The Association between Immune Checkpoint Proteins and Therapy Outcomes in Acute Myeloid Leukaemia Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Characteristics
2.2. Study Material
2.3. Flow Cytometry
2.4. Biostatistical Analysis
3. Results
3.1. Baseline Levels of Selected Immune Checkpoint Proteins within Th Lymphocytes and Blast Cells of Patients with AML
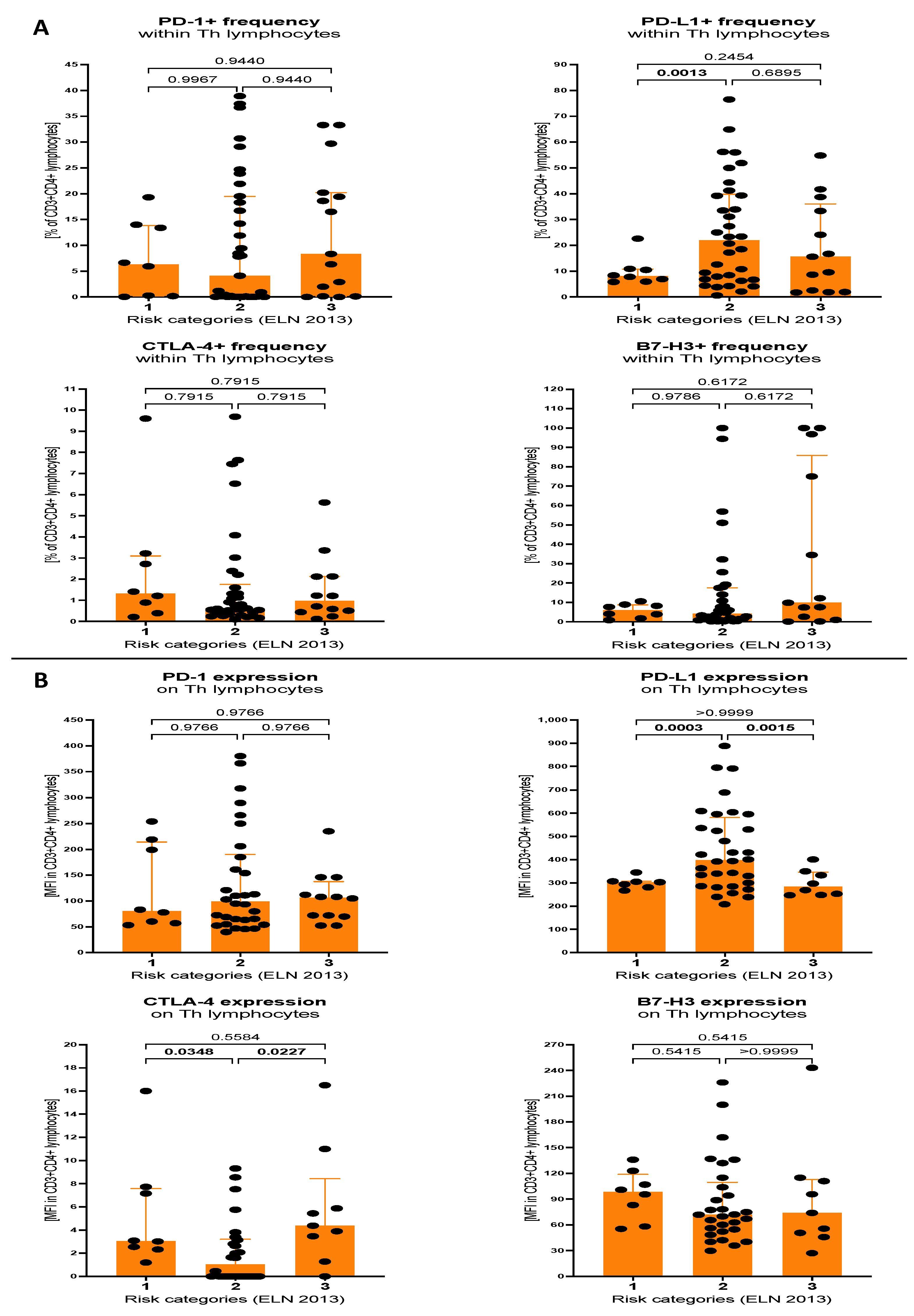
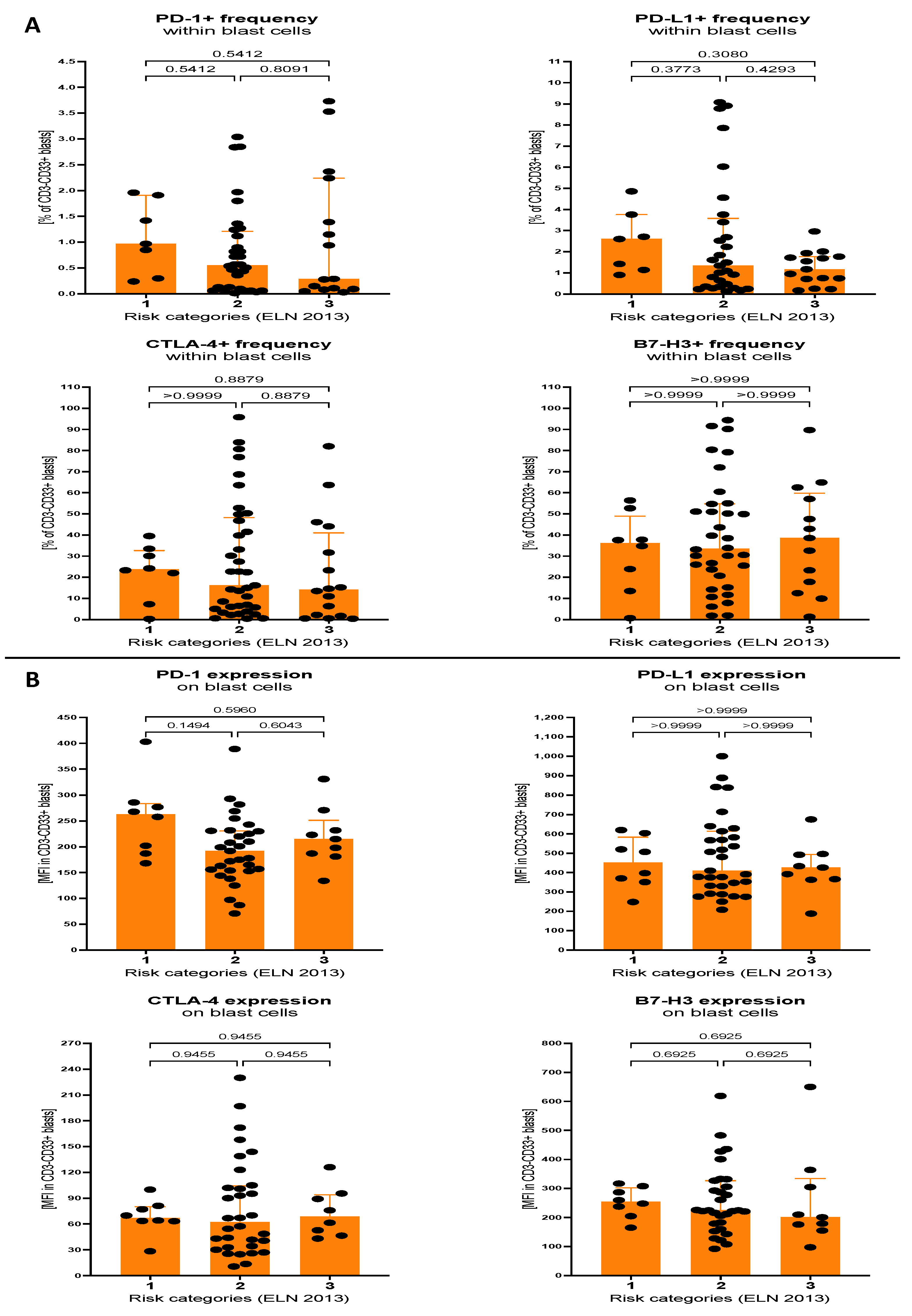
3.2. Expression of Selected Immune Checkpoint Proteins within Th Lymphocytes and Blast Cells of AML Patients Stratified on the Basis of Molecular Cytogenetic Risk
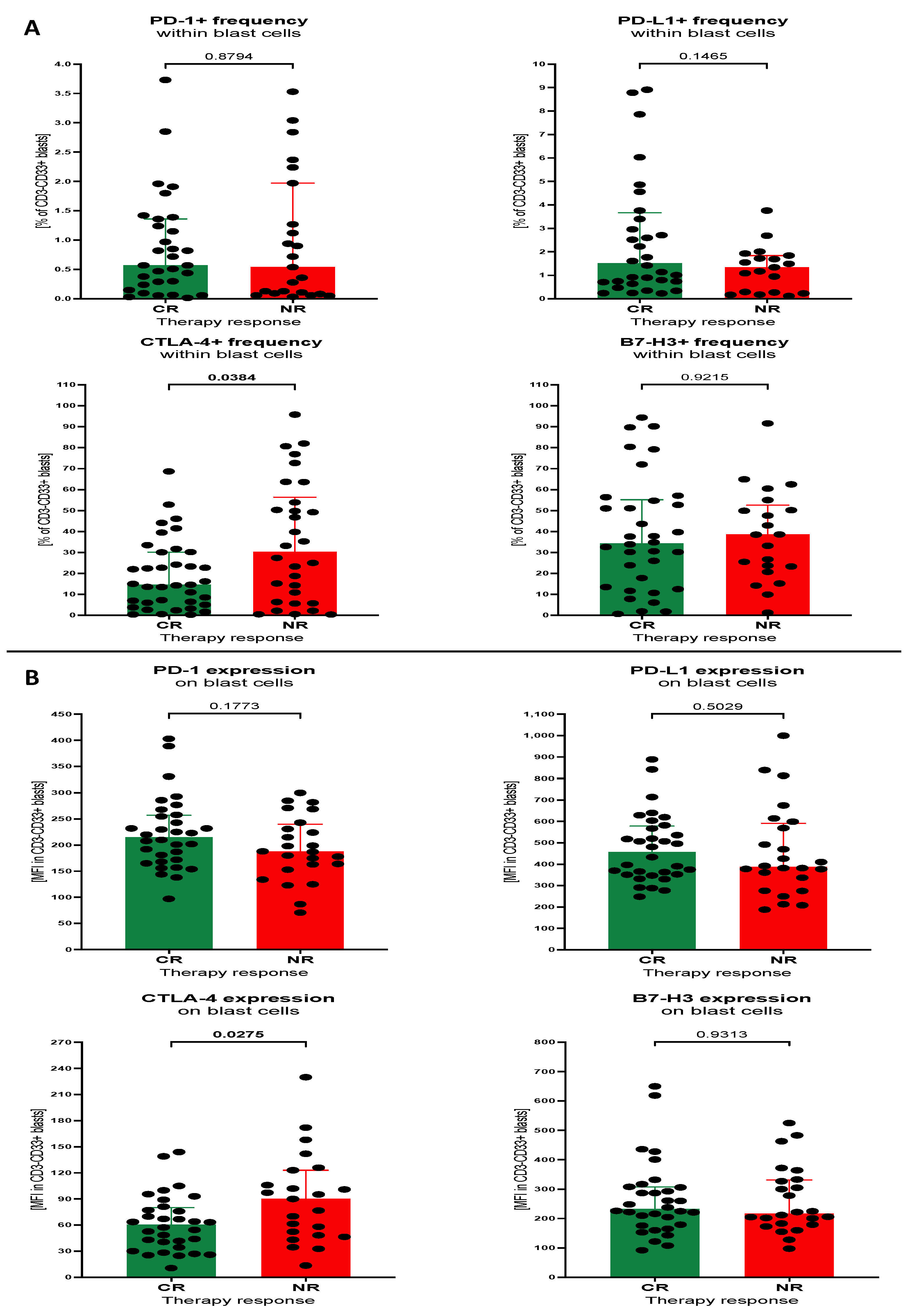
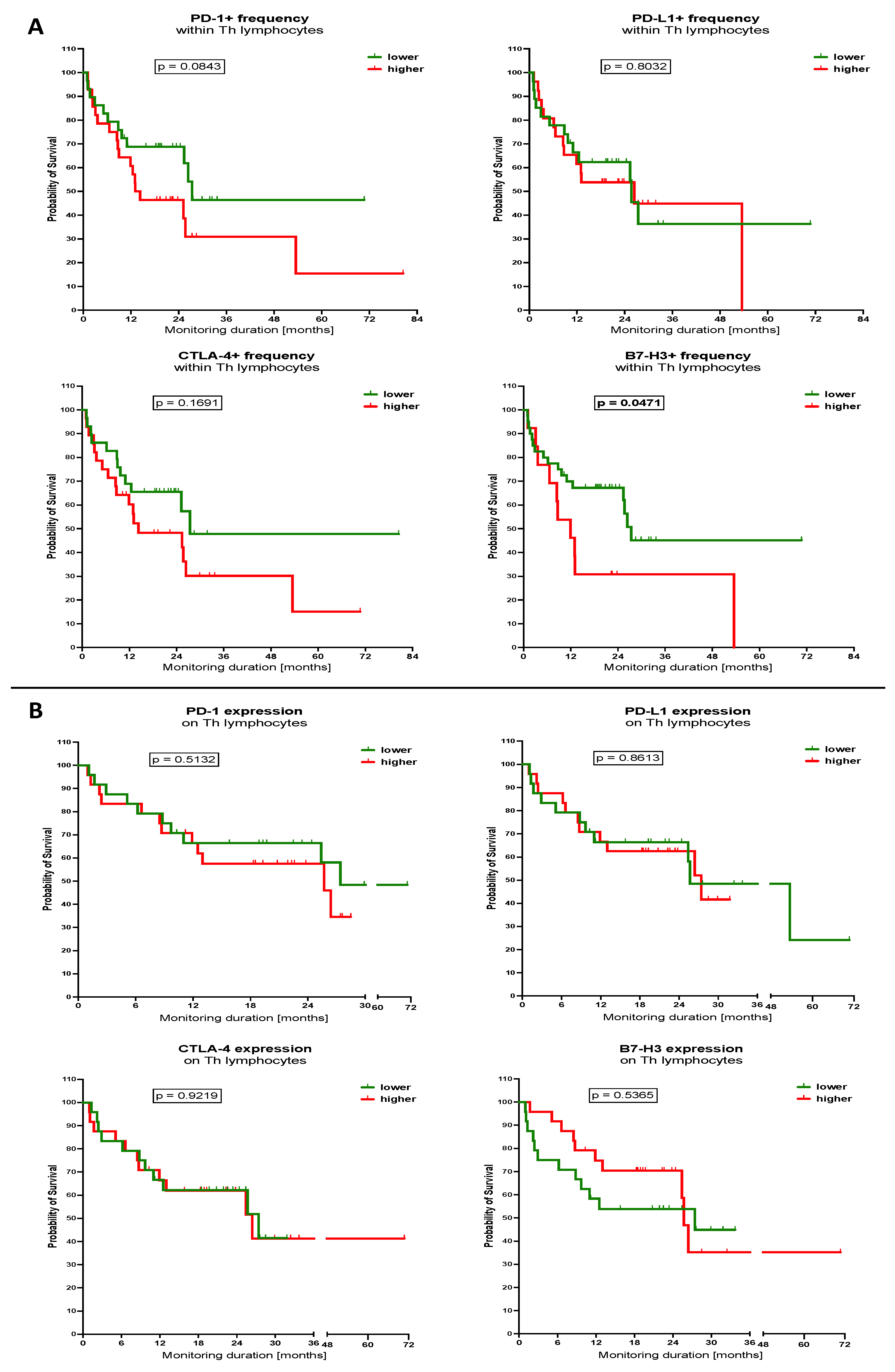
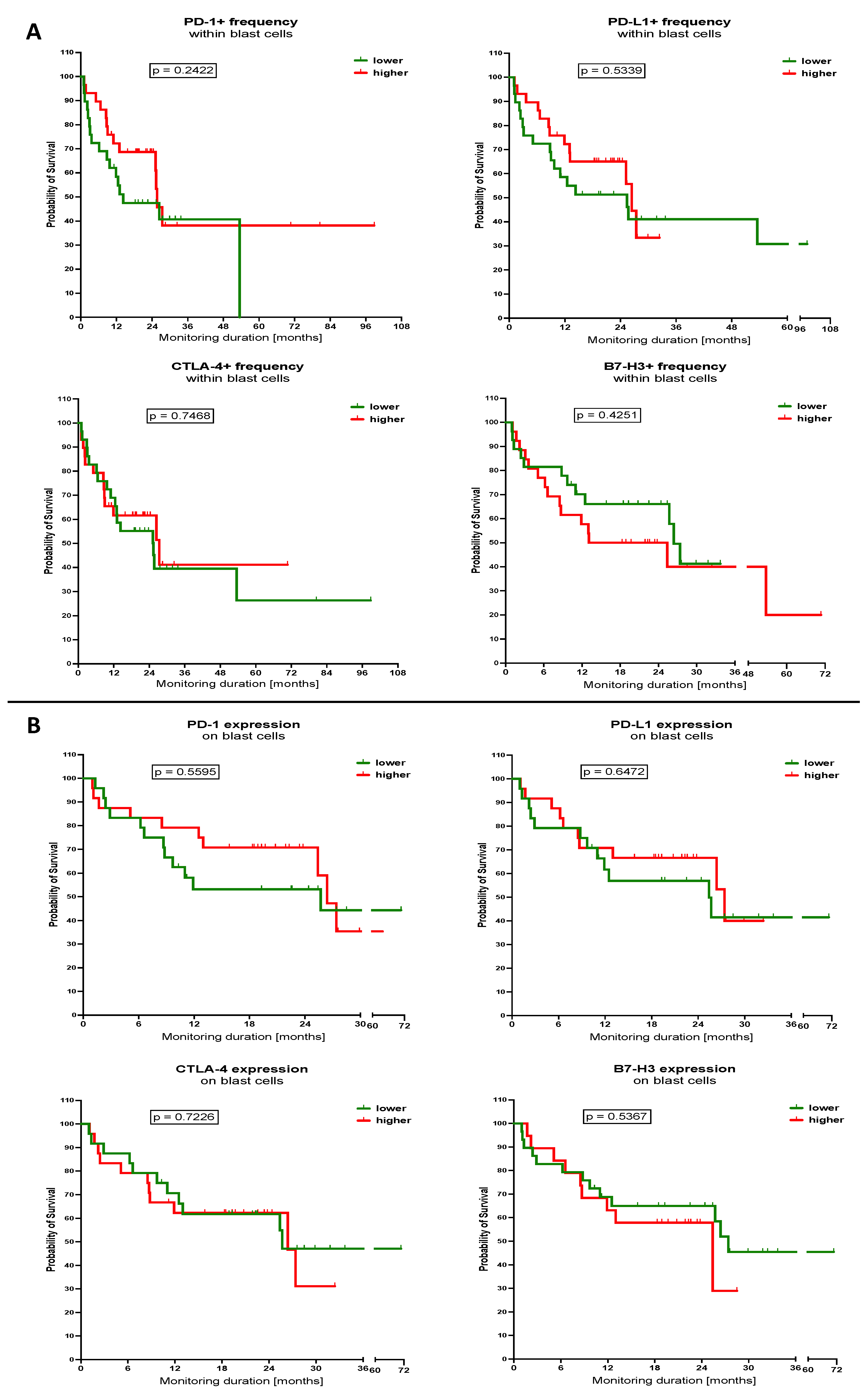
3.3. Influence of Selected Immune Checkpoint Proteins Levels within Th Lymphocytes and Blast Cells on the Survival of the AML Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Allison, Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Lichtenegger, F.S.; Krupka, C.; Haubner, S.; Köhnke, T.; Subklewe, M. Immunotherapy for Acute Myeloid Leukemia. Semin. Hematol. 2015, 52, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Böhme, M.; Kayser, S. Immune-Based Therapeutic Strategies for Acute Myeloid Leukemia. Cancers 2021, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Pentcheva-Hoang, T.; Egen, J.G.; Wojnoonski, K.; Allison, J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 2004, 21, 401–413. