State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine
Abstract
Simple Summary
Abstract
1. Introduction
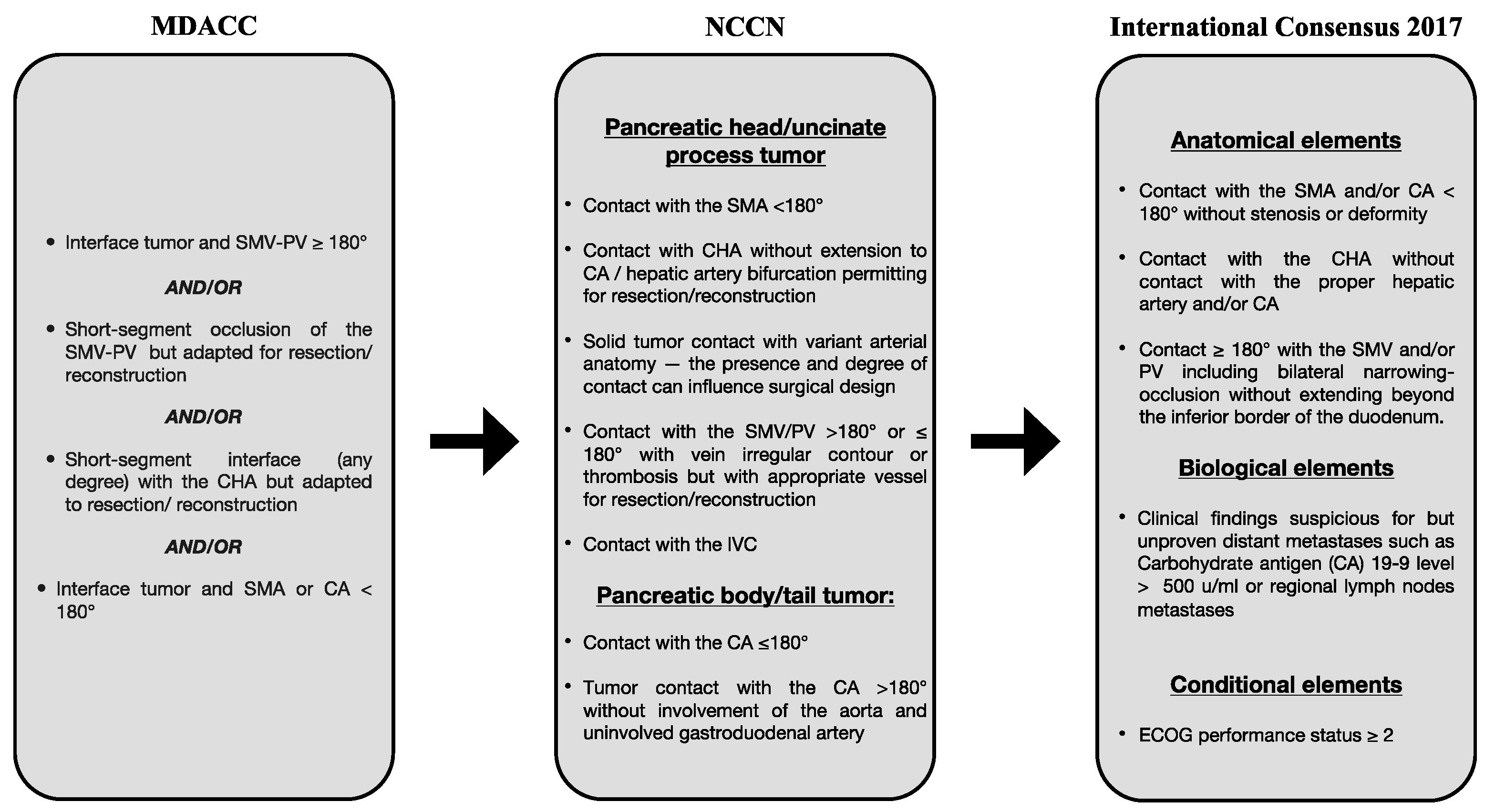
2. Advances in Pancreatic Surgery
2.1. Surgical Approaches
2.2. Laparoscopy and Robotics Are the New Advances in Pancreatic Surgery
3. Locoregional Therapy in Unresectable Pancreatic Cancer
3.1. Brachytherapy
3.2. Ablative Therapies
3.3. EUS-Guided Fiducial Marker Implantation and Intratumoral Delivery of Chemotherapeutic Agents
4. Chemotherapy
5. The Quality of Life
5.1. Pain Management
5.2. Nutrition
5.3. Psychological Assessment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prades, J.; Arnold, D.; Brunner, T.; Cardone, A.; Carrato, A.; Coll-Ortega, C.; De Luze, S.; Garel, P.; Goossens, M.E.; Grilli, R.; et al. Bratislava Statement: Consensus recommendations for improving pancreatic cancer care. ESMO Open 2020, 5, e001051. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Michl, P.; Löhr, M.; Neoptolemos, J.P.; Capurso, G.; Rebours, V.; Malats, N.; Ollivier, M.; Ricciardiello, L. UEG position paper on pancreatic cancer. Bringing pancreatic cancer to the 21st century: Prevent, detect, and treat the disease earlier and better. United Eur. Gastroenterol. J. 2021, 9, 860–871. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Lowenfels, A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiol. 2014, 44, 186–198. [Google Scholar] [CrossRef]
- Rosato, V.S.; Polesel, J.S.; Bosetti, C.; Serraino, D.; Negri, E.S.; La Vecchia, C. Population Attributable Risk for Pancreatic Cancer in Northern Italy. Pancreas 2015, 44, 216–220. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Gomez-Rubio, P.; Márquez, M.; Rava, M.; Löhr, M.; Michalski, C.W.; Molero, X.; Farré, A.; Perea, J.; Greenhalf, W.; et al. Risk of pancreatic cancer associated with family history of cancer and other medical conditions by accounting for smoking among relatives. Int. J. Epidemiol. 2018, 47, 473–483. [Google Scholar] [CrossRef]
- Greenhalf, W.; Lévy, P.; Gress, T.; Rebours, V.; Brand, R.E.; Pandol, S.; Chari, S.; Jørgensen, M.T.; Mayerle, J.; Lerch, M.M.; et al. International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association. Pancreatology 2020, 20, 910–918. [Google Scholar] [CrossRef]
- Solomon, S.M.; Das, S.B.; Brand, R.; Whitcomb, D.C. Inherited Pancreatic Cancer Syndromes. Cancer J. 2012, 18, 485–491. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer. A Review. J. Am. Med. Assoc. 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Schmidt-Hansen, M.; Berendse, S.; Hamilton, W. Symptoms of Pancreatic Cancer in Primary Care. Pancreas 2016, 45, 814–818. [Google Scholar] [CrossRef]
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic Ductal Adenocarcinoma Radiology Reporting Template: Consensus Statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014, 270, 248–260. [Google Scholar] [CrossRef]
- Vachiranubhap, B.; Kim, Y.H.; Balci, N.C.; Semelka, R.C. Magnetic Resonance Imaging of Adenocarcinoma of the Pancreas. Top. Magn. Reson. Imaging 2009, 20, 3–9. [Google Scholar] [CrossRef]
- Tzeng, C.-W.D.; Balachandran, A.; Ahmad, M.; Lee, J.E.; Krishnan, S.; Wang, H.; Crane, C.H.; Wolff, R.A.; Varadhachary, G.R.; Pisters, P.W.; et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB 2014, 16, 430–438. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology 2021, 160, 1373–1383.e6. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.S.; Lee, Y.K.; Norton, J.A.; Jeffrey, S.S. Liquid biopsy in pancreatic ductal adenocarcinoma: Current status of circulating tumor cells and circulating tumorDNA. Mol. Oncol. 2019, 13, 1623–1650. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Gilbert, J.; Wolpin, B.; Clancy, T.; Wang, J.; Mamon, H.; Shinagare, A.; Jagannathan, J.; Rosenthal, M. Borderline resectable pancreatic cancer: Conceptual evolution and current approach to image-based classification. Ann. Oncol. 2017, 28, 2067–2076. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Marsh, R.; Herman, J.M.; Shi, Q.; Collison, E.; Venook, A.P.; Kindler, H.L.; Alberts, S.R.; Philip, P.; Lowy, A.M.; et al. Borderline Resectable Pancreatic Cancer: Need for Standardization and Methods for Optimal Clinical Trial Design. Ann. Surg. Oncol. 2013, 20, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Castillo, C.F.-D.; Hackert, T.; Hayasaki, A.; Katz, M.H.; Kim, S.-W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.T.; Evans, D.B.; Wolff, R.A. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Demir, I.E.; Jäger, C.; Schlitter, A.M.; Konukiewitz, B.; Stecher, L.; Schorn, S.; Tieftrunk, E.; Scheufele, F.; Calavrezos, L.; Schirren, R.; et al. R0 Versus R1 Resection Matters after Pancreaticoduodenectomy, and Less after Distal or Total Pancreatectomy for Pancreatic Cancer. Ann. Surg. 2018, 268, 1058–1068. [Google Scholar] [CrossRef]
