Simple Summary
In clinical trials, patients treated with idecabtagene vicleucel (ide-cel) chimeric antigen receptor T-cell therapy (CAR T) have reported meaningful improvements in patient-reported outcomes, such as health-related quality of life. To test whether these findings are generalizable to the broader, real-world patient population, this study aimed to prospectively characterize patient-reported outcomes (i.e., health-related quality of life, symptom burden) among patients with relapsed/refractory multiple myeloma treated with ide-cel CAR T in standard of care. Patient-reported outcomes were assessed across 14 timepoints from pre-CAR T infusion through day 90 post-infusion. Patients reported significant and meaningful improvements in health-related quality of life and physical well-being by day 60 after CAR T infusion. Overall, most patients had meaningful improvement or maintenance of patient-reported outcomes collected over time. Findings have implications for treatment decision-making, patient education, and supportive interventions to improve patient outcomes post-CAR T.
Abstract
Idecabtagene vicleucel (ide-cel) was the first FDA-approved chimeric antigen receptor T-cell therapy for relapsed/refractory multiple myeloma (RRMM) patients. This was the first study to evaluate patient-reported outcomes (PROs) among RRMM patients receiving ide-cel in standard of care (SOC). We prospectively assessed health-related quality of life (HRQOL) and symptoms from pre-infusion (baseline) through day (D)90 post-infusion. Baseline PRO associations with patient characteristics, mean PRO changes, and time to stable change were evaluated with t-tests, linear mixed-effects models, and Kaplan–Meier analyses, respectively. Within-person change scores and minimally important difference thresholds determined clinical and meaningful significance. Participants (n = 42) were a median of 66 years old (range: 43–81). At baseline, extramedullary disease was associated with worse physical well-being (p = 0.008), global pain (p < 0.001), performance status (p = 0.002), and overall symptom burden (p < 0.001). Fatigue (p < 0.001) and functional well-being (p = 0.003) worsened by D7 before returning to baseline levels. Overall HRQOL (p = 0.008) and physical well-being (p < 0.001) improved by D60. Most participants reported PRO improvement (10–57%) or maintenance (23–69%) by D90. The median time it took to stabile deterioration in functional well-being was 14 days. The median time it took to stabile improvement in physical and emotional well-being was 60 days. Overall, RRMM patients reported improvements or maintenance of HRQOL and symptom burden after SOC ide-cel.
1. Introduction
Multiple myeloma is the second most common hematologic malignancy in the United States (US) [1] and is incurable, as most patients develop relapsed or refractory multiple myeloma (RRMM) after initial therapy [2,3,4]. Multiple myeloma negatively affects patients’ health-related quality of life (HRQOL) or overall wellbeing, due in part to common and distressing disease- and treatment-related symptoms that can interfere with physical and social functioning [5,6,7,8]. While first-line treatments may improve these patient-reported outcomes (PROs), subsequent treatments for RRMM are less likely to improve HRQOL and symptom burden [9,10].
In March 2021, idecabtagene vicleucel (ide-cel) became the first FDA-approved chimeric antigen receptor T-cell therapy (CAR T) for RRMM patients [11]. FDA approval was based on the Phase II KarMMa trial that showed a 73% overall response rate (ORR), complete response (CR) or better among 33% of patients, and a median 10.7 months response duration [12]. This is a striking improvement in clinical efficacy relative to prior treatments for similar patients, which a recent study showed had an ORR of approximately 32% [13]. In addition, ide-cel resulted in clinically meaningful improvements in PROs, such as pain, fatigue, physical function, and global HRQOL [14]. Most recently, the Phase III KarMMa-3 randomized controlled trial showed that RRMM patients treated with ide-cel had better overall HRQOL, cognitive function, fatigue, and pain relative to patients treated with standard regimens at 20 months post-treatment [15]. Thus, the introduction of ide-cel into standard of care (SOC) offers RRMM patients renewed hope for durable remission and improved PROs.
With the introduction of any therapy into SOC, a key question is whether it performs as well in the real-world as it does in clinical trials [16,17]. Trials often have stringent eligibility criteria that are not necessarily representative of real-world patients. Trial participants also tend to be younger and have better overall health, which can affect downstream outcomes [18]. Recently, our team evaluated 159 RRMM patients treated with SOC ide-cel across 11 institutions in the US Multiple Myeloma Immunotherapy Consortium. Clinical outcomes in SOC were comparable to those reported in KarMMa (e.g., 84% ORR, 42% CR or better, and a median 8.6 months response duration), despite 75% of real-world patients not meeting KarMMa eligibility criteria [19]. Building on this work, this subsequent study aimed to prospectively characterize PROs among real-world RRMM patients treated with SOC ide-cel at a single institution. Consistent with the KarMMa trial, we hypothesized that RRMM patients treated with SOC ide-cel would report improvements in PROs.