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, R.K.; Doss, D.; Khan, P.; Nasser, M.W.; Mahapatra, S. To kill a cancer: Targeting the immune inhibitory checkpoint molecule, B7-H3. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188783. [Google Scholar] [CrossRef]
- Schnorfeil, F.M.; Lichtenegger, F.S.; Emmerig, K.; Schlueter, M.; Neitz, J.S.; Draenert, R.; Hiddemann, W.; Subklewe, M. T cells are functionally not impaired in AML: Increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J. Hematol. Oncol. 2015, 8, 93. [Google Scholar] [CrossRef]
- Knaus, H.A.; Berglund, S.; Hackl, H.; Blackford, A.L.; Zeidner, J.F.; Montiel-Esparza, R.; Mukhopadhyay, R.; Vanura, K.; Blazar, B.R.; Karp, J.E.; et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight 2018, 3, e120974. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Munger, M.E.; Highfill, S.L.; Tolar, J.; Weigel, B.J.; Riddle, M.; Sharpe, A.H.; Vallera, D.A.; Azuma, M.; Levine, B.L.; et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 2010, 116, 2484–2493. [Google Scholar] [CrossRef]
- Guinn, B.-A.; Schuler, P.J.; Schrezenmeier, H.; Hofmann, S.; Weiss, J.; Bulach, C.; Götz, M.; Greiner, J. A Combination of the Immunotherapeutic Drug Anti-Programmed Death 1 with Lenalidomide Enhances Specific T Cell Immune Responses against Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 9285. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, L.; Xiao, M.; Zhang, Z.; Shen, J.