- Strobel, O.; Hank, T.; Hinz, U.; Bergmann, F.; Schneider, L.; Springfeld, C.; Jäger, D.; Schirmacher, P.; Hackert, T.; Büchler, M.W. Pancreatic Cancer Surgery. Ann. Surg. 2017, 265, 565–573. [Google Scholar] [CrossRef]
- Tol, J.A.; Gouma, D.J.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bockhorn, M.; Büchler, M.W.; et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014, 156, 591–600. [Google Scholar] [CrossRef]
- Hackert, T.; Klaiber, U.; Pausch, T.; Mihaljevic, A.L.; Büchler, M.W. Fifty Years of Surgery for Pancreatic Cancer. Pancreas 2020, 49, 1005–1013. [Google Scholar] [CrossRef]
- Stoop, T.F.; Ateeb, Z.; Ghorbani, P.; Scholten, L.; Arnelo, U.; Besselink, M.G.; Del Chiaro, M. Surgical Outcomes After Total Pancreatectomy: A High-Volume Center Experience. Ann. Surg. Oncol. 2021, 28, 1543–1551. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital Volume and Surgical Mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally advanced pancreatic cancer: Neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann. Surg. 2016, 264, 457–463. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Wang, H.; Fleming, J.B.; Sun, C.C.; Hwang, R.F.; Wolff, R.A.; Varadhachary, G.; Abbruzzese, J.L.; Crane, C.H.; Krishnan, S.; et al. Long-Term Survival After Multidisciplinary Management of Resected Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2009, 16, 836–847. [Google Scholar] [CrossRef]
- Kolbeinsson, H.; Hoppe, A.; Bayat, A.; Kogelschatz, B.; Mbanugo, C.; Chung, M.; Wolf, A.; Assifi, M.M.; Wright, G.P. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery 2021, 169, 649–654. [Google Scholar] [CrossRef]
- Macedo, F.I.; Ryon, E.; Maithel, S.K.; Lee, R.M.; Kooby, D.A.; Fields, R.C.; Hawkins, W.G.; Williams, G.; Maduekwe, U.; Kim, H.J.; et al. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann. Surg. 2019, 270, 400–413. [Google Scholar] [CrossRef]
- Kim, R.Y.; Christians, K.K.; Aldakkak, M.; Clarke, C.N.; George, B.; Kamgar, M.; Khan, A.H.; Kulkarni, N.; Hall, W.A.; Erickson, B.A.; et al. Total Neoadjuvant Therapy for Operable Pancreatic Cancer. Ann. Surg. Oncol. 2021, 28, 2246–2256. [Google Scholar] [CrossRef]
- Artinyan, A.; Anaya, D.A.; McKenzie, S.; Ellenhorn, J.D.I.; Kim, J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer 2011, 117, 2044–2049. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Tonini, V.; Zanni, M. Pancreatic cancer in 2021: What you need to know to win. World J. Gastroenterol. 2021, 27, 5851–5889. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2022, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hüttner, F.J.; Fitzmaurice, C.; Schwarzer, G.; Seiler, C.M.; Antes, G.; Büchler, M.W.; Diener, M.K. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst. Rev. 2016, 2016, CD006053. [Google Scholar] [CrossRef] [PubMed]
- Ishchenko, R.V.; Ivanov, Y.V.; Smirnov, A.V.; Danilina, E.S.; Lysenko, A.; Hospital, V.C.C. ‘Artery-first’ approaches to pancreatoduodenectomy. Clin. Exp. Surg. 2022, 99, 1027–1035. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, Z.; Ma, Z.; Deng, H.; Ren, W.; Shi, W.; Jiao, Z. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: A meta-analysis. Int. J. Surg. 2020, 73, 14–24. [Google Scholar] [CrossRef]
- Schneider, M.; Hackert, T.; Strobel, O.; Büchler, M.W. Technical advances in surgery for pancreatic cancer. Br. J. Surg. 2021, 108, 777–785. [Google Scholar] [CrossRef]
- Zwart, E.; Yilmaz, B.; Halimi, A.; Ahola, R.; Kurlinkus, B.; Laukkarinen, J.; Ceyhan, G. Venous resection for pancreatic cancer, a safe and feasible option? A systematic review and meta-analysis. Pancreatology 2022, 22, 803–809. [Google Scholar] [CrossRef]
- Gagner, M.; Pomp, A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg. Endosc. 1994, 8, 408–410. [Google Scholar] [CrossRef]
- Poves, I.; Burdío, F.; Morató, O.; Iglesias, M.; Radosevic, A.; Ilzarbe, L.; Visa, L.; Grande, L. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: The Padulap randomized controlled trial. Ann. Surg. 2018, 268, 731–739. [Google Scholar] [CrossRef]
- de Rooij, T.; van Hilst, J.; van Santvoort, H.; Boerma, D.; van den Boezem, P.; Daams, F.; van Dam, R.; Dejong, C.; van Duyn, E.; Dijkgraaf, M.; et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann. Surg. 2019, 269, 2–9. [Google Scholar] [CrossRef]
