A New Risk Prediction Model for Venous Thromboembolism and Death in Ambulatory Lung Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Collection and Plasma Preparation
2.3. Hemostatic Biomarkers
2.4. Thrombin Generation (TG) Assay
2.5. Study Outcomes
2.6. Statistical Analysis
2.7. KRS, New-Vienna CATS, PROTECHT, and CONKO Score Calculation
3. Results
3.1. General Characteristics of the Study Cohort
3.2. Thromboembolic Events and Mortality during Follow-Up
3.3. Hemostatic Biomarkers and Thrombin Generation
3.4. Clinical and Laboratory Predictors of VTE
3.5. Clinical and Laboratory Predictors of OS
3.6. Published RAMS for VTE and Mortality Risk Prediction
3.7. Accuracy of RAMs for VTE and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khorana, A.A.; Mackman, N.; Falanga, A.; Pabinger, I.; Noble, S.; Ageno, W.; Moik, F.; Lee, A.Y.Y. Cancer-associated venous thromboembolism. Nat. Rev. Dis. Primers 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Baldwin, D.R.; Card, T.R.; Powell, H.A.; Hubbard, R.B.; Grainge, M.J. Risk of venous thromboembolism in people with lung cancer: A cohort study using linked UK healthcare data. Br. J. Cancer 2017, 116, e1. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Card, T.R.; West, J.; Crooks, C.; Grainge, M.J. Incidence of venous thromboembolism in patients with cancer—A cohort study using linked United Kingdom databases. Eur. J. Cancer 2013, 49, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Davies, A.M.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. The incidence of venous thromboembolism among patients with primary lung cancer. J. Thromb. Haemost. 2008, 6, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Osanto, S.; Rosendaal, F.R. The risk of a venous thrombotic event in lung cancer patients: Higher risk for adenocarcinoma than squamous cell carcinoma. J. Thromb. Haemost. 2004, 2, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wu, S.G.; Lin, Y.T.; Chen, C.Y.; Shih, J.Y. Risk of thromboembolism in non-small-cell lung cancers patients with different oncogenic drivers, including ROS1, ALK, and EGFR mutations. ESMO Open 2022, 7, 100742. [Google Scholar] [CrossRef] [PubMed]