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S.; Xiao, Z. PD-L1 regulates cell proliferation and apoptosis in acute myeloid leukemia by activating PI3K-AKT signaling pathway. Sci. Rep. 2022, 12, 11444. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, Z.; Wang, H. Expression of PD-L1 on regulatory B cells in patients with acute myeloid leukaemia and its effect on prognosis. J. Cell Mol. Med. 2022, 26, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Jin, Y.; Xia, P.H.; Lin, J.; Ma, J.C.; Li, T.; Liu, Z.; Xiang, H.; Cheng, C.; Xu, Z.; et al. Integrated analysis reveals distinct molecular, clinical, and immunological features of B7-H3 in acute myeloid leukemia. Cancer Med. 2021, 10, 7831–7846. [Google Scholar] [CrossRef] [PubMed]
- Armand, P. Immune checkpoint blockade in hematologic malignancies. Blood 2015, 125, 3393–3400. [Google Scholar] [CrossRef]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.A.; Thompson, P.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; Salmons, J.; Nowak, A.K.; Rozali, E.N.; Khong, A.; Dick, I.M.; Harken, J.A.; Robinson, B.W.; Lake, R.A. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS ONE 2013, 8, e61895. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for older patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Herbrich, S.M.; Pemmaraju, N.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Pierce, S.A.; Jabbour, E.; Wang, S.A.; Bueso-Ramos, C.; et al. A phase 1b/2 study of azacitidine with PD-L1 antibody avelumab in relapsed/refractory acute myeloid leukemia. Cancer 2021, 127, 3761–3771. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef]