2. Materials & Methods
2.1. Participants and Procedures
PRO data were pooled across two observational studies at Moffitt Cancer Center (Moffitt), an NCI-designated comprehensive cancer center in Tampa, FL. PROs were combined with clinical and outcomes data collected in a retrospective electronic medical record (EMR) review study. Each protocol was approved by the Advarra Institutional Review Board (Pro00046848) or deemed exempt from IRB oversight due to minimal risk (Pro00055609, Pro00044602) and was conducted in accordance with Helsinki Declaration ethical standards. Data are available from the corresponding author upon reasonable request. Participants were adults (≥18 years old) with RRMM, scheduled to receive SOC ide-cel, able to speak and read English, without documented or observable psychiatric or neurologic diagnoses that could preclude participation (e.g., dementia), and able to provide informed consent. Between May 2021 and August 2022, trained research coordinators identified potentially eligible patients in collaboration with providers in Moffitt’s Immune Cell Therapy program. Coordinators screened patients’ EMRs for eligibility and approached patients in person or via telephone to introduce the study, confirm eligibility, and solicit informed consent. Participants were asked to complete PRO assessments at 14 timepoints (Supplementary Table S1): baseline (i.e., enrollment, before CAR T infusion), day of CAR T infusion (day (D)0), daily for one week post-infusion (D1–D6), weekly for one month (D7, D14, D21), and monthly for three months (D30, D60, D90). This timeline was informed by recommendations for monitoring PROs after CAR T [20] and our past work, which showed feasibility [21]. Most participants (93%) completed the baseline assessment on or before the day of conditioning chemotherapy. Assessments were completed using REDCap, a HIPAA-compliant and internet-based data capture tool [22].
2.2. Measures
2.2.1. Participant Characteristics and Clinical Outcomes
At baseline, participants reported their demographic characteristics (e.g., date of birth, sex, race, ethnicity) and completed the Charlson Comorbidity Index [23,24]. EMR reviews were conducted for baseline clinical characteristics (e.g., diagnosis, treatment history, KarMMa eligibility) and safety and clinical outcomes through D90 (e.g., toxicities, treatment response, date/cause of progression/death). Moffitt physicians assessed cytokine release syndrome (CRS) and neurotoxicity per the American Society for Transplantation and Cellular Therapy criteria [25], hematologic toxicities per the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [26], and CAR T response per International Myeloma Working Group criteria [27].
2.2.2. HRQOL
At all timepoints, except D1–D6, participants completed the 27-item Functional Assessment of Cancer Therapy-General (FACT-G), which assesses overall HRQOL and four well-being domains (i.e., physical, functional, emotional, social) [28]. Participants responded to items on a Likert-type scale from 0 to 4. Higher scale scores indicated better HRQOL. We used published thresholds to indicate clinically low individual scores for overall HRQOL (≤62), functional well-being (≤11), physical well-being (≤15), emotional well-being (≤13), and social well-being (≤16) [29]. We also used published thresholds to indicate clinically low average group-level overall HRQOL (≤70), functional well-being (≤14), physical well-being (≤18), emotional well-being (≤15), and social well-being (≤19) [29]. Minimally important differences (MIDs) of ±4 points for overall HRQOL and ±2 points for each well-being domain determined clinically meaningful changes [29,30,31].
On D1–D6, to reduce burden, participants completed the FACT-G7, which includes 7 items from the FACT-G and assesses cancer patients’ top-priority concerns [32]. Participants responded to items on a Likert-type scale from 0 to 4. Higher scale scores indicated better HRQOL. Average scores ≤ 13 indicated low HRQOL, and an MID of ±2 points determined clinically meaningful changes [29]. A FACT-G7 score was derived from the FACT-G on D0 to facilitate statistical analyses.
2.2.3. Symptom Burden
At all timepoints, except D1–D6, participants completed the 31-item PRO Measurement Information System (PROMIS)-29+2 Profile v2.1, which assesses fatigue, pain interference, sleep disturbance, depression, anxiety, physical function, social function (4 items each), cognitive function (2 items), and global pain (1 item) [33,34]. Participants rated their global pain from 0 to 10, and higher scores indicated worse pain. For all other scales, participants responded to items on Likert-type scales from 1 to 5, and standardized T-scores were calculated (normative M = 50, SD = 10). Higher scores were worse for fatigue, pain interference, sleep disturbance, depression, anxiety (55–59 mild, 60–69 moderate, ≥70 severe), and global pain (1–4 mild, 5–6 moderate, ≥7 severe). Lower scores were worse for physical, cognitive, and social function (≤30 severe, 31–40 moderate, 41–45 mild). MIDs of ±2 points for global pain and ±5 points for all other scales determined clinically meaningful changes [35,36]. To minimize confusion between PROMIS physical function and FACT-G physical well-being, we herein refer to PROMIS physical function as “performance status”.
At all timepoints, except D1–D6, participants completed items from the PRO version of the CTCAE (PRO-CTCAE). PRO-CTCAE is a library of 124 items assessing the frequency, severity, and/or interference of 78 toxicities and was designed for investigators to select items that are relevant to specific treatments and/or diagnoses [37,38]. We assessed 31 toxicities (e.g., decreased appetite, nausea, constipation, diarrhea), with 47 items based on consensus among study team experts. Participants responded to items on Likert-type scales from 0 to 4. For each toxicity, we calculated a within-person composite grade that mapped onto clinician-rated CTCAE grades [39], where 0 indicates that a toxicity is absent, 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening, and 5 = toxicity-related death. Composite grades for patient-reported toxicities were capped at 3 (severe) as recommended [39] and were used to calculate a Toxicity Index to indicate overall symptom burden as follows [40,41]:
In this equation, toxicities are ranked in descending order of severity, assigned decreasing weights, and summed. This accommodates the differential impact of multiple toxicities and yields an easily interpretable score, wherein the number before the decimal indicates the highest toxicity grade reported, and the numbers after the decimal indicate other toxicities beyond the highest grade, with lower grade toxicities contributing less to the final score. For example, a participant reporting a single grade 3 toxicity would have a score of 3.0, and a participant reporting one grade 3 and two grade 2 toxicities would have a score of 3.67. We defined the MID threshold for overall symptom burden as a change from one severity category to another (i.e., from ≥3 severe to 2.0–2.9 moderate).