- Croome, K.P.; Farnell, M.B.; Que, F.G.; Reid-Lombardo, K.; Truty, M.J.; Nagorney, D.M.; Kendrick, M.L. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma oncologic advantages over open approaches? Ann. Surg. 2014, 260, 633–640. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Zhang, R.-C.; Zhou, Y.-C. Comparison of overall survival and perioperative outcomes of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. BMC Cancer 2019, 19, 781. [Google Scholar] [CrossRef]
- Stauffer, J.A.; Coppola, A.; Villacreses, D.; Mody, K.; Johnson, E.; Li, Z.; Asbun, H.J. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: Long-term results at a single institution. Surg. Endosc. 2017, 31, 2233–2241. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, X.; Peng, B.; Li, B. Is total laparoscopic pancreaticoduodenectomy superior to open procedure? A meta-analysis. World J. Gastroenterol. 2019, 25, 5711–5731. [Google Scholar] [CrossRef]
- Melvin, W.; Needleman, B.; Krause, K.; Ellison, E. Robotic Resection of Pancreatic Neuroendocrine Tumor. J. Laparoendosc. Adv. Surg. Tech. 2003, 13, 33–36. [Google Scholar] [CrossRef]
- Zureikat, A.H.; Postlewait, L.M.; Liu, Y.; Gillespie, T.W.; Weber, S.M.; Abbott, D.E.; Ahmad, S.A.; Maithel, S.K.; Hogg, M.E.; Zenati, M.; et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann. Surg. 2016, 264, 640–649. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.-M.; You, L.; Zhao, Y.-P. Robotic versus Open Pancreatectomy: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2013, 20, 1774–1780. [Google Scholar] [CrossRef]
- Peng, L.; Lin, S.; Li, Y.; Xiao, W. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surg. Endosc. 2017, 31, 3085–3097. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J.; Marc, O.S.; Jiao, L.R.; Manas, D.; Abu Hilal, M.; White, S.A. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 6–14. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Lim, C.; Menahem, B.; Lahat, E.; Salloum, C.; Azoulay, D. Robotic versus laparoscopic distal pancreatectomy—The first meta-analysis. HPB 2016, 18, 567–574. [Google Scholar] [CrossRef]
- Lof, S.; van der Heijde, N.; Abuawwad, M.; Al-Sarireh, B.; Boggi, U.; Butturini, G.; Capretti, G.; Coratti, A.; Casadei, R.; D’hondt, M.; et al. Robotic versus laparoscopic distal pancreatectomy: Multicentre analysis. Br. J. Surg. 2021, 108, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Caruso, R.; D’Ovidio, A.; Núñez-Alfonsel, J.; Pinilla, F.B.; Collazo, Y.Q.; Vicente, E.; Ielpo, B. Robotic versus laparoscopic distal pancreatectomies: A systematic review and meta-analysis on costs and perioperative outcome. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2295. [Google Scholar] [CrossRef] [PubMed]
- Van Hilst, J.; De Rooij, T.; Klompmaker, S.; Rawashdeh, M.; Aleotti, F.; Al-Sarireh, B.; Alseidi, A.; Ateeb, Z.; Balzano, G.; Berrevoet, F.; et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): A Pan-European Propensity Score Matched Study. Ann. Surg. 2019, 269, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; Del Chiaro, M.; Van Lienden, K.P.; Meijerink, M.R.; Van Tienhoven, G.; et al. Locally advanced pancreatic cancer: Work-up, staging, and local intervention strategies. Cancers 2019, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Herman, J.; Klapman, J.; Tuli, R.; El-Haddad, G.; Hoffe, S.; Wong, F.L.; Chasen, B.; Fogelman, D.; Lo, S.; et al. An open-label, single-arm pilot study of EUS-guided brachytherapy with phosphorus-32 microparticles in combination with gemcitabine +/- nab-paclitaxel in unresectable locally advanced pancreatic cancer (OncoPaC-1): Technical details and study protocol. Endosc. Ultrasound 2020, 9, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gillen, S.; Schuster, T.; Büschenfelde, C.M.Z.; Friess, H.; Kleeff, J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef]
- Paiella, S.; Salvia, R.; Girelli, R.; Frigerio, I.; Giardino, A.; D’onofrio, M.; De Marchi, G.; Bassi, C. Role of local ablative techniques (Radiofrequency ablation and Irreversible Electroporation) in the treatment of pancreatic cancer. Updat. Surg. 2016, 68, 307–311. [Google Scholar] [CrossRef]
- Sun, X.; Lu, Z.; Wu, Y.; Min, M.; Bi, Y.; Shen, W.; Xu, Y.; Li, Z.; Jin, Z.; Liu, Y. An endoscopic ultrasonography-guided interstitial brachytherapy based special treatment-planning system for unresectable pancreatic cancer. Oncotarget 2017, 8, 79099–79110. [Google Scholar] [CrossRef]
- Wang, J.; Chai, S.; Zheng, G.; Jiang, Y.; Ji, Z.; Guo, F.; Zhuang, H.; Zhang, K. Expert consensus statement on computed tomography-guided 125I radioactive seeds permanent interstitial brachytherapy. J. Cancer Res. Ther. 2018, 14, 12–17. [Google Scholar] [CrossRef]