- Sankar, K.; Ye, J.C.; Li, Z.; Zheng, L.; Song, W.; Hu-Lieskovan, S. The role of biomarkers in personalized immunotherapy. Biomark. Res. 2022, 10, 32. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Holowiecki, J.; Grosicki, S.; Robak, T.; Kyrcz-Krzemien, S.; Giebel, S.; Hellmann, A.; Skotnicki, A.; Jedrzejczak, W.W.; Konopka, L.; Kuliczkowski, K.; et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia 2004, 18, 989–997. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, H.; Li, C.; Fang, J.-Y.; Xu, J. Cancer Cell-Intrinsic PD-1 and Implications in Combinatorial Immunotherapy. Front. Immunol. 2018, 9, 1774. [Google Scholar] [CrossRef]
- Kim, M.J.; Ha, S.J. Differential Role of PD-1 Expressed by Various Immune and Tumor Cells in the Tumor Immune Microenvironment: Expression, Function, Therapeutic Efficacy, and Resistance to Cancer Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 767466. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, B.; Annageldiyev, C.; Minagawa, K.; Zhao, C.; Mineishi, S.; Ehmann, W.C.; Naik, S.G.; Cioccio, J.; Wirk, B.; et al. CD26 low PD-1 + CD8 T cells are terminally exhausted and associated with leukemia progression in acute myeloid leukemia. Front. Immunol. 2023, 14, 1169144. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; Zhu, L.; Zeng, H.; Schell, T.D.; Zheng, H. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015, 5, e330. [Google Scholar] [CrossRef] [PubMed]