2.3. Statistical Analysis
Analyses were performed using SAS Version 9.4. We used descriptive statistics to characterize the sample and summarize the PRO data and independent-sample t-tests to evaluate associations between participant characteristics and PROs at baseline. Our analytic approach was informed by the KarMMa trial PRO analyses [14]. First, we used PROC MIXED to calculate linear mixed models, examining changes in average PRO scores from baseline to each follow-up timepoint with maximum likelihood estimation and all available data for calculating estimates. We also used MIDs to determine whether average score changes were clinically meaningful (i.e., exceeded the MID). Second, we used PROC GLIMMIX to calculate logistic regression models, examining differences in the proportion of participants who exceeded clinical thresholds at baseline vs. D90. For HRQOL outcomes, participants were categorized as having low vs. normal HRQOL. For symptom outcomes, participants were categorized as having at least moderate symptoms vs. mild or none. We also used MIDs to quantify the proportion of participants with PRO improvement, maintenance (i.e., no change), or deterioration from baseline to D90. Third, we used Kaplan–Meier analyses to evaluate time to stable PRO change, defined as ≥2 consecutive assessments with clinically meaningful improvement or deterioration [14,42]. Participants who did not achieve stable change by D90 were censored. For all statistical tests, significance was indicated by two-sided p < 0.01 to account for multiple comparisons.
3. Results
Sixty-three eligible patients were approached (Figure 1). Of those, 49 (78%) consented to participate, and 42 (67%) provided at least baseline data for analysis. PRO completion rates were lowest during the week post-infusion (D0–D6; range: 50–88%) and high otherwise (D7–D90; range: 88–98%) (Figure 2). Follow-up exploratory analyses compared toxicities between participants who did (n = 21) vs. did not complete the D1 assessment (n = 21). Participants who did not complete D1 were more likely to develop any grade neurotoxicity (19% vs. 0%) (p = 0.035).
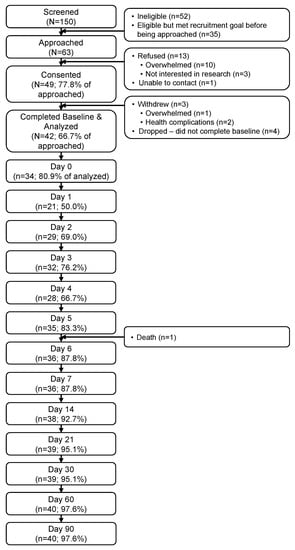
Figure 1.
Participant flow through the study.
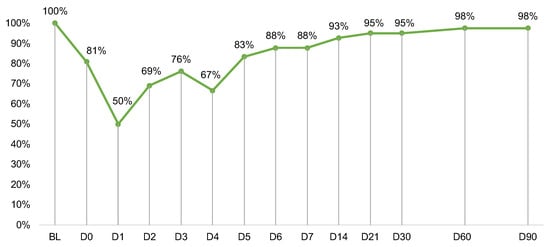
Figure 2.
Measure completion rates at each timepoint out of n = 42 participants. Day (D)0 was the day of CAR T infusion. Completion rates after D6 were calculated out of n = 41 due to one participant death. Abbreviations: BL, baseline.
3.1. Participant Characteristics
As shown in Table 1, participants were a median of 66 years old (range: 43–81), half were male (50%), most identified as non-Hispanic White (69%), and most had earned at least a college degree (57%). The median of prior lines of therapy was 6 (range: 4–16). At least one-third had extramedullary disease (40.5%) and penta-refractory disease (38%). Most (71%) did not meet KarMMa trial eligibility criteria. As shown in Table 2, 38% had a CR or better by D90 post-ide-cel. One participant (2%) died due to disease progression. Most (81%) developed any grade CRS (12% grade ≥ 2), and 10% developed any grade neurotoxicity (5% grade ≥ 2).

Table 1.
Baseline sociodemographic and clinical characteristics (n = 42).

Table 2.
Safety and clinical outcomes in the first 90 days post-CAR T-cell infusion (n = 42).
3.2. Baseline PROs
Table 3 shows average PRO scores over time. At baseline, participants on average reported severe overall symptom burden (M = 3.2, SD = 0.8), mild pain interference (M = 55.0, SD = 10.0), mild global pain (M = 3.7, SD = 2.5), and mildly impaired performance status (M = 41.9, SD = 9.4). At baseline, extramedullary disease was associated with worse physical well-being (p = 0.008), worse global pain (p < 0.001), worse performance status (p = 0.002), and worse overall symptom burden (p < 0.001) (Supplementary Table S2). Having achieved at least a college degree was associated with better social well-being (p < 0.001).

Table 3.
Means (standard deviations) of patient-reported outcomes at each timepoint.
3.3. Mean PRO Changes from Baseline
Figure 3 shows the estimated mean changes from baseline for HRQOL outcomes compared to MIDs. See Supplementary Table S3 for details on the linear mixed models. In the week post-infusion, daily HRQOL significantly and meaningfully improved on D6 (p = 0.008, Figure 3A). Overall HRQOL and physical well-being significantly and meaningfully improved on D60 (p = 0.008, Figure 3B and p < 0.001, Figure 3C, respectively), and improvements were sustained at D90 (p = 0.006 and p = 0.002, respectively). Functional well-being significantly and meaningfully worsened on D7 (p = 0.003), D14 (p < 0.001), and D21 (p = 0.001) before returning to baseline levels (Figure 3D). Emotional well-being showed significant but not meaningful improvements on D14 (p = 0.009), D21 (p = 0.007), and D60 (p = 0.008) (Figure 3E). Social well-being did not change significantly or meaningfully (Figure 3F).