- Gai, B.; Zhang, F. Chinese expert consensus on radioactive 125I seeds interstitial implantation brachytherapy for pancreatic cancer. J. Cancer Res. Ther. 2018, 14, 1455–1462. [Google Scholar] [CrossRef]
- Jia, S.-N.; Wen, F.-X.; Gong, T.-T.; Li, X.; Wang, H.-J.; Sun, Y.-M.; Yang, Z.-C. A review on the efficacy and safety of iodine-125 seed implantation in unresectable pancreatic cancers. Int. J. Radiat. Biol. 2020, 96, 383–389. [Google Scholar] [CrossRef]
- Jin, K.; Xing, B. Comments on National guidelines for diagnosis and treatment of pancreatic cancer 2022 in China (English version). Chin. J. Cancer Res. 2022, 34, 637–643. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Wang, Z.; Zhang, T.; Cai, F.; Tang, P.; Meng, J.; Du, H.; Wang, H.; Li, M.; et al. The role of seed implantation in patients with unresectable pancreatic carcinoma after relief of obstructive jaundice using ERCP. Brachytherapy 2020, 19, 97–103. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Wu, J.; Zhu, R.; Ji, W. A Systematic Review and Meta-analysis of Intraluminal Brachytherapy Versus Stent Alone in the Treatment of Malignant Obstructive Jaundice. Cardiovasc. Interv. Radiol. 2017, 41, 206–217. [Google Scholar] [CrossRef]
- Naidu, J.; Bartholomeusz, D.; Zobel, J.; Safaeian, R.; Hsieh, W.; Crouch, B.; Ho, K.; Calnan, D.; Singhal, N.; Ruszkiewicz, A.; et al. Combined chemotherapy and endoscopic ultrasound-guided intratumoral 32P implantation for locally advanced pancreatic adenocarcinoma: A pilot study. Endoscopy 2021, 54, 75–80. [Google Scholar] [CrossRef]
- Lv, W.-F.; Lu, D.; Xiao, J.-K.; Mukhiya, G.; Tan, Z.-X.; Cheng, D.-L.; Zhou, C.-Z.; Zhang, X.-M.; Zhang, Z.-F.; Hou, C.-L. The side effects and complications of percutaneous iodine-125 seeds implantation under CT-guide for patients with advanced pancreatic cancer. Medicine 2017, 96, e9535. [Google Scholar] [CrossRef]
- Marinova, M.; Wilhelm-Buchstab, T.; Strunk, H. Advanced Pancreatic Cancer: High-Intensity Focused Ultrasound (HIFU) and Other Local Ablative Therapies. RoFo Fortschr. Auf Dem Geb. Rontgenstrahlen Bildgeb. Verfahren. 2019, 191, 216–227. [Google Scholar] [CrossRef]
- Frigerio, I.; Paiella, S.; Barbi, E.; Bianco, R.; Boz, G.; Butturini, G.; Cantore, M.; Cardarelli, N.; Mirko, D.; Fiorentini, G.; et al. Open radiofrequency ablation as upfront treatment for locally advanced pancreatic cancer: Requiem from a randomized controlled trial. Pancreatology 2021, 21, 1342–1348. [Google Scholar] [CrossRef]
- Giardino, A.; Innamorati, G.; Ugel, S.; Perbellini, O.; Girelli, R.; Frigerio, I.; Regi, P.; Scopelliti, F.; Butturini, G.; Paiella, S.; et al. Immunomodulation after radiofrequency ablation of locally advanced pancreatic cancer by monitoring the immune response in 10 patients. Pancreatology 2017, 17, 962–966. [Google Scholar] [CrossRef]
- Walma, M.S.; Dutch Pancreatic Cancer Group; Rombouts, S.J.; Brada, L.J.H.; Rinkes, I.H.B.; Bosscha, K.; Bruijnen, R.C.; Busch, O.R.; Creemers, G.J.; Daams, F.; et al. Radiofrequency ablation and chemotherapy versus chemotherapy alone for locally advanced pancreatic cancer (PELICAN): Study protocol for a randomized controlled trial. Trials 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Paiella, S.; Malleo, G.; Cataldo, I.; Gasparini, C.; De Pastena, M.; De Marchi, G.; Marchegiani, G.; Rusev, B.; Scarpa, A.; Girelli, R.; et al. Radiofrequency ablation for locally advanced pancreatic cancer: SMAD4 analysis segregates a responsive subgroup of patients. Langenbeck’s Arch. Surg. 2017, 403, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Reccia, I.; Sodergren, M.H.; Kusano, T.; Zanellato, A.; Pai, M.; Spalding, D.; Zacharoulis, D.; Habib, N. Radiofrequency assisted pancreaticoduodenectomy for palliative surgical resection of locally advanced pancreatic adenocarcinoma. Oncotarget 2018, 9, 15732–15739. [Google Scholar] [CrossRef]
- Farmer, W.; Hannon, G.; Ghosh, S.; Prina-Mello, A. Thermal ablation in pancreatic cancer: A scoping review of clinical studies. Front. Oncol. 2022, 12, 1066990. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, D. Preoperative ultrasound ablation for borderline resectable pancreatic cancer: A report of 30 cases. Ultrason. Sonochemistry 2015, 27, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Fergadi, M.P.; Magouliotis, D.E.; Rountas, C.; Vlychou, M.; Athanasiou, T.; Symeonidis, D.; Pappa, P.A.; Zacharoulis, D. A meta-analysis evaluating the role of high-intensity focused ultrasound (HIFU) as a fourth treatment modality for patients with locally advanced pancreatic cancer. Abdom. Imaging 2022, 47, 254–264. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.; Zheng, L.; Meng, Z. Retrospective analysis of high intensity focused ultrasound combined with S-1 in the treatment of metastatic pancreatic cancer after failure of gemcitabine. Am. J. Cancer Res. 2016, 6, 84–90. [Google Scholar]
