Prognostic Factors in Extremity Soft Tissue Sarcomas Treated with Radiotherapy: Systematic Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
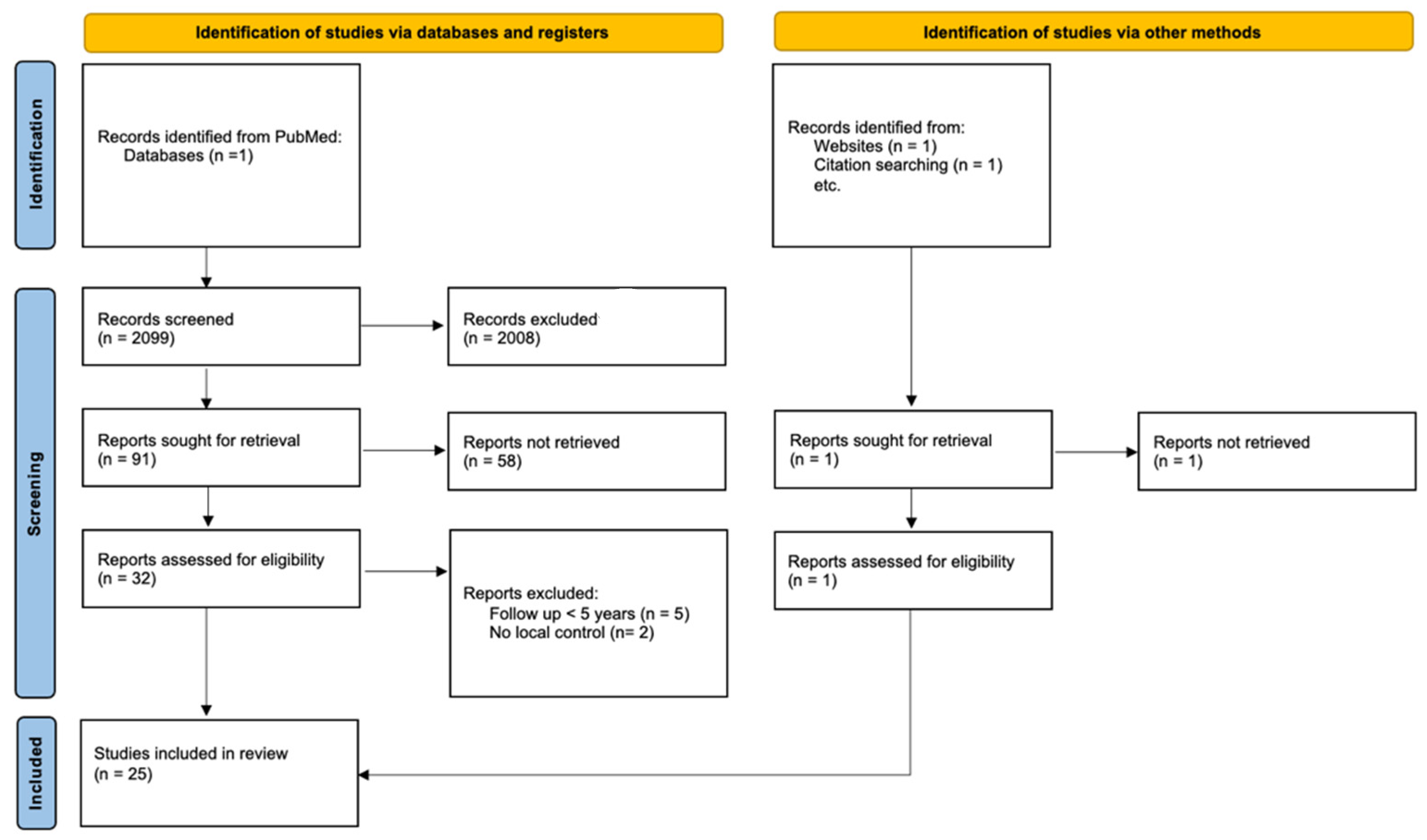
2. Materials and Methods
3. Results
3.1. Identified Studies
3.2. Patient Population
| Series | Country | Sample Size | Age | Sex M:F | Location Type LL:UL | Median Follow-Up (Months) | More Frequent Histologic Subtype in Series | Tumor Size (cm) | Tumor Grade | Margin Status | Total Median Dose (Gy) ±Boost /Dose per Fraction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannon et al. [34], 2006 | USA | 412 | 49 (8–92) | 1:1 (206–206) | LL:412 | 111.6 (14.4–372) | UPS 42% Liposarcoma 22% Synovial sarcoma 13% | 8 (1.2–30) >5:304 <5:107 | I:17 II:119 III:276 | Positive/uncertain: 63 Negative:349 |
|
| Folkert et al. [35], 2014 | USA | 319 (EBRT: 154; IMRT 165) | 54 (17–89) | NA | 2.9:1 (238–81) | 60 | UPS 37% Liposarcoma 28% Synovial sarcoma 9% Leiomyosarcoma 5% | <10:EBRT 84, IMRT 92 >10:EBRT 70, IMRT 73 | High grade: EBRT 120; IMRT 143 Low grade: EBRT 34, IMRT 22 | Positive/close margin No: EBRT 93, IMRT 8 Yes: EBRT 61, IMRT 85 |
|
| Roeder et al. [36], 2018 | Germany | 259 | 55 (3–89) | 1.7:1 (162–97) | 4.2:1 (209–50) | 54 (2–231) | Liposarcoma 31% UPS 27% Synovial sarcoma 15% Leiomyosarcoma 7% | Median: 8 | High grade:236 | R0:185 R1:74 | 45 (20–60.4) + 12 (7.5–20)/1.8–2 |
| Alektiar et al. [28], 2002 | USA | 204 | 49 (16–89) | 1:1 (103–101) | 1.7:1 (128–76) | 67 | NA | 3.2 <3:109 >3:88 | High:204 | NA |
|
| Goertz et al. [27], 2020 | Germany | 192 | 64.5 (18.3–89.9) | 1.2:1 (106–86) | 1.9:1 (126–66) | 61.2 | UPS 100% | ≤5:69 >5:123 | I:8 II:69 III:115 | R0:179 R1:11 R2:2 | 60 (25–70) |
| McGee et al. [37], 2012 | USA | 173 | 57 (18–86) | 1.2:1 (94–79) | 1.9:1 (114–59) | 124.8 (3.6–385.2) | UPS 51% Liposarcoma 18% | <5:8 >5–10:33 >10:13 Unknown:44 | High grade: 154 | Negative: 70% Marginal or microscopically positive: 30% | 65 (49–74)/once or twice daily |
| Kneisl et al. [38], 2017 | USA | 162 | ≤50:61 >50:101 | 0.9:1 (78–84) | 3.4:1 (125–37) | 61.2 (9.6–243.6) | NA | ≤5:56 >5:106 | II–III:120 I:42 | Positive:16 Close:26 Negative:117 Unknown:3 |