- Leiva, O.; Connors, J.M.; Al-Samkari, H. Impact of Tumor Genomic Mutations on Thrombotic Risk in Cancer Patients. Cancers 2020, 12, 1958. [Google Scholar] [CrossRef]
- Deschenes-Simard, X.; Richard, C.; Galland, L.; Blais, F.; Desilets, A.; Malo, J.; Cvetkovic, L.; Belkaid, W.; Elkrief, A.; Gagne, A.; et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: A retrospective multicentric cohort study. Thromb. Res. 2021, 205, 29–39. [Google Scholar] [CrossRef]
- Khorana, A.A.; Palaia, J.; Rosenblatt, L.; Pisupati, R.; Huang, N.; Nguyen, C.; Barron, J.; Gallagher, K.; Bond, T.C. Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. J. Immunother. Cancer 2023, 11, e006072. [Google Scholar] [CrossRef]
- Lyman, G.H. Venous thromboembolism in the patient with cancer: Focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011, 117, 1334–1349. [Google Scholar] [CrossRef]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Tafur, A.J.; Wang, C.E.; Kourelis, T.V.; Wysokinska, E.M.; Yang, P. Predictors of active cancer thromboembolic outcomes: Validation of the Khorana score among patients with lung cancer. J. Thromb. Haemost. 2016, 14, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Verzeroli, C.; Giaccherini, C.; Russo, L.; Bolognini, S.; Gamba, S.; Tartari, C.J.; Schieppati, F.; Ticozzi, C.; Vignoli, A.; Masci, G.; et al. Utility of the Khorana and the new-Vienna CATS prediction scores in cancer patients of the HYPERCAN cohort. J. Thromb. Haemost. 2023, 21, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Poniewierski, M.S.; Culakova, E.; Lyman, G.H.; Khorana, A.A.; Pabinger, I.; Agnelli, G.; Liebman, H.A.; Vicaut, E.; Meyer, G.; et al. Predictors of Venous Thromboembolism and Early Mortality in Lung Cancer: Results from a Global Prospective Study (CANTARISK). Oncologist 2018, 23, 247–255. [Google Scholar] [CrossRef]
- Van Es, N.; Ventresca, M.; Di Nisio, M.; Zhou, Q.; Noble, S.; Crowther, M.; Briel, M.; Garcia, D.; Lyman, G.H.; Macbeth, F.; et al. The Khorana score for prediction of venous thromboembolism in cancer patients: An individual patient data meta-analysis. J. Thromb. Haemost. 2020, 18, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Barni, S.; Gasparini, G.; LaBianca, R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern. Emerg. Med. 2012, 7, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, U.; Sinn, M.; Stieler, J.; Riess, H. Primary pharmacological prevention of thromboembolic events in ambulatory patients with advanced pancreatic cancer treated with chemotherapy? Dtsch. Med. Wochenschr. 2013, 138, 2084–2088. [Google Scholar]