- Noviello, M.; Manfredi, F.; Ruggiero, E.; Perini, T.; Oliveira, G.; Cortesi, F.; De Simone, P.; Toffalori, C.; Gambacorta, V.; Greco, R.; et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat. Commun. 2019, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, S.D.; Shin, H.; Freeman, G.J.; Wherry, E.J. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc. Natl. Acad. Sci. USA 2008, 105, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mineishi, S.; Claxton, D.; Zhu, J.; Zhao, C.; Jia, B.; Ehmann, W.C.; Rybka, W.B.; Naik, S.; Songdej, N.; et al. A phase I clinical trial of avelumab in combination with decitabine as first line treatment of unfit patients with acute myeloid leukemia. Am. J. Hematol. 2021, 96, E46–E50. [Google Scholar] [CrossRef]
- Geng, S.; Xu, R.; Huang, X.; Li, M.; Deng, C.; Lai, P.; Wang, Y.; Chen, X.; Weng, J.; Du, X. Dynamics of PD-1 expression are associated with treatment efficacy and prognosis in patients with intermediate/high-risk myelodysplastic syndromes under hypomethylating treatment. Front. Immunol. 2022, 13, 950134. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Liu, L. Prognostic significance of PD-L1 in solid tumor: An updated meta-analysis. Medicine 2017, 96, e6369. [Google Scholar] [CrossRef] [PubMed]
- Lipson, E.J.; Vincent, J.G.; Loyo, M.; Kagohara, L.T.; Luber, B.S.; Wang, H.; Xu, H.; Nayar, S.K.; Wang, T.S.; Sidransky, D.; et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: Association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol. Res. 2013, 1, 54–63. [Google Scholar] [CrossRef]