- Rai, Z.L.; Feakins, R.; Pallett, L.J.; Manas, D.; Davidson, B.R. Irreversible electroporation (Ire) in locally advanced pancreatic cancer: A review of current clinical outcomes, mechanism of action and opportunities for synergistic therapy. J. Clin. Med. 2021, 10, 1609. [Google Scholar] [CrossRef]
- Oikonomou, D.M.; Karamouzis, M.V.M.; Moris, D.M.; Dimitrokallis, N.M.; Papamichael, D.; Kountourakis, P.; Astras, G.; Davakis, S.; Papalampros, A.M.; Schizas, D.M.; et al. Irreversible Electroporation (IRE) Combined With Chemotherapy Increases Survival in Locally Advanced Pancreatic Cancer (LAPC). Am. J. Clin. Oncol. 2021, 44, 325–330. [Google Scholar] [CrossRef]
- Simmerman, E.; Chung, J.; Lawson, A.; Kruse, E. Application of Irreversible Electroporation Ablation as Adjunctive Treatment for Margin Enhancement: Safety and Efficacy. J. Surg. Res. 2020, 246, 260–268. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Y.; Lin, X.; Li, S. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: A propensity score matching analysis. Pancreatology 2020, 20, 477–484. [Google Scholar] [CrossRef]
- Kozak, O.; Hać, S.; Pieńkowska, J.; Studniarek, M. Benefitial role of electrochemotherapy in locally advanced pancreatic cancer —Radiological perspective. Pol. J. Radiol. 2022, 87, 30–42. [Google Scholar] [CrossRef]
- Rudno-Rudzińska, J.; Kielan, W.; Guziński, M.; Płochocki, M.; Antończyk, A.; Kulbacka, J. New therapeutic strategy: Personalization of pancreatic cancer treatment-irreversible electroporation (IRE), electrochemotherapy (ECT) and calcium electroporation (CaEP)—A pilot preclinical study. Surg. Oncol. 2021, 38, 101634. [Google Scholar] [CrossRef]
- Ma, Y.; Xing, Y.; Li, H.; Liang, B.; Li, R.; Li, J.; Li, Z.; Lin, M.; Niu, L. Simultaneous Gemcitabine and Percutaneous CT-Guided Irreversible Electroporation for Locally Advanced Pancreatic Cancer. J. Oncol. 2022, 2022, 3523769. [Google Scholar] [CrossRef]
- van Veldhuisen, E.; Vroomen, L.G.; Ruarus, A.H.; Derksen, T.C.; Busch, O.R.; de Jong, M.C.; Kazemier, G.; Puijk, R.S.; Sorgedrager, N.S.; Vogel, J.A.; et al. Value of CT-Guided Percutaneous Irreversible Electroporation Added to FOLFIRINOX Chemotherapy in Locally Advanced Pancreatic Cancer: A Post Hoc Comparison. J. Vasc. Interv. Radiol. 2020, 31, 1600–1608. [Google Scholar] [CrossRef]
- Moris, D.; Machairas, N.; Tsilimigras, D.I.; Prodromidou, A.; Ejaz, A.; Weiss, M.; Hasemaki, N.; Felekouras, E.; Pawlik, T.M. Systematic Review of Surgical and Percutaneous Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer. Ann. Surg. Oncol. 2019, 26, 1657–1668. [Google Scholar] [CrossRef]
- Liot, S.; Balas, J.; Aubert, A.; Prigent, L.; Mercier-Gouy, P.; Verrier, B.; Bertolino, P.; Hennino, A.; Valcourt, U.; Lambert, E. Stroma Involvement in Pancreatic Ductal Adenocarcinoma: An Overview Focusing on Extracellular Matrix Proteins. Front. Immunol. 2021, 12, 612271. [Google Scholar] [CrossRef]
- Levy, M.J.; Alberts, S.R.; Bamlet, W.; Burch, P.A.; Farnell, M.B.; Gleeson, F.C.; Haddock, M.G.; Kendrick, M.L.; Oberg, A.L.; Petersen, G.M.; et al. EUS-guided fine-needle injection of gemcitabine for locally advanced and metastatic pancreatic cancer. Gastrointest. Endosc. 2017, 86, 161–169. [Google Scholar] [CrossRef]
- Fujisawa, T.; Tsuchiya, T.; Kato, M.; Mizuide, M.; Takakura, K.; Nishimura, M.; Kutsumi, H.; Matsuda, Y.; Arai, T.; Ryozawa, S.; et al. STNM01, the RNA oligonucleotide targeting carbohydrate sulfotransferase 15, as second-line therapy for chemotherapy-refractory patients with unresectable pancreatic cancer: An open label, phase I/IIa trial. Eclinicalmedicine 2023, 55, 101731. [Google Scholar] [CrossRef]
- Matsuda, Y.; Fujii, Y.; Matsukawa, M.; Ishiwata, T.; Nishimura, M.; Arai, T. Overexpression of carbohydrate sulfotransferase 15 in pancreatic cancer stroma is associated with worse prognosis. Oncol. Lett. 2019, 18, 4100–4105. [Google Scholar] [CrossRef]
- Nishimura, M.; Matsukawa, M.; Fujii, Y.; Matsuda, Y.; Arai, T.; Ochiai, Y.; Itoi, T.; Yahagi, N. Effects of EUS-guided intratumoral injection of oligonucleotide STNM01 on tumor growth, histology, and overall survival in patients with unresectable pancreatic cancer. Gastrointest. Endosc. 2018, 87, 1126–1131. [Google Scholar] [CrossRef]