- Gerotziafas, G.T.; Mahe, I.; Lefkou, E.; AboElnazar, E.; Abdel-Razeq, H.; Taher, A.; Antic, D.; Elalamy, I.; Syrigos, K.; Van Dreden, P. Overview of risk assessment models for venous thromboembolism in ambulatory patients with cancer. Thromb. Res. 2020, 191, S50–S57. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Y.; Du, H.; Wang, Y.; Xu, M.; Guo, X. Optimal authoritative risk assessment score of Cancer-associated venous thromboembolism for hospitalized medical patients with lung Cancer. Thromb. J. 2021, 19, 95. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; McCrae, K.; Milentijevic, D.; Germain, G.; Laliberte, F.; MacKnight, S.D.; Lefebvre, P.; Lyman, G.H.; Streiff, M.B. Cancer associated thrombosis and mortality in patients with cancer stratified by khorana score risk levels. Cancer Med. 2020, 9, 8062–8073. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Tokushige, A.; Imamura, M.; Ikeda, Y.; Ohishi, M. Evaluating the Khorana risk score of gastrointestinal cancer patients during initial chemotherapy as a predictor of patient mortality: A retrospective study. J. Cardiol. 2022, 79, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Vathiotis, I.; Dimakakos, E.P.; Boura, P.; Ntineri, A.; Charpidou, A.; Gerotziafas, G.; Syrigos, K. Khorana Score: Nuew Predictor of Early Mortality in Patients with Lung Adenocarcinoma. Clin. Appl. Thromb. Hemost. 2018, 24, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Falanga, A.; Santoro, A.; Labianca, R.; De Braud, F.; Gasparini, G.; D’Alessio, A.; Barni, S.; Iacoviello, L.; Group, H.S. Hypercoagulation screening as an innovative tool for risk assessment, early diagnosis and prognosis in cancer: The HYPERCAN study. Thromb. Res. 2016, 140, S55–S59. [Google Scholar] [CrossRef] [PubMed]
