increase the secretion of cytokines GM-CSF, CXCL1, IL-8 in cancer cells | Colorectal | [69,70] |
| Pancreatic | |||
| Treponema denticola | Dentilisin degradation of IL-8 and TNF-α, cleavage of pro-MMP-8 and 9 | Esophageal | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baima, G.; Ribaldone, D.G.; Romano, F.; Aimetti, M.; Romandini, M. The Gum–Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers. Cancers 2023, 15, 4594. https://doi.org/10.3390/cancers15184594
Baima G, Ribaldone DG, Romano F, Aimetti M, Romandini M. The Gum–Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers. Cancers. 2023; 15(18):4594. https://doi.org/10.3390/cancers15184594
Chicago/Turabian StyleBaima, Giacomo, Davide Giuseppe Ribaldone, Federica Romano, Mario Aimetti, and Mario Romandini. 2023. "The Gum–Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers" Cancers 15, no. 18: 4594. https://doi.org/10.3390/cancers15184594
APA StyleBaima, G., Ribaldone, D. G., Romano, F., Aimetti, M., & Romandini, M. (2023). The Gum–Gut Axis: Periodontitis and the Risk of Gastrointestinal Cancers. Cancers, 15(18), 4594. https://doi.org/10.3390/cancers15184594