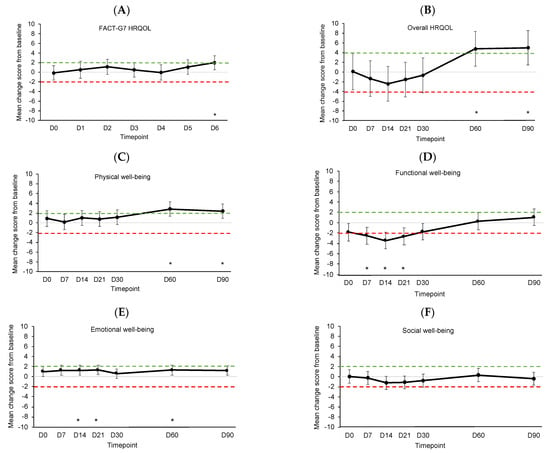
Figure 3.
Estimated mean changes from baseline for (A) daily HRQOL, (B) overall HRQOL, (C) physical well-being, (D) functional well-being, (E) emotional well-being, and (F) social well-being. Dotted lines indicate clinically meaningful improvement (green) or worsening (red). Error bars represent 95% confidence intervals. D, day. D0 was the day of CAR T-cell infusion. * p < 0.01.
Figure 4 shows the estimated mean changes from baseline for symptom outcomes compared to MIDs. See Supplementary Table S4 for details of the linear mixed models. Fatigue significantly and meaningfully worsened from baseline on D7 (p < 0.001) before returning to baseline levels (Figure 4A). There were no significant or meaningful changes in pain interference, sleep disturbance, depression, or anxiety (Figure 4B–E). Global pain showed significant but not meaningful improvement on D30 (p = 0.007, Figure 4F). Performance status showed significant but not meaningful worsening on D7 (p < 0.001), D14 (p < 0.001), D21 (p = 0.001), and D30 (p = 0.001, Figure 4G). Cognitive function showed significant but not meaningful improvement on D90 (p = 0.004, Figure 4H). Changes in social function were not significant or meaningful (Figure 4I). Symptom burden showed significant but not meaningful improvement on D90 (p = 0.004, Figure 4J).
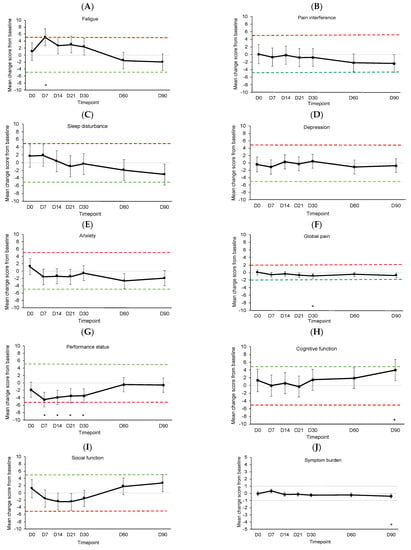
Figure 4.
Estimated mean change from baseline for (A) fatigue, (B) pain interference, (C) sleep disturbance, (D) depression, (E) anxiety, (F) global pain, (G) performance status, (H) cognitive function, (I) social function, and (J) overall symptom burden. Dotted lines indicate clinically meaningful improvement (green) or worsening (red). Error bars represent 95% confidence intervals. D, day. D0 was the day of CAR T-cell infusion. * p < 0.01.
3.4. Proportions with Clinically Meaningful PRO Scores
Figure 5 shows the proportions of participants with normal vs. low HRQOL across timepoints. There were no differences in the proportion of participants with low HRQOL at baseline vs. D90 for any HRQOL outcome (logistic regression p-values > 0.01). Functional well-being appeared most impaired, with up to 39% of participants reporting low functional well-being (D14; Figure 5D).
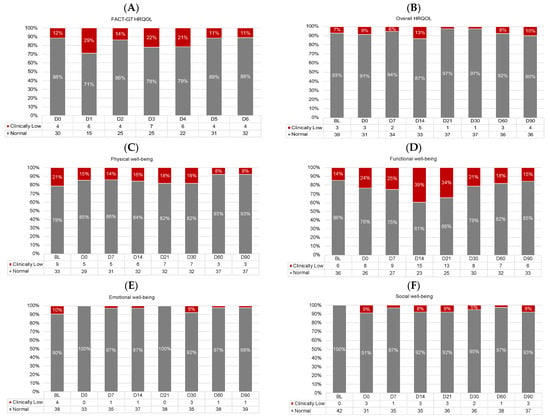
Figure 5.
Proportions of participants with normal (gray) and clinically low (red) HRQOL scores at each timepoint for (A) daily HRQOL, (B) overall HRQOL, (C) physical well-being, (D) functional well-being, (E) emotional well-being, and (F) social well-being. Proportions < 5% are not labeled. All frequencies are shown in the legends. D, day. D0 was the day of CAR T-cell infusion.