|
| Beane et al. [39], 2014 | USA | 141 (71:No RT, 70:RT) | No RT:59.9 ± 2.2 RT:58.6 ± 3.2 | 1.2:1 (78–63) | 3.1:1 (107–35) | 214.8 (12–348) | NA | 0–1.9:No RT 6, RT 5 2–4.9:No RT 19; RT 24 5–9.9:No RT 25, RT 27 >10:No RT 21, RT 13 | I:No RT 19; RT 22 II:No RT 26; RT 24 III:No RT 21; RT 20 | Positive (<1 mm): No RT11, RT 7 Negative; close (≤1 cm): No RT 20, RT 12 Negative; wide (>1 cm) No RT 5, RT 13 Negative; not specified:No RT 7, RT 11 R0:No RT 27, RT 27 | 45 + 18/1.8 |
| Khanfir et al. [40], 2003 | France | 133 | 44 (16–88) | 1.2:1 (73–60) | 2.5:1 (92–37) | 120 (36–300) | UPS 30% Synovial sarcoma 21% | 6 (1–20) | I:36 II:55 III:36 | R0:100% | 50 (36–65) |
| Choong et al. [32], 2001 | Australia | 132 | 43.8 (10.1–83.9) | 1.2:1 (71–61) | 1.5:1 (79–53) | 98.4 (18–210) | UPS 35% Liposarcoma 34% Fibrosarcoma 15% Leiomyosarcoma 7% | 5 (0.7–30) | I:59 II:73 | Marginal:39 Wide:91 Radical:2 | 62 (30–71) ±13.5 (3.6–20) |
| Felderhof et al. [41], 2013 | Netherlands | 118 | NA | 1:1 (58–60) | 3.1:1 (89–29) | 93 (9–192) | Myxoid liposarcoma 14% Leiomyosarcoma 13% Synovial sarcoma 12% UPS 6% | <5.0:46 5.1–10.0:43 >10.0:29 | I–II:28 III:90 | Involved:29 Marginal:75 Wide:12 Unknown:2 |
|
| Dogan et al. [42], 2019 | Turkey | 114 | 44 (15–82) | 1.1:1 (60–54) | 2.6:1 (82–32) | 60 | UPS 26% Liposarcoma 25% Synovial sarcoma 13%, Fibrosarcoma 11% | 7 (3–26) <5:41 5-<15:44 >15:29 | I–II:13 III:101 | Involved:25 Marginal:72 Wide:12 Unknown:5 | 60.9 (44–70)/1.8–2 |
| Cheng et al. [43], 1996 | USA | 112 | 18–88 | 1.3:1 (63–49) | NA | 63.6 (16–192) | UPS 45% Liposarcoma 21% Synovial sarcoma 12% | NA | NA | Intralesional:20 Marginal:26 Wide:66 | 48.2 ± 16.6 |
| Mullen et al. [30], 2012 | USA | 96 | 49 (26–75) | 1.2:1 (53–43) | 5:1 (80–16) | 111.3 | Liposarcoma 26% UPS 22% Leiomyosarcoma 3% | 14.2 (8–35) | II:25 III:23 | R0:80 R1:15 R2:1 | 44 ± 16/2 |
| Tanabe et al. [29], 1994 | USA | 95 | 52 (17–97) | 1.8:1 (61–34) | 6.7:1 (87–13) | 66 (16–236) | UPS 43% Liposarcoma 24%, Synovial sarcoma 8% | 0.1–5:16 5.1–10:27 10.1–15:33 15.1–20:9 >20:15 | II:46 III:54 | Positive:24 Negative:71 | 50 (38–70)/2 |