- Giaccherini, C.; Marchetti, M.; Masci, G.; Verzeroli, C.; Russo, L.; Celio, L.; Sarmiento, R.; Gamba, S.; Tartari, C.J.; Diani, E.; et al. Thrombotic biomarkers for risk prediction of malignant disease recurrence in patients with early stage breast cancer. Haematologica 2020, 105, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Castoldi, E.; Spronk, H.M.; van Oerle, R.; Balducci, D.; Barbui, T.; Rosing, J.; Ten Cate, H.; Falanga, A. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood 2008, 112, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Allignol, A.; Fine, J.P. The number of primary events per variable affects estimation of the subdistribution hazard competing risks model. J. Clin. Epidemiol. 2017, 83, 75–84. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat. Methods Med. Res. 2017, 26, 796–808. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Eckert, L.; Wang, Y.; Wang, H.; Cohen, A. Venous thromboembolism risk in patients with cancer receiving chemotherapy: A real-world analysis. Oncologist 2013, 18, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Martini, F.; Portarena, I.; Massimiani, G.; Riondino, S.; La Farina, F.; Mariotti, S.; Guadagni, F.; Roselli, M. Novel high-sensitive D-dimer determination predicts chemotherapy-associated venous thromboembolism in intermediate risk lung cancer patients. Clin. Lung Cancer 2012, 13, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Ball, D.; Solomon, B.; MacManus, M.; Manser, R.; Riedel, B.; Westerman, D.; Evans, S.M.; Wolfe, R.; Burbury, K. Dynamic Thromboembolic Risk Modelling to Target Appropriate Preventative Strategies for Patients with Non-Small Cell Lung Cancer. Cancers 2019, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007, 25, 70–76. [Google Scholar] [CrossRef]
- Kourelis, T.V.; Wysokinska, E.M.; Wang, Y.; Yang, P.; Mansfield, A.S.; Tafur, A.J. Early venous thromboembolic events are associated with worse prognosis in patients with lung cancer. Lung Cancer 2014, 86, 358–362. [Google Scholar] [CrossRef]