Figure 6 shows the proportions of participants with normal/none, mild, moderate, and severe symptoms across timepoints. There were no differences in the proportion of participants with at least moderate symptoms at baseline vs. D90 for any symptom outcome (logistic regression p-values > 0.01). Performance status and overall symptom burden appeared most impaired, with ≥50% of participants reporting at least moderately impaired performance status at all timepoints (Figure 6G) and up to 97% of participants reporting at least moderate overall symptom burden (D7; Figure 6J).
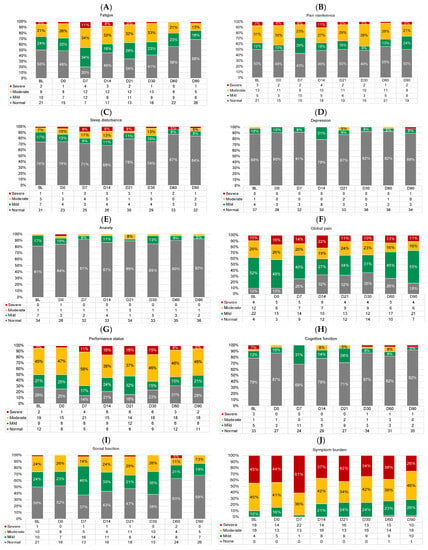
Figure 6.
Proportions of participants with normal/none (gray), mild (green), moderate (yellow), and severe (red) symptoms at each timepoint for (A) fatigue, (B) pain interference, (C) sleep disturbance, (D) depression, (E) anxiety, (F) global pain, (G) performance status, (H) cognitive function, (I) social function, and (J) overall symptom burden. Proportions < 5% are not labeled. All frequencies are shown in the legend. D, day. D0 was the day of CAR T-cell infusion.
Figure 7 shows the proportion of participants with clinically meaningful PRO improvement, deterioration, and maintenance from baseline to D90. Overall, most participants had clinically meaningful improvement (range: 10–57%) or maintenance (range: 23–69%). The proportion of participants with meaningful improvement was largest for overall HRQOL (54%) and physical well-being (57%). The proportion with meaningful deterioration was largest for functional well-being (33%).
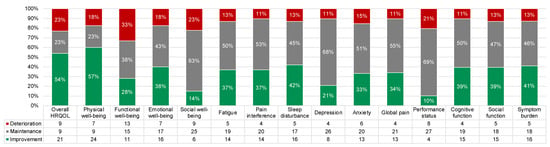
Figure 7.
Proportions of participants with clinically meaningful PRO improvement (green), deterioration (red), and maintenance (i.e., no change; gray) from baseline to day 90. All frequencies are shown in the legend.
3.5. Time to Stable PRO Change
Median time to stable deterioration was 14 days for functional well-being, and median time to stable improvement was 60 days for physical and emotional well-being (Supplementary Table S5). Median time to stable change was not reached for the other PROs (i.e., <50% of participants had stable change by D90).
4. Discussion
This was the first study to evaluate PROs of SOC ide-cel among real-world RRMM patients [18]. In this study, 71% of participants would not have met eligibility criteria for the phase 2 KarMMa trial. We observed significant and meaningful worsening of PROs (i.e., fatigue, functional well-being) as early as D7 post-infusion, which later rebounded to baseline levels. We also observed significant and meaningful improvements in PROs (i.e., overall HRQOL, physical well-being) by D60, which were sustained through D90. Most participants reported clinically meaningful improvement or maintenance of PROs from baseline to D90.
Our results are similar to those of the KarMMa trial but tempered in the context of shorter follow-up. The KarMMa trial found significant and meaningful improvements in most PROs by month 1 or 2 that were sustained over 12 months or more, (e.g., overall HRQOL, physical and cognitive function, pain, fatigue) [14]. Similarly, we found significant, meaningful, and sustained improvements in overall HRQOL and physical well-being by D60 (i.e., month 2) and significant improvements in cognitive function and global pain that did not exceed MID thresholds. In contrast to the KarMMa trial, participants reported worsened fatigue in the week post-infusion before rebounding to baseline levels. Discrepancies between studies could possibly be explained by using different PRO measures. Alternatively, participant cohorts may be demographically and clinically different. For example, real-world RRMM patients in our study were older than KarMMa trial participants (median 66 vs. 61 years), and our study included more patients with ECOG performance status ≥ 2 (10% vs. 2%) and penta-refractory disease (38% vs. 26%) [14]. These differences may reflect characteristics of patients deemed ineligible vs. eligible to participate in clinical trials, and thus, findings from our study are likely more generalizable to the broader real-world population of RRMM patients treated in SOC.
Findings have direct implications for patient education and clinical care, as PROs should be considered throughout the clinical management of RRMM [43]. Clinicians rely on real-world evidence when considering treatment approaches and educating patients, and patient education is a critical component of patient-centered care and informed treatment decision-making [44,45]. Our findings can inform patient education for SOC ide-cel with regard to the potential impacts on key PROs. In addition, findings can be used to identify targets for supportive behavioral and pharmacologic interventions to maximize survivorship outcomes for individual patients post-ide-cel.
Strengths of this study include a rigorous schedule of prospective PRO data collection using validated measures from pre-treatment through D90 post-infusion, which allowed for a nuanced evaluation of how PRO scores changed in the first three months post-ide-cel. Assessment response rates were high, supporting the feasibility of collecting PROs in real-world settings. In addition, we considered both statistical significance and clinical meaningfulness, and we documented a high prevalence of clinically meaningful symptomatology in this population.