- Godfrey, J.; Liu, H.; Yu, J.; Tallarico, M.; Curran, E.; Artz, A.S.; Riedell, P.A.; Stock, W.; Karrison, T.; Fitzpatrick, C.; et al. Pembrolizumab for the treatment of disease relapse after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2023, 7, 963–970. [Google Scholar] [CrossRef]
- Soltani, M.; Ghanadian, M.; Ghezelbash, B.; Shokouhi, A.; Zamyatnin, A.A.; Bazhin, A.V.; Ganjalikhani-Hakemi, M. PD-L1 stimulation can promote proliferation and survival of leukemic cells by influencing glucose and fatty acid metabolism in acute myeloid leukemia. BMC Cancer 2023, 23, 447. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Norde, W.J.; Maas, F.; Hobo, W.; Korman, A.; Quigley, M.; Kester, M.G.; Hebeda, K.; Falkenburg, J.H.F.; Schaap, N.; de Witte, T.M.; et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011, 71, 5111–5122. [Google Scholar] [CrossRef]
- Tyagi, A.; Ly, S.; El-Dana, F.; Yuan, B.; Jaggupilli, A.; Grimm, S.; Konopleva, M.Y.; Bühring, H.-J.; Battula, V.L. Evidence supporting a role for the immune checkpoint protein B7-H3 in NK cell-mediated cytotoxicity against AML. Blood 2022, 139, 2782–2796. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cai, Q.; Long, Q.; Pan, S.; Zhou, W.; Deng, T.; Mo, W.; Wang, S.; Zhang, Y.; Wang, C.; et al. Optimal combination of immune checkpoint and senescence molecule predicts adverse outcomes in patients with acute myeloid leukemia. Ann. Med. 2023, 55, 2201507. [Google Scholar] [CrossRef]