- Bhutani, M.S.; Herman, J.M. Endoscopic Ultrasound-Guided Fiducial Placement for Gastrointestinal Malignancies. Gastroenterol. Hepatol. 2019, 15, 167–170. [Google Scholar]
- Kothary, N.; Heit, J.J.; Louie, J.D.; Kuo, W.T.; Loo, B.W.; Koong, A.; Chang, D.T.; Hovsepian, D.; Sze, D.Y.; Hofmann, L.V. Safety and Efficacy of Percutaneous Fiducial Marker Implantation for Image-guided Radiation Therapy. J. Vasc. Interv. Radiol. 2009, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Pishvaian, A.C.; Collins, B.; Gagnon, G.; Ahlawat, S.; Haddad, N.G. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest. Endosc. 2006, 64, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Jaoude, J.A.; Kouzy, R.; Lin, D.; Nguyen, N.D.; Garcia, C.J.G.; Phan, J.L.; Avila, S.; Smani, D.; Cazacu, I.M.; et al. Impact of Fiducial Marker Placement Before Stereotactic Body Radiation Therapy on Clinical Outcomes in Patients With Pancreatic Cancer. Adv. Radiat. Oncol. 2021, 6, 100621. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Abdelrahim, M. The Latest Advancement in Pancreatic Ductal Adenocarcinoma Therapy: A Review Article for the Latest Guidelines and Novel Therapies. Biomedicines 2021, 9, 389. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2324–2328. [Google Scholar] [CrossRef]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Lee, B.; Lipton, L.; Cohen, J.; Tie, J.; Javed, A.; Li, L.; Goldstein, D.; Burge, M.; Cooray, P.; Nagrial, A.; et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann. Oncol. 2019, 30, 1472–1478. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Boku, N.; Uesaka, K. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer. Eur. J. Cancer 2017, 72, S77. [Google Scholar] [CrossRef]
- Tas, F.; Sen, F.; Keskin, S.; Kilic, L.; Yildiz, I. Prognostic factors in metastatic pancreatic cancer: Older patients are associated with reduced overall survival. Mol. Clin. Oncol. 2013, 1, 788–792. [Google Scholar] [CrossRef]
- Riazy, M.; E Kalloger, S.; Sheffield, B.S.; Peixoto, R.D.; Li-Chang, H.H.; Scudamore, C.H.; Renouf, D.J.; Schaeffer, D.F. Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Mod. Pathol. 2015, 28, 1383–1389. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Wattenberg, M.M.; Asch, D.; Yu, S.; O’dwyer, P.J.; Domchek, S.M.; Nathanson, K.L.; Rosen, M.A.; Beatty, G.L.; Siegelman, E.S.; Reiss, K.A. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br. J. Cancer 2020, 122, 333–339. [Google Scholar] [CrossRef]
- Golan, T.; Kanji, Z.S.; Epelbaum, R.; Devaud, N.; Dagan, E.; Holter, S.; Aderka, D.; Paluch-Shimon, S.; Kaufman, B.; Gershoni-Baruch, R.; et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 2014, 111, 1132–1138. [Google Scholar] [CrossRef]
- Park, J.H.; Jo, J.H.; Jang, S.I.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y.; Lee, H.S.; Cho, J.H. BRCA 1/2 Germline Mutation Predicts the Treatment Response of FOLFIRINOX with Pancreatic Ductal Adenocarcinoma in Korean Patients. Cancers 2022, 14, 236. [Google Scholar] [CrossRef]
- Dalmasso, B.; Puccini, A.; Catalano, F.; Borea, R.; Iaia, M.L.; Bruno, W.; Fornarini, G.; Sciallero, S.; Rebuzzi, S.E.; Ghiorzo, P. Beyond BRCA: The Emerging Significance of DNA Damage Response and Personalized Treatment in Pancreatic and Prostate Cancer Patients. Int. J. Mol. Sci. 2022, 23, 4709. [Google Scholar] [CrossRef]
- O’reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- McMullen, M.; Karakasis, K.; Madariaga, A.; Oza, A.M. Overcoming Platinum and PARP-Inhibitor Resistance in Ovarian Cancer. Cancers 2020, 12, 1607. [Google Scholar] [CrossRef]
- Principe, D.R. Precision Medicine for BRCA/PALB2-Mutated Pancreatic Cancer and Emerging Strategies to Improve Therapeutic Responses to PARP Inhibition. Cancers 2022, 14, 897. [Google Scholar] [CrossRef]
- Buisson, R.; Niraj, J.; Rodrigue, A.; Ho, C.K.; Kreuzer, J.; Foo, T.K.; Hardy, E.J.-L.; Dellaire, G.; Haas, W.; Xia, B.; et al. Coupling of Homologous Recombination and the Checkpoint by ATR. Mol. Cell 2017, 65, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Gralewska, P.; Gajek, A.; Marczak, A.; Mikuła, M.; Ostrowski, J.; Śliwińska, A.; Rogalska, A. PARP Inhibition Increases the Reliance on ATR/CHK1 Checkpoint Signaling Leading to Synthetic Lethality—An Alternative Treatment Strategy for Epithelial Ovarian Cancer Cells Independent from HR Effectiveness. Int. J. Mol. Sci. 2020, 21, 9715. [Google Scholar] [CrossRef] [PubMed]