- Jin, Y.F.; Ye, Y.Q.; Jin, Y.J.; Zhu, X.Y.; Sha, M.; Liu, R.; Chen, C. Risk Factors and Impact on Outcomes of Lung Cancer Patients Concurrent with Deep Vein Thrombosis. Cancer Control 2022, 29, 10732748221145074. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Chen, W.; Guo, L.; Liang, L.; Zhai, Z.; Wang, C.; China Venous Thromboembolism (VTE) Study Group. Prevalence and associations of VTE in patients with newly diagnosed lung cancer. Chest 2014, 146, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Quan, X.; Xu, C.; Lv, J.; Li, C.; Dong, L.; Liu, M. Lung cancer in young adults aged 35 years or younger: A full-scale analysis and review. J. Cancer 2019, 10, 3553–3559. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, D.; Liang, D.; He, Y. Epidemiology and prognosis in young lung cancer patients aged under 45 years old in northern China. Sci. Rep. 2021, 11, 6817. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, I.T.; Barco, S.; Mavromanoli, A.C.; Konstantinides, S.V.; Valerio, L. Performance Status and Long-Term Outcomes in Cancer-Associated Pulmonary Embolism: Insights from the Hokusai-VTE Cancer Study. JACC CardioOncol. 2022, 4, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Noventa, F.; Milan, M. Aspirin and recurrent venous thromboembolism. Phlebology 2013, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Larocca, A.; Cavallo, F.; Bringhen, S.; Di Raimondo, F.; Falanga, A.; Evangelista, A.; Cavalli, M.; Stanevsky, A.; Corradini, P.; Pezzatti, S.; et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012, 119, 933–939; quiz 1093. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Larran, A.; Pereira, A.; Guglielmelli, P.; Hernandez-Boluda, J.C.; Arellano-Rodrigo, E.; Ferrer-Marin, F.; Samah, A.; Griesshammer, M.; Kerguelen, A.; Andreasson, B.; et al. Antiplatelet therapy versus observation in low-risk essential thrombocythemia with a CALR mutation. Haematologica 2016, 101, 926–931. [Google Scholar] [CrossRef]
- Shai, A.; Rennert, H.S.; Lavie, O.; Ballan-Haj, M.; Bitterman, A.; Steiner, M.; Keren, S.; Rennert, G. Statins, aspirin and risk of venous thromboembolic events in breast cancer patients. J. Thromb. Thrombolysis 2014, 38, 32–38. [Google Scholar] [CrossRef]
- Shai, A.; Rennert, H.S.; Rennert, G.; Sagi, S.; Leviov, M.; Lavie, O. Statins, aspirin and risk of thromboembolic events in ovarian cancer patients. Gynecol. Oncol. 2014, 133, 304–308. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Buller, H.R.; van Es, N.; CAT-prediction collaborators. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef]