| Blaes et al. [33], 2010 | USA | 89 | 50 (7–88) | 1.4:1 (52–37) | LL: 89 | 87.6 (9.6–262) | NA | NA | NA | NA | 63 (20–70.2)/1.8–2 |
| Talbert et al. [24], 1990 | USA | 78 | NA | 1.4:1 (45–32) | 1:1 (39–39) | 94.8 | Synovial sarcoma 32% UPS 11% Epithelioid sarcoma 9% | <2:16% 2–4.9:56% >5:28% | I–II:5 III:73 | NA | 62 (45–75)/2 |
| Dickie et al. [31], 2009 | Canada | 74 | 58–63 | 0.8:1 (32–42) | LL:74 | 89 | NA | NA | NA | NA | 64 (57–71) |
| Wanebo et al. [44], 1995 | USA | 66 | 48 (17–77) | 1.1:1 (34–32) | 2.7:1 (48–18) | 84 | UPS 20% Synovial sarcoma 18.2% Liposarcoma 16.7% | <5:40% 5.1–10:25% 10.1–15:22% >15:13% | I:2 II:9 III:55 | Wide:38 Radical:19 Amputation:4 Limited:2 |
|
| Le Péchoux et al. [45], 1999 | France | 62 | 44 (15–76) | 1.6:1 (38–24) | 3.4:1 (48–14) | 72 | Synovial sarcoma 27% UPS 21% Liposarcoma 11% Neurosarcoma 10% | 9.5 (1.0 21.0) >5:43 | I:10% II:52% III:38% | Marginal:24 Incomplete:16 |
|
| Dincbas et al. [46], 2014 | Turkey | 60 | <50:35 ≥50:25 | 1.6:1 (37–23) | 7.6:1 (53–7) | 67 (8–268) | Synovial cell sarcoma 35% Liposarcoma 23% UPS 22% Leiomyosarcoma 7% | <12:23 ≥12:37 | I:18 II:14 III:28 | Marginal:31 Wide:24 Radical:5 |
|
| Pao et al. [47], 1990 | USA | 50 | 52 (18–91) | 1.5:1 (30–20) | 1.8:1 (32–18) | 70 (28–168) | Liposarcoma and UPS 60% | <5:22 5–10:18 >10:10 | I:11 II:8 III:31 | R0:10 R1:31 R2:8 | 60 (45–69) |
| Lee et al. [48], 2012 | South Korea | 43 | NA | 1.3:1 (24–19) | 3.3:1 (33–10) | 70 (5–302) | Liposarcoma 33% Synovial sarcoma 23% UPS 19% | 7 (1.1–20) | I:11 II:13 III:19 | Negative:20 Close (<2 cm):12 Positive:11 | 60 (50–74.4) /1.8–2 |
| Issakov et al. [26], 2006 | Israel | 38 | 51.1 (18–84) | 1.1 (20–18) | 11.7:1 (35–3) | 67 (9–123) | Liposarcoma 100%:
| NA | II–III:100% | Wide:10 Marginal:3 Involved:25 2nd attempt for marginal/involved: Wide 13, marginal 12, involved 3 |
|