- Mohni, K.N.; Thompson, P.; Luzwick, J.W.; Glick, G.G.; Pendleton, C.S.; Lehmann, B.; Pietenpol, J.A.; Cortez, D. A Synthetic Lethal Screen Identifies DNA Repair Pathways that Sensitize Cancer Cells to Combined ATR Inhibition and Cisplatin Treatments. PLoS ONE 2020, 10, e0125482. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; O’reilly, E.M. New Treatment Strategies for Metastatic Pancreatic Ductal Adenocarcinoma. Drugs 2020, 80, 647–669. [Google Scholar] [CrossRef]
- Dai, M.; Jahanzaib, R.; Liao, Y.; Yao, F.; Li, J.; Teng, X.; Chen, K.; Cheng, W. Prognostic value of KRAS subtype in patients with PDAC undergoing radical resection. Front. Oncol. 2022, 12, 1074538. [Google Scholar] [CrossRef]
- Strickler, J.H.; Satake, H.; Hollebecque, A.; Sunakawa, Y.; Tomasini, P.; Bajor, D.L.; Schuler, M.H.; Yaeger, R.; George, T.J.; Garrido-Laguna, I.; et al. First data for sotorasib in patients with pancreatic cancer with KRAS p.G12C mutation: A phase I/II study evaluating efficacy and safety. J. Clin. Oncol. 2022, 40, 360490. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Spira, A.I.; Yaeger, R.; Buchschacher, G.L.; McRee, A.J.; Sabari, J.K.; Johnson, M.L.; Barve, M.A.; Hafez, N.; Velastegui, K.; et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. J. Clin. Oncol. 2022, 40, 519. [Google Scholar] [CrossRef]
- Strickler, J.H.; Satake, H.; George, T.J.; Yaeger, R.; Hollebecque, A.; Garrido-Laguna, I.; Schuler, M.; Burns, T.F.; Coveler, A.L.; Falchook, G.S.; et al. Sotorasib in KRAS p.G12C–Mutated Advanced Pancreatic Cancer. N. Engl. J. Med. 2023, 388, 33–43. [Google Scholar] [CrossRef]
- Leidner, R.; Silva, N.S.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.-P.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef]
- Schultheis, B.; Reuter, D.; Ebert, M.P.; Siveke, J.; Kerkhoff, A.; Berdel, W.E.; Hofheinz, R.; Behringer, D.M.; Schmidt, W.E.; Goker, E.; et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen inKRAS wildtype patients with locally advanced or metastatic pancreatic cancer: A multicenter, randomized phase IIb study. Ann. Oncol. 2017, 28, 2429–2435. [Google Scholar] [CrossRef]
- Philip, P.A.; Buyse, M.E.; Alistar, A.T.; Lima, C.M.S.P.R.; Luther, S.; Pardee, T.S.; Van Cutsem, E. Avenger 500, a phase III open-label randomized trial of the combination of CPI-613 with modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas. J. Clin. Oncol. 2019, 37, TPS479. [Google Scholar] [CrossRef]
- Hakim, N.; Patel, R.; DeVoe, C.; Saif, M.W. Why HALO 301 Failed and Implications for Treatment of Pancreatic Cancer. Pancreas 2019, 3, e1–e4. [Google Scholar] [CrossRef]
- Petrioli, R.; Torre, P.; Pesola, G.; Paganini, G.; Paolelli, L.; Miano, S.T.; Martellucci, I.; Francini, G.; Francini, E. Gemcitabine plus nab-paclitaxel followed by maintenance treatment with gemcitabine alone as first-line treatment for older adults with locally advanced or metastatic pancreatic cancer. J. Geriatr. Oncol. 2020, 11, 647–651. [Google Scholar] [CrossRef]
- Dahan, L.; Williet, N.; Le Malicot, K.; Phelip, J.-M.; Desrame, J.; Bouché, O.; Petorin, C.; Malka, D.; Rebischung, C.; Aparicio, T.; et al. Randomized Phase II Trial Evaluating Two Sequential Treatments in First Line of Metastatic Pancreatic Cancer: Results of the PANOPTIMOX-PRODIGE 35 Trial. J. Clin. Oncol. 2021, 39, 3242–3250. [Google Scholar] [CrossRef]
- Chakladar, J.; Kuo, S.Z.; Castaneda, G.; Li, W.T.; Gnanasekar, A.; Yu, M.A.; Chang, E.Y.; Wang, X.Q.; Ongkeko, W.M. The Pancreatic Microbiome Is Associated with Carcinogenesis and Worse Prognosis in Males and Smokers. Cancers 2020, 12, 2672. [Google Scholar] [CrossRef]
- Nista, E.C.; Del Gaudio, A.; Del Vecchio, L.E.; Mezza, T.; Pignataro, G.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Pancreatic Cancer Resistance to Treatment: The Role of Microbiota. Biomedicines 2023, 11, 157. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Fu, Y.; Ricciardiello, F.; Yang, G.; Qiu, J.; Huang, H.; Xiao, J.; Cao, Z.; Zhao, F.; Liu, Y.; Luo, W.; et al. The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells. Cells 2021, 10, 497. [Google Scholar] [CrossRef]
- Voorde, J.V.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing Enzymes in Mycoplasma-infected Tumor Cell Cultures Compromise the Cytostatic Activity of the Anticancer Drug Gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef]
- Weniger, M.; Hank, T.; Qadan, M.; Ciprani, D.; Michelakos, T.; Niess, H.; Heiliger, C.; Ilmer, M.; D’Haese, J.G.; Ferrone, C.R.; et al. Influence of Klebsiella pneumoniae and quinolone treatment on prognosis in patients with pancreatic cancer. Br. J. Surg. 2021, 108, 709–716. [Google Scholar] [CrossRef]