Limitations include a relatively small sample size of n = 42. This study was limited to a single institution offering commercial ide-cel, which only became available in March 2021. Participants were mostly non-Hispanic White and highly educated, which reflects the characteristics of patients who had access to and received CAR T at an NCI-designated comprehensive cancer center. Studies show that racial and ethnic minority RRMM patients are underrepresented in CAR T clinical trials and are less likely to receive commercial CAR T [46,47]. Thus, future studies should replicate our findings among diverse cohorts that are representative of the broader RRMM population. Finally, follow-up was limited to D90 post-infusion. Studies with longer follow-up are needed to understand PROs further into the trajectory of real-world post-CAR T survivorship.
5. Conclusions
This study was the first to investigate the effects of SOC ide-cel on PROs among real-world RRMM patients who largely would not have been eligible for clinical trials. Overall, participants reported significant and meaningful improvements or maintenance of HRQOL and symptom burden up to 90 days post-treatment. The results can be used to inform patient education approaches, treatment decision-making, and early supportive interventions to improve post-CAR T survivorship outcomes among real-world patients with RRMM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15194711/s1, Table S1. Schedule and content of patient-reported outcome assessments. Table S2. Associations between patient characteristics and patient-reported outcomes at baseline (N = 42). Table S3. Results of linear mixed models evaluating estimated mean change from baseline for the HRQOL outcomes. Table S4. Results of linear mixed models evaluating estimated mean changes from baseline for the symptom outcomes. Table S5. Proportions of participants with and median time to stable improvement and deterioration for each patient-reported outcome.
Author Contributions
Conceptualization, L.B.O., L.C.P., M.A., H.S.L.J. and D.K.H.; data curation, L.M.G., X.L., G.D.A., A.I.H. and D.K.H.; formal analysis, L.M.G. and X.L.; funding acquisition, L.B.O., K.K. and D.K.H.; investigation, O.N. and Y.R.; resources, R.C.B., K.H.S., M.A., F.L.L., C.F., O.C.P., T.N., H.L., B.B. and A.G.-C.; supervision, L.B.O., Y.R., H.S.L.J. and D.K.H.; visualization, L.M.G. and X.L.; writing—original draft, L.B.O., L.M.G., L.C.P. and D.K.H.; writing—review and editing, L.B.O., L.M.G., X.L., G.D.A., L.C.P., K.K., B.D.G., A.I.H., O.N., Y.R., R.C.B., K.H.S., M.A., F.L.L., C.F., O.C.P., T.N., H.L., B.B., A.G.-C., H.S.L.J. and D.K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a 2020 Moffitt Cancer Center Team Science Award to L.B.O and K.K., the Brenda T. Myers and Family Multiple Myeloma Fund, Moffitt Cancer Center’s Participant Research, Interventions, and Measurement (PRISM) Core and Biostatistics and Bioinformatics Shared Resource (BBSR), and a National Institutes of Health Cancer Center Support Grant (grant number P30CA076292).
Institutional Review Board Statement
Patient-reported outcome data were pooled across two observational studies at Moffitt Cancer Center and combined with clinical and outcome data collected in a retrospective electronic medical record review study. Each study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Advarra Institutional Review Board (Pro00046848 on 25 September 2020) of Moffitt Cancer Center or deemed exempt from IRB oversight, with ethical review and approval waived due to minimal risk (Pro00055609 on 2 July 2021, Pro00046602 on 15 September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
Dr. Oswald: Research funding from the Pentecost Family Myeloma Research Center. Dr. Peres: Research funding to the institution from Bristol-Myers Squibb, Karyopharm, the Pentecost Family Myeloma Research Center, and the National Institutes of Health; support for attending Tandem ASTCT/CIMBTR Annual Meeting (2023). Dr. Kirtane: Owns stock in Seattle Genetics, Oncternal Therapeutics, and Veru; received consulting fees from A2Bio; part of the scientific advisory board for MyCareGorithm. Dr. Gonzalez: Former consultant for SureMed Compliance; advisory board member for Elly Health, Inc. Dr. Baz: Advisory board: Janssen, Bristol-Myers Squibb, Pfizer, and GSK; research funding: Bristol-Myers Squibb, Janssen, Abbvie, Karyopharm, and Regeneron. Dr. Shain: Advisory board/received honoraria for Sanofi, Bristol Myers Squibb, GlaxoSmithKline, Karyopharm, Takeda, Janssen, and Amgen; research funding to the institution from Abbvie and Karyopharm, outside the submitted work. Dr. Alsina: Advisory board: Janssen, Bristol-Myers Squibb, and Sanofi; research funding: Janssen, Bristol-Myers Squibb, and Sanofi. Dr. Locke: Scientific Advisory Role/Consulting Fees: A2, Allogene, Amgen, Bluebird Bio, Bristol-Myers Squibb/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group (GLG), Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, Umoja, and Phizer; contracts for Service: Kite Pharma (Institutional), Allogene (Institutional), CERo Therapeutics (Institutional), Novartis (Institutional), BlueBird Bio (Institutional), Bristol-Myers Squibb (Institutional), National Cancer Institute, and the Leukemia and Lymphoma Society; patents, royalties, other intellectual property: several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy; education or editorial activity: Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, and the Society for Immunotherapy of Cancer. Dr. Freeman: Honoraria/consulting for: Bristol-Myers Squibb, Seattle Genetics, Cellgene, AbbVie, Sanofi, Incyte, Amgen, ONK therapeutics, and Janssen; Research funding: Bristol-Myers Squibb, Janssen, and Roche/Genentech. Dr. Castaneda Puglianini: Speaker bureau for Adaptive Biotechnologies. Dr. Nishihori: Clinical trial support from Novartis