| Schoenfeld et al. [25], 2006 | USA | 23 | 64 | 0.8:1 (10–13) | 0.9:1 (11–12) | 132 (14.4–310) | UPS 39% Synovial sarcoma 17%, Dermatofibrosarcoma 9%, Leiomyosarcoma 9% | NA | High grade:18 Low grade:4 Undetermined: 1 | Intralesional:0 Marginal:10 Wide:11 Radical:2 |
|
3.3. Treatments
3.3.1. Schedule of Radiotherapy
3.3.2. Irradiation Technique
3.3.3. Set Up
3.3.4. Radiation Therapy Prescription
3.3.5. Chemotherapy
3.4. Local Control
3.4.1. Local Control with Only Preoperative Radiotherapy
3.4.2. Local Control with Only Postoperative Radiotherapy
3.4.3. Local Control with Both Pre- and Postoperative Radiotherapy
3.4.4. Chemotherapy and Local Control
3.5. Disease-Free Survival and Distant Control
| More Frequent Histologic Subtype in Series | Preoperative RT | Postoperative RT | Preoperative RT | Postoperative RT | |||||
| 5 y DFS | 10 y DFS | 5 y DFS | 10 y DFS | 5 y DC | 10 y DC | 5 y DC | 10 y DC | ||
| Wanebo et al. [44] | UPS 20%, Synovial sarcoma 18%, Liposarcoma 17% | 44% (7 y) | NA | 46% (7 y) | NA | ||||
| Dincbas et al. [46] | Synovial sarcoma 35%, liposarcoma 24% UPS 22% | 48.1% | NA | 51.8% | NA | ||||
| Talber et al. [24] | Synovial sarcoma 32%, UPS 11%, Epithelioid sarcoma 9% | 61% | 51% | 83% | 74% | ||||
| Le Péchoux et al. [45] | Synovial sarcoma 27%, UPS 21%, Liposarcoma 11% | 42% (30–54) | NA | NA | NA | ||||
| Alektiar et al. [28] | NA | NA | NA | 80% (74–86) | NA | ||||
| Khanfir et al. [40] | UPS 30%, Synovial sarcoma 21% | NA | NA | 71% (63–78) | 59% (48–68) | ||||
| Issakov et al. [26] | Liposarcomas 100% | NA | 51% | NA | 61% | ||||
| Lee et al. [48] | Liposarcoma 33% Synovial sarcoma 23%, UPS 19% | 67.9% | NA | 73.3% | NA | ||||
| McGee et al. [37] | UPS 51%, Liposarcoma 18% | NA | NA | 82% | 81% | ||||
| Felderhof et al. [41] | Myxoid liposarcoma 14%, Leiomyosarcoma 13%, Synovial sarcoma 12% | 64% | 44% | 69% | 63% | ||||
| Dogan et al. [42] | UPS 26%, Liposarcoma 25%, Synovial sarcoma 13% | 60% | 52% | NA | NA | ||||
| Preoperative + Postoperative RT | |||||||||
| 5 y DFS | 10 y DFS | 5 y DC | 10 y DC | ||||||
| Choong et al. [32] | UPS 35%, Liposarcoma 34%, Fibrosarcoma 15% | NA | NA | 95.2% ± 2% | NA | ||||
| Schoenfeldet al. [25] | UPS 40