- Hu, Y.; Lv, X.; Wu, Y.; Xu, J.; Wang, L.; Chen, W.; Zhang, W.; Li, J.; Zhang, S.; Qiu, H. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology 2015, 20, 187–195. [Google Scholar] [CrossRef]

| Number of Patients | 72 |
|---|---|
| Mean (range) age, year | 54 (18–67) |
| Mean (±SD) white blood cell count (G/l) | 38.1 (2.0–212.3) |
| Mean (range) percentage of blast cells in peripheral blood | 47 (0–98) |
| Mean (range) of blastic cell percentage in bone marrow | 56 (20–93) |
| 1. AML with recurrent genetic abnormalities | n (%) |
| t(8;21) (q22;q22);(AML1/ETO) | 3 (4%) |
| inv(16) (p13;q22) or t(16;16) (p13;q22); (CBFß/ MYH11) | 3 (4%) |
| Biallelic CEBPAmut | 1 (1%) |
| NPM1mut without FLT3-ITD | 1 (1%) |
| t(9;11); MLLT3-MLL | 2 (2%) |
| NPM1wtFLT3low/NPM1mutFLT3high | 11 (15%) |
| NPM1wtFLT3high | 5 (6.5%) |
| 2. AML with multilineage dysplasia without antecedent MDS | 6 (7.5%) |
| 3. AML therapy-related | 0 (0%) |
| 4. AML not otherwise categorized (FAB classification) | n (%) |
| AML, minimally differentiated | 6 (7.5%) |
| AML without maturation | 10 (13%) |
| AML with maturation | 15 (20%) |
| Acute myelomonocytic leukaemia (AMMoL) | 7 (9%) |
| Acute monocytic leukaemia | 8 (10%) |
| Induction therapy protocols: DAC/DA, n | 60/12 |
| CR achieved after the first induction, n (%) | 45 (63%) |
| Favourable risk | 8 (11%) |
| Intermediate I and II risk | 42 (58%) |