- Rupa-Matysek, J.; Lembicz, M.; Rogowska, E.K.; Gil, L.; Komarnicki, M.; Batura-Gabryel, H. Evaluation of risk factors and assessment models for predicting venous thromboembolism in lung cancer patients. Med. Oncol. 2018, 35, 63. [Google Scholar] [CrossRef]
- Noble, S.R.A.; Alikhan, R.; Hood, K.; Macbeth, F. Prediction of venous thromboembolism in lung cancer patients receiving chemotherapy OR 130. Int. Soc. Thromb. Haemost. 2015, 13, 143. [Google Scholar]
| Overall Cohort (n = 568) | VTE (n = 62) | Death (n = 167) | |
|---|---|---|---|
| Male sex (n, %) Age (years, mean [SD]) BMI (kg/m2, mean [SD]) BMI ≥ 35kg/m2 (n, %) ECOG (n, %) 0 1 2 | 381 (67) 65 (9.5) 25 (4.3) 11 (2) 236 (42) 252 (44) 51 (9) | 45 (73) 63 (8.9) 25 (4.4) 2 (13) 24 (39) 26 (42) 9 (15) | 121 (73) 66 (9.7) 25 (4.3) 2 (1) 41 (25) 84 (50) 33 (20) |
| Smoking (n, %) Active Previous | 194 (34) 250 (44) | 22 (36) 28 (45) | 53 (32) 79 (47) |
| Comorbidities ≥ 1 risk factor (n, %) Diabetes Hypertension Dyslipidemia Cardiopathy CVA history | 432 (76) 61 (11) 241 (42) 86 (15) 48 (9) 10 (2) | 45 (73) 8 (13) 26 (42) 5 (8) 4 (7) 1 (2) | 129 (77) 22 (13) 83 (50) 24 (14) 12 (7) 2 (1) |
| Antithrombotic therapy (n, %) * Antiplatelet drugs Anticoagulants | 108 (19) 35 (6) | 3 (5) 5 (8) | 30 (18) 14 (8) |
| Histological subtypes (n, %) Squamous Large-cell carcinoma Neuroendocrine Sarcomatoid Adenocarcinoma Mixed Mucinous Acinar Solid Papilar Non-differentiated Non classified | 84 (15) 5 (1) 1 (0.2) 5 (1) 262 (46) 2 (0.4) 6 (1) 1 (0.2) 2 (0.4) 7 (1) 22 (4) 171 (30) | 9 (15) 27 (44) 4 (6.5) 21 (34) | 22 (13) 67 (40) 11 (7) 56 (34) |
| Metastatic site (n, %) Intrathoracic Bone Suprarenal Encephalic | 401 (71) 189 (33) 92 (16) 124 (22) | 41 (66) 22 (36) 11 (18) 20 (32) | 121 (73) 65 (39) 41 (25) 44 (26) |
| Blood Count (median [95%CI]) Leukocyte, 109/L Hemoglobin, g/dL Hematocrit, % Platelets, 109/L | 9.2 (4.6–19.2) 13.3 (9.8–15.5) 40 (31–47) 280 (145–507) | 9.6 (3.0–26.9) 13.8 (9.8–15.4) 41 (31–46) 269 (125–431) | 10.2 (4.8–27.7) 12.7 (9.3–15.1) 39 (29–46) 297 (127–524) |