- Bronckaers, A.; Balzarini, J.; Liekens, S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: Implications for cancer therapy. Biochem. Pharmacol. 2008, 76, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heshiki, Y.; Vazquez-Uribe, R.; Li, J.; Ni, Y.; Quainoo, S.; Imamovic, L.; Li, J.; Sørensen, M.; Chow, B.K.C.; Weiss, G.J.; et al. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Ruiz-Cordero, R.; Devine, W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef]
- Chung, V.; Sun, V.; Ruel, N.; Smith, T.J.; Ferrell, B.R. Improving Palliative Care and Quality of Life in Pancreatic Cancer Patients. J. Palliat. Med. 2022, 25, 720–727. [Google Scholar] [CrossRef]
- Westermann, A.; Matrisian, L.M.; Rahib, L. The need for improvement in the management of fatigue, depression and pain in pancreatic cancer. J. Clin. Oncol. 2019, 37, 429. [Google Scholar] [CrossRef]
- Smith, H. Potential Analgesic Mechanisms of Acetaminophen. Pain Physician 2009, 12, 269–280. [Google Scholar] [CrossRef]
- Magee, D.; Jhanji, S.; Poulogiannis, G.; Farquhar-Smith, P.; Brown, M. Nonsteroidal anti-inflammatory drugs and pain in cancer patients: A systematic review and reappraisal of the evidence. Br. J. Anaesth. 2019, 123, e412–e423. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst. Rev. 2017, 2020, CD012508. [Google Scholar] [CrossRef]
- Straube, C.; Derry, S.; Jackson, K.C.; Wiffen, P.J.; Bell, R.F.; Strassels, S.; Straube, S. Codeine, alone and with paracetamol (acetaminophen), for cancer pain. Cochrane Database Syst. Rev. 2014, 2018, CD006601. [Google Scholar] [CrossRef]
- Benyamin, R. Opioid Complications and Side Effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef]
- Porta-Sales, J.; Garzón-Rodríguez, C.; Llorens-Torromé, S.; Brunelli, C.; Pigni, A.; Caraceni, A. Evidence on the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: A systematic review within the European Association for Palliative Care guidelines project. Palliat. Med. 2017, 31, 5–25. [Google Scholar] [CrossRef]
- Lamer, T.J. Treatment of Cancer-Related Pain: When Orally Administered Medications Fail. Mayo Clin. Proc. 1994, 69, 473–480. [Google Scholar] [CrossRef]
- Pérez-Aguado, G.; de la Mata, D.M.-A.; Valenciano, C.M.-L.; Sainz, I.F.-U. Endoscopic ultrasonography-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer: An update. World J. Gastrointest. Endosc. 2021, 13, 460–472. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Kasvis, P.M.; Kilgour, R.D. Diet and Exercise Interventions in Patients With Pancreatic Cancer. Pancreas 2021, 50, 657–666. [Google Scholar] [CrossRef]
- Vujasinovic, M.; Valente, R.; Del Chiaro, M.; Permert, J.; Löhr, J.-M. Pancreatic Exocrine Insufficiency in Pancreatic Cancer. Nutrients 2017, 9, 183. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.-M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.L.; Loscalzo, M.; Trask, P.C.; Zabora, J.; Philip, E.J. Psychological distress in patients with pancreatic cancer-an understudied group. Psychooncology 2010, 19, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Gooden, H.; Tiller, K.; Mumford, J.; White, K. Integrated psychosocial and supportive care needed for patients with pancreatic cancer. Cancer Forum. 2016, 40, 66–69. [Google Scholar]
- Rehman, M.; Khaled, A.; Noel, M. Cytotoxic Chemotherapy in Advanced Pancreatic Cancer. Hematol. Clin. N. Am. 2022, 36, 1011–1018. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepis, T.; De Lucia, S.S.; Pellegrino, A.; del Gaudio, A.; Maresca, R.; Coppola, G.; Chiappetta, M.F.; Gasbarrini, A.; Franceschi, F.; Candelli, M.; et al. State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers 2023, 15, 3423. https://doi.org/10.3390/cancers15133423
Schepis T, De Lucia SS, Pellegrino A, del Gaudio A, Maresca R, Coppola G, Chiappetta MF, Gasbarrini A, Franceschi F, Candelli M, et al. State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers. 2023; 15(13):3423. https://doi.org/10.3390/cancers15133423
Chicago/Turabian StyleSchepis, Tommaso, Sara Sofia De Lucia, Antonio Pellegrino, Angelo del Gaudio, Rossella Maresca, Gaetano Coppola, Michele Francesco Chiappetta, Antonio Gasbarrini, Francesco Franceschi, Marcello Candelli, and et al. 2023. "State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine" Cancers 15, no. 13: 3423. https://doi.org/10.3390/cancers15133423
APA StyleSchepis, T., De Lucia, S. S., Pellegrino, A., del Gaudio, A., Maresca, R., Coppola, G., Chiappetta, M. F., Gasbarrini, A., Franceschi, F., Candelli, M., & Nista, E. C. (2023). State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine. Cancers, 15(13), 3423. https://doi.org/10.3390/cancers15133423