and Karyopharm. Dr. Liu: Consulting honoraria for BiolineRx. Dr. Blue: Consulting: Pfizer Pharmaceutics, Janssen Pharmaceutics, Oncopeptides, and Sanofi Pharmaceutics; Honoraria: Abbvie. Dr. Grajales-Cruz: Member of Advisory Boards for Cellectar, Janssen, and Sanofi; Speaker bureau for Amgen and Sanovi. Dr. Jim: Consultant for SBR Biosciences; grant funding from Kite Pharma. Dr. Hansen: Member of the Bristol-Myers Squibb IMW Ide-Cel Academic Advisory Board, Bristol-Myers Squibb Multiple Myeloma ASH Steering Committee, and Multiple Myeloma Pfizer Advisory Board; net honoraria for Onc Live and Survivorship; research funding from Bristol-Myers Squibb, Karyopharm, International Myeloma Society Young Investigator Award, and the Pentecost Family Myeloma Research Center; consulting for Bristol-Myers Squibb, Karyopharm, Janssen, and Pfizer. The other authors have no conflict of interest to disclose.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, I.S.; van de Donk, N.; Zweegman, S.; Lokhorst, H.M. Current and New Therapeutic Strategies for Relapsed and Refractory Multiple Myeloma: An Update. Drugs 2018, 78, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Rifkin, R.M.; Gasparetto, C.J.; Toomey, K.; Durie, B.G.M.; Hardin, J.W.; Terebelo, H.R.; Wagner, L.; Narang, M.; Ailawadhi, S.; et al. Treatment Journeys of Patients With Newly Diagnosed Multiple Myeloma (NDMM): Results From The Connect MM Registry. Clin. Lymphoma Myeloma Leuk. 2020, 20, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 396–406. [Google Scholar] [CrossRef]
- Baz, R.; Lin, H.M.; Hui, A.M.; Harvey, R.D.; Colson, K.; Gallop, K.; Swinburn, P.; Laubach, J.; Berg, D.; Richardson, P. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support. Care Cancer 2015, 23, 2789–2797. [Google Scholar] [CrossRef]
- Johnsen, A.T.; Tholstrup, D.; Petersen, M.A.; Pedersen, L.; Groenvold, M. Health related quality of life in a nationally representative sample of haematological patients. Eur. J. Haematol. 2009, 83, 139–148. [Google Scholar] [CrossRef]
- Boland, E.; Eiser, C.; Ezaydi, Y.; Greenfield, D.M.; Ahmedzai, S.H.; Snowden, J.A. Living with advanced but stable multiple myeloma: A study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J. Pain. Symptom Manag. 2013, 46, 671–680. [Google Scholar] [CrossRef]
- Jordan, K.; Proskorovsky, I.; Lewis, P.; Ishak, J.; Payne, K.; Lordan, N.; Kyriakou, C.; Williams, C.D.; Peters, S.; Davies, F.E. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: Results of a European, multicenter cohort study. Support. Care Cancer 2014, 22, 417–426. [Google Scholar] [CrossRef]
- Robinson Jr, D.; Esseltine, D.L.; Regnault, A.; Meunier, J.; Liu, K.; van de Velde, H. The influence of baseline characteristics and disease stage on health-related quality of life in multiple myeloma: Findings from six randomized controlled trials. Br. J. Haematol. 2016, 174, 368–381. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jarden, M.; Andersen, C.L.; Frederiksen, H.; Abildgaard, N. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. Eur. J. Haematol. 2017, 99, 3–17. [Google Scholar] [CrossRef]
- ABECMA (Idecabtagene Vicleucel) [Package Insert]; Celgene; Bristol-Myers Squibb Company; Bluebird Bio: Summit, NJ, USA, 2021.
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Jagannath, S.; Lin, Y.; Goldschmidt, H.; Reece, D.; Nooka, A.; Senin, A.; Rodriguez-Otero, P.; Powles, R.; Matsue, K.; Shah, N. KarMMa-RW: Comparison of idecabtagene vicleucel with real-world outcomes in relapsed and refractory multiple myeloma. Blood Cancer J. 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Shah, N.; Miguel, J.S.F.; Braverman, J.; Dhanda, D.S.; Shi, L.; Guo, S.; Yu, P.; Liao, W.; Campbell, T.B. Health-related quality of life with idecabtagene vicleucel in relapsed and refractory multiple myeloma. Blood Adv. 2022, 6, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Delforge, M.; Patel, K.K.; Eliason, L.; Dhanda, D.; Shi, L.; Guo, S.; Marshall, T.; Arnulf, B.; Cavo, M.; Nooka, A.K. Health related quality of life (HRQoL) in patients with triple-class-exposed relapsed/refractory multiple myeloma (TCE RRMM) treated with idecabtagene vicleucel (ide-cel) versus standard regimens: Patient-reported outcomes (PROs) from KarMMa-3 phase 3 randomized controlled trial (RCT). J. Clin. Oncol. 2023, 41, 8032. [Google Scholar]
- Booth, C.M.; Tannock, I.F. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br. J. Cancer 2014, 110, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.P.; Harrison, M.R.; Walker, M.S.; George, D.J.; Abernethy, A.P.; Hirsch, B.R. Clinical Trial Participants With Metastatic Renal Cell Carcinoma Differ From Patients Treated in Real-World Practice. J. Oncol. Prac. 2015, 11, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D. Idecabtagene vicleucel for relapsed/refractory multiple myeloma: Real-world experience from the myeloma CAR T consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sidana, S.; Shah, G.L.; Scordo, M.; Hamilton, B.K.; Majhail, N.S. Patient-Reported Outcomes with Chimeric Antigen Receptor T Cell Therapy: Challenges and Opportunities. Biol. Blood Marrow Transpl. 2019, 25, e155–e162. [Google Scholar] [CrossRef]
- Oswald, L.B.; Li, X.; Carvajal, R.; Hoogland, A.I.; Gudenkauf, L.M.; Hansen, D.K.; Alsina, M.; Locke, F.L.; Rodriguez, Y.; Irizarry-Arroyo, N.; et al. Longitudinal Collection of Patient-Reported Outcomes and Activity Data during CAR-T Therapy: Feasibility, Acceptability, and Data Visualization. Cancers 2022, 14, 2742. [Google Scholar] [CrossRef]
- Patridge, E.F.; Bardyn, T.P. Research electronic data capture (REDCap). J. Med. Libr. Assoc. JMLA 2018, 106, 142. [Google Scholar] [CrossRef]
- Katz, J.N.; Chang, L.C.; Sangha, O.; Fossel, A.H.; Bates, D.W. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care 1996, 34, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 1 March 2021).