| Chemotherapy (n, %) Platinum or Gemcitabine Platinum with Gemcitabine Other Immunotherapy (n, %) Target therapy (n, %) Radiotherapy (n, %) | 377 (66) 127 (22) 48 (9) 85 (15) 54 (10) 281 (49) | 41 (66) 15 (24) 6 (10) 10 (6) 10 (6) 40 (65) | 112 (67) 40 (24) 15 (9) 9 (5) 7 (4) 70 (42) |
| Reference Value | VTE Free (n = 506) | VTE (n = 62) | p-Value | Survivors (n = 401) | Non-Survivors (n = 167) | p-Value | |
|---|---|---|---|---|---|---|---|
| F1 + 2, pmol/L | 215 (126–478) | 255 (128–826) | 338 (135–1122) | 0.001 | 283 (144–824) | 293 (141–1033) | 0.151 |
| D-dimer, ng/mL | 110 (40–280) | 1330 (170–5620) | 2030 (160–8340) | 0.002 | 620 (150–4800) | 1230 (270–7600) | <0.001 |
| Fibrinogen, mg/dL | 150–400 | 475 (248–922) | 444 (201–800) | 0.231 | 475 (245–908) | 524 (240–1061) | <0.001 |
| FVIII, % | 104 (73–145) | 155 (75–295) | 190 (92–362) | 0.007 | 150 (71–250) | 186 (101–280) | <0.001 |
| Free PS, % | 90 (70–120) | 86 (59–116) | 87 (58–116) | 0.645 | 86 (59–116) | 89 (59–110) | 0.316 |
| PC, % | 98 (72–125) | 120 (82–182) | 132 (78–204) | 0.025 | 120 (82–202) | 124 (82–188) | 0.951 |
| TG lag time, min | 3.1 (2.2–4.5) | 3.3 (2.2–5.4) | 3.1 (2.0–4.6) | 0.023 | 3.2 (2.1–4.8) | 3.3 (2.3–5.0) | 0.042 |
| TG ETP, nM*min | 1702 (962–2601) | 1847 (1228–2730) | 1836 (1124–2993) | 0.736 | 1853 (1279–2874) | 1837 (1164–3033) | 0.630 |
| TG ttP, min | 6.7 (4.7–8.8) | 5.7 (4.1–8.6) | 5.1 (3.7–7.4) | 0.001 | 5.5 (4.0–8.2) | 5.4 (4.1–8.1) | 0.647 |
| TG peak, nM | 237 (128–404) | 390 (210–598) | 432 (278–592) | 0.002 | 400 (206–591) | 424 (244–642) | 0.025 |
| D-Dimer Levels (ng/mL) | Points |
|---|---|
| >4000 | 3 |
| >1500–4000 | 2 |
| 500–1500 | 1 |
| <500 | 0 |
| ECOG performance | |
| 2 | 1 |
| 0–1 | 0 |
| Risk 0–1 point = low, ≥2 points high | |
| 6-Month VTE | 6-Month Death | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAM | Risk Category | Cumulative Incidence (95% CI) | Log-Rank (p-Value) | ROC AUC (p-Value) | Sen (%) | Spe (%) | PPV (%) | NPV (%) | Cumulative Incidence (95% CI) | Log-Rank (p-Value) | ROC AUC (p-Value) | Sen (%) | Spe (%) | PPV (%) | NPV (%) |
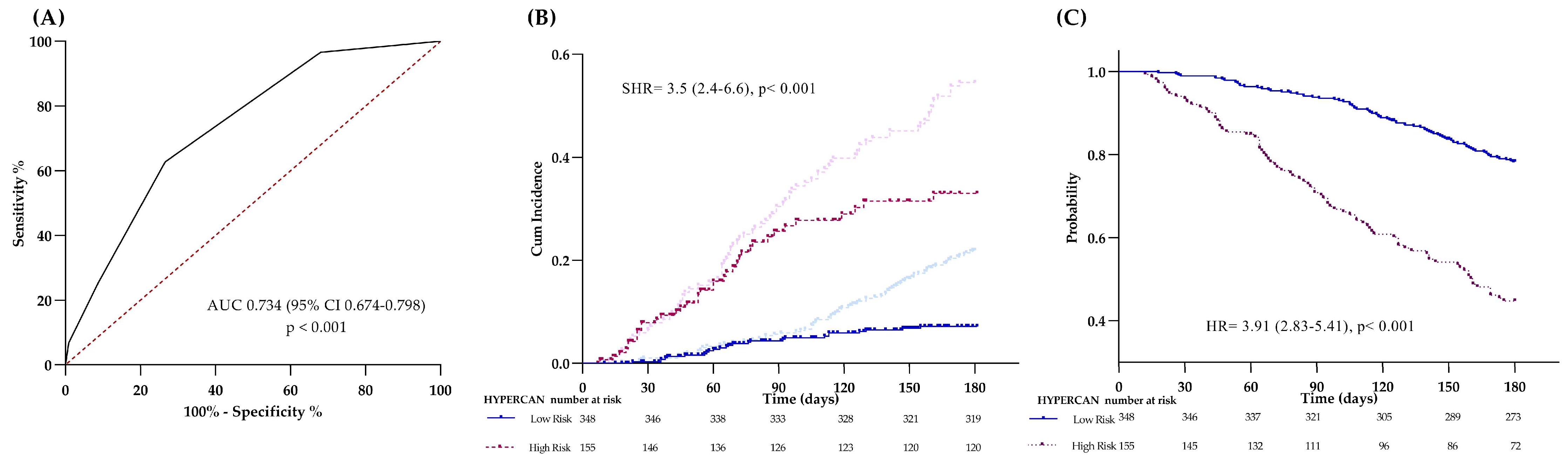
HYPERCAN
| Low High | 6 (4–10) 25 (24–42) | < 0.001 | 0.734 (<0.001) | 63 | 74 | 25 | 93 | 19 (15–23) 55 (47–63) | <0.001 | 0.726 (<0.001) | 56 | 80 | 55 | 81 |
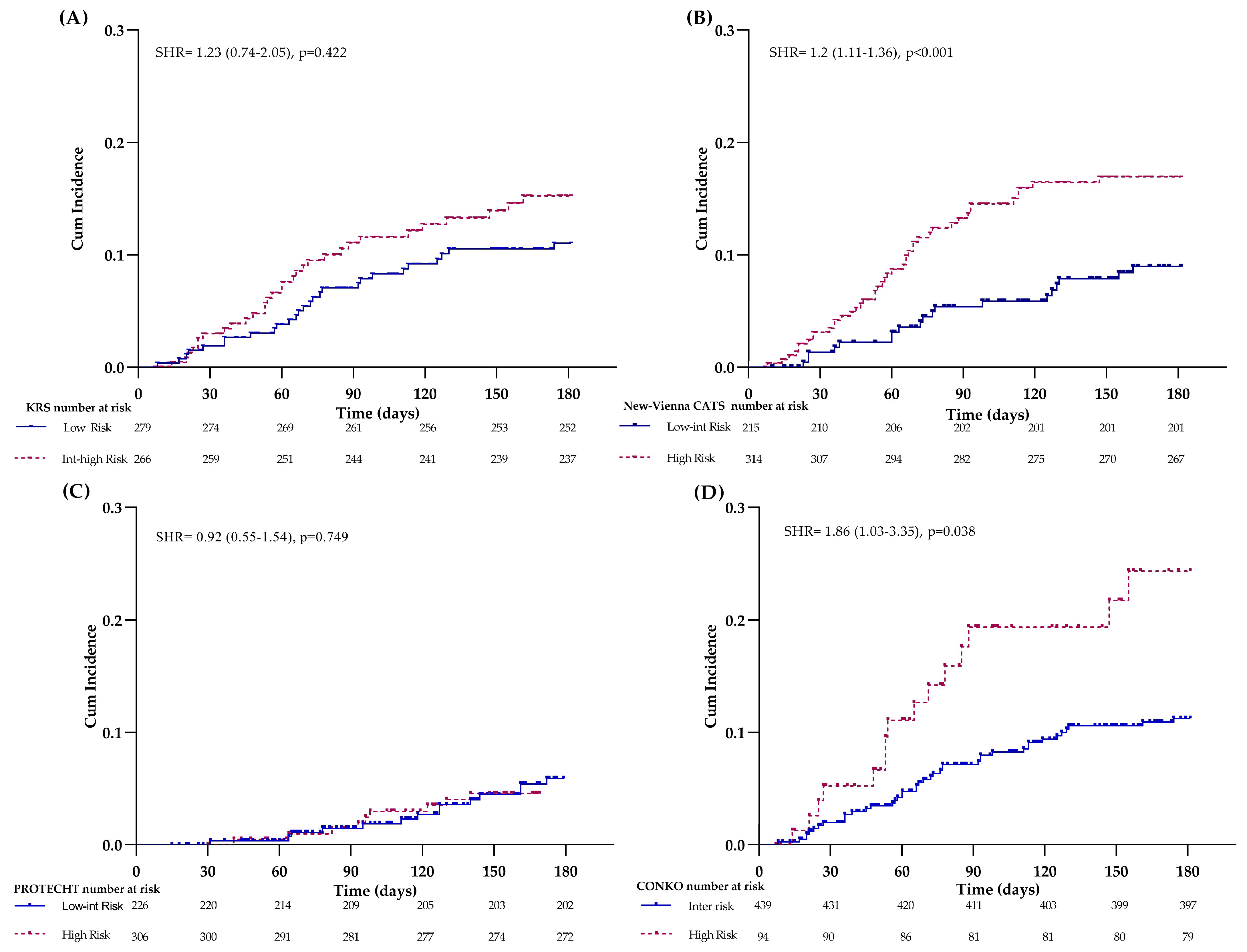