| Unfavourable risk | 22 (31%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolkun, L.; Tynecka, M.; Walewska, A.; Bernatowicz, M.; Piszcz, J.; Cichocka, E.; Wandtke, T.; Czemerska, M.; Wierzbowska, A.; Moniuszko, M.; et al. The Association between Immune Checkpoint Proteins and Therapy Outcomes in Acute Myeloid Leukaemia Patients. Cancers 2023, 15, 4487. https://doi.org/10.3390/cancers15184487
Bolkun L, Tynecka M, Walewska A, Bernatowicz M, Piszcz J, Cichocka E, Wandtke T, Czemerska M, Wierzbowska A, Moniuszko M, et al. The Association between Immune Checkpoint Proteins and Therapy Outcomes in Acute Myeloid Leukaemia Patients. Cancers. 2023; 15(18):4487. https://doi.org/10.3390/cancers15184487
Chicago/Turabian StyleBolkun, Lukasz, Marlena Tynecka, Alicja Walewska, Malgorzata Bernatowicz, Jaroslaw Piszcz, Edyta Cichocka, Tomasz Wandtke, Magdalena Czemerska, Agnieszka Wierzbowska, Marcin Moniuszko, and et al. 2023. "The Association between Immune Checkpoint Proteins and Therapy Outcomes in Acute Myeloid Leukaemia Patients" Cancers 15, no. 18: 4487. https://doi.org/10.3390/cancers15184487
APA StyleBolkun, L., Tynecka, M., Walewska, A., Bernatowicz, M., Piszcz, J., Cichocka, E., Wandtke, T., Czemerska, M., Wierzbowska, A., Moniuszko, M., Grubczak, K., & Eljaszewicz, A. (2023). The Association between Immune Checkpoint Proteins and Therapy Outcomes in Acute Myeloid Leukaemia Patients. Cancers, 15(18), 4487. https://doi.org/10.3390/cancers15184487