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Pearman, T.; Yanez, B.; Peipert, J.; Wortman, K.; Beaumont, J.; Cella, D. Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer 2014, 120, 2902–2909. [Google Scholar] [CrossRef]
- King, M.T.; Cella, D.; Osoba, D.; Stockler, M.; Eton, D.; Thompson, J.; Eisenstein, A. Meta-analysis provides evidence-based interpretation guidelines for the clinical significance of mean differences for the FACT-G, a cancer-specific quality of life questionnaire. Patient Relat. Outcome Meas. 2010, 1, 119–126. [Google Scholar] [CrossRef]
- Cella, D.; Hahn, E.A.; Dineen, K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual. Life Res. 2002, 11, 207–221. [Google Scholar] [CrossRef]
- Yanez, B.; Pearman, T.; Lis, C.G.; Beaumont, J.L.; Cella, D. The FACT-G7: A rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann. Oncol. 2013, 24, 1073–1078. [Google Scholar] [CrossRef]
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Ader, D.; Fries, J.F.; Bruce, B.; Rose, M.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med. Care 2007, 45 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; Spritzer, K.L.; Schalet, B.D.; Cella, D. PROMISA (R)-29 v2.0 profile physical and mental health summary scores. Qual. Life Res. 2018, 27, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Peipert, J.D.; Chapman, R.; Lai, J.-S.; Terluin, B.; Cella, D.; Griffith, P.; Mokkink, L.B. Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual. Life Res. 2021, 30, 2729–2754. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.J.; Eton, D.T.; Garcia, S.F.; Cella, D. Minimally important differences were estimated for six PROMIS-Cancer scales in advanced-stage cancer patients. J. Clin. Epidemiol. 2011, 64, 507. [Google Scholar] [CrossRef]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef]
- Basch, E.; Becker, C.; Rogak, L.J.; Schrag, D.; Reeve, B.B.; Spears, P.; Smith, M.L.; Gounder, M.M.; Mahoney, M.R.; Schwartz, G.K.; et al. Composite grading algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Clin. Trials 2021, 18, 104–114. [Google Scholar] [CrossRef]
- Gresham, G.; Diniz, M.A.; Razaee, Z.S.; Luu, M.; Kim, S.; Hays, R.D.; Piantadosi, S.; Tighiouart, M.; Yothers, G.; Ganz, P.A.; et al. Evaluating Treatment Tolerability in Cancer Clinical Trials Using the Toxicity Index. J. Natl. Cancer Inst. 2020, 112, 1266–1274. [Google Scholar] [CrossRef]
- Rogatko, A.; Babb, J.S.; Wang, H.; Slifker, M.J.; Hudes, G.R. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin. Cancer Res. 2004, 10, 4645–4651. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar]
- Niscola, P.; Scaramucci, L.; Efficace, F. Towards the Integration of Patient-Reported Outcomes into the Global Clinical Management of Multiple Myeloma. Expert Rev. Hematology. 2019, 12, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.P.; Ganz, P.A.; Murphy, S.B.; Nass, S.J.; Ferrell, B.R.; Stovall, E. Patient-Centered Cancer Treatment Planning: Improving the Quality of Oncology Care. Summary of an Institute of Medicine Workshop. Oncologist 2011, 16, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.C.; Studts, J.L.; Hayslip, J.W. Shared decision making in oncology practice: What do oncologists need to know? Oncologist 2012, 17, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Shahzad, M.; Shippey, E.; Bansal, R.; Mushtaq, M.U.; Mahmoudjafari, Z.; Faisal, M.S.; Hoffmann, M.; Abdallah, A.O.; Divine, C.; et al. Socioeconomic and Racial Disparity in Chimeric Antigen Receptor T Cell Therapy Access. Transpl. Cell Ther. 2022, 28, 358–364. [Google Scholar] [CrossRef]
- Emole, J.; Lawal, O.; Lupak, O.; Dias, A.; Shune, L.; Yusuf, K. Demographic differences among patients treated with chimeric antigen receptor T-cell therapy in the United States. Cancer Med. 2022, 11, 4440–4448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).