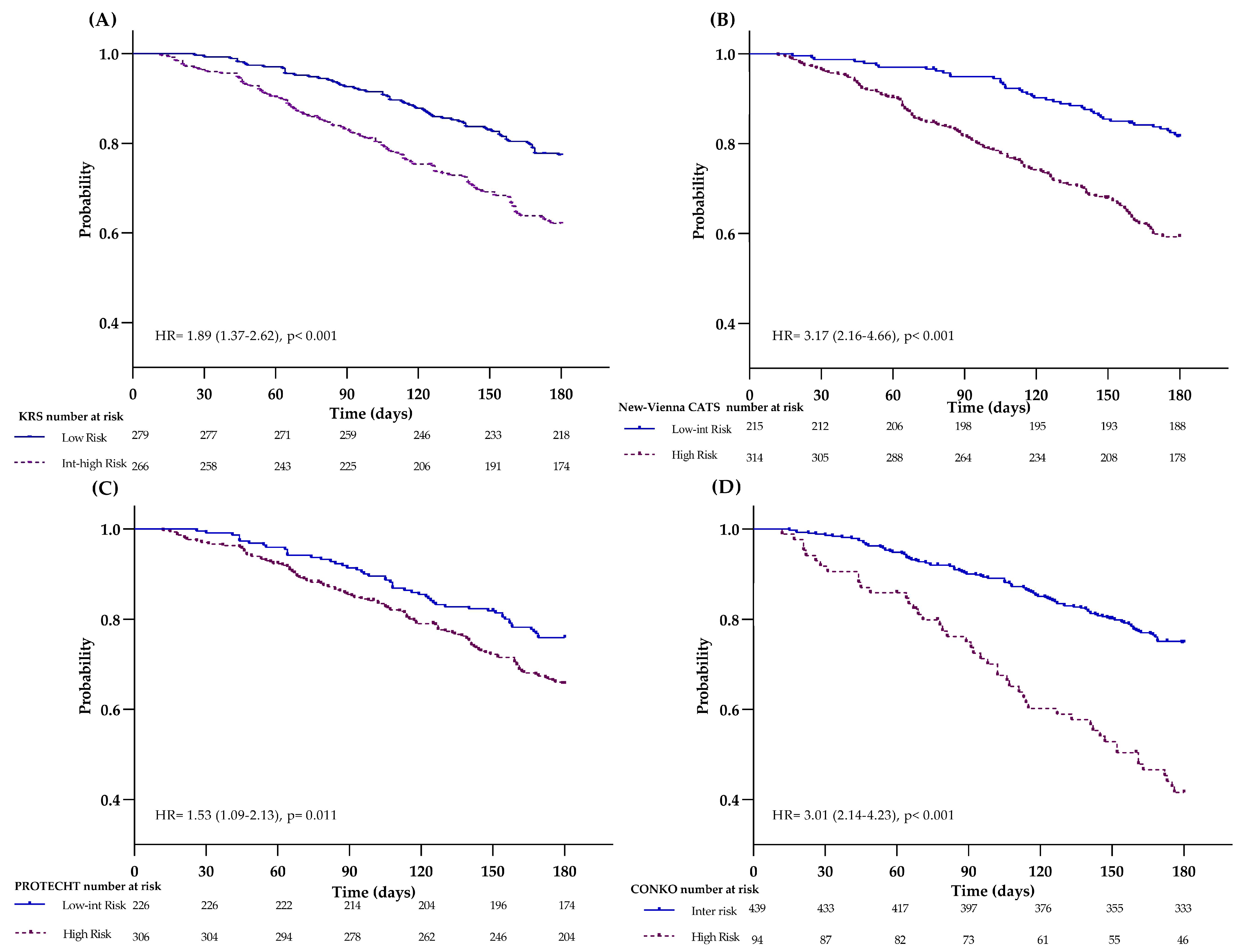
KRS
| Low Int-High | 11 (9–15) 16 (9–30) | 0.089 | 0.543 (0.290) | 21 | 86 | 16 | 89 | 26 (22–30) 49 (39–62) | <0.001 | 0.609 (<0.001) | 25 | 89 | 49 | 74 |
New-Vienna CATS *
| Low-Int High | 9 (5–13) 14 (12–22) | 0.008 | 0.642 (0.001) | 70 | 43 | 14 | 92 | 15 (11–20) 40 (35–46) | <0.001 | 0.670 (<0.001) | 79 | 50 | 40 | 85 |
PROTECHT
| Low-Int High | 11 (8–17) 12 (9–18) | 0.730 | 0.527 (0.504) | 59 | 42 | 12 | 89 | 24 (18–30) 34 (29–40) | 0.012 | 0.584 (0.002) | 66 | 46 | 34 | 76 |
CONKO
| Int High | 10 (8–14) 19 (13–36) | 0.004 | 0.558 (0.156) | 26 | 85 | 19 | 90 | 25 (21–29) 57 (48–69) | <0.001 | 0.647 (<0.001) | 31 | 90 | 56 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Rosas, P.; Giaccherini, C.; Russo, L.; Verzeroli, C.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Ticozzi, C.; Schieppati, F.; Barcella, L.; et al. A New Risk Prediction Model for Venous Thromboembolism and Death in Ambulatory Lung Cancer Patients. Cancers 2023, 15, 4588. https://doi.org/10.3390/cancers15184588
Gomez-Rosas P, Giaccherini C, Russo L, Verzeroli C, Gamba S, Tartari CJ, Bolognini S, Ticozzi C, Schieppati F, Barcella L, et al. A New Risk Prediction Model for Venous Thromboembolism and Death in Ambulatory Lung Cancer Patients. Cancers. 2023; 15(18):4588. https://doi.org/10.3390/cancers15184588
Chicago/Turabian StyleGomez-Rosas, Patricia, Cinzia Giaccherini, Laura Russo, Cristina Verzeroli, Sara Gamba, Carmen Julia Tartari, Silvia Bolognini, Chiara Ticozzi, Francesca Schieppati, Luca Barcella, and et al. 2023. "A New Risk Prediction Model for Venous Thromboembolism and Death in Ambulatory Lung Cancer Patients" Cancers 15, no. 18: 4588. https://doi.org/10.3390/cancers15184588
APA StyleGomez-Rosas, P., Giaccherini, C., Russo, L., Verzeroli, C., Gamba, S., Tartari, C. J., Bolognini, S., Ticozzi, C., Schieppati, F., Barcella, L., Sarmiento, R., Masci, G., Tondini, C., Petrelli, F., Giuliani, F., D’Alessio, A., Minelli, M., De Braud, F., Santoro, A., ... on behalf of the HYPERCAN Investigators. (2023). A New Risk Prediction Model for Venous Thromboembolism and Death in Ambulatory Lung Cancer Patients. Cancers, 15(18), 4588. https://doi.org/10.3390/cancers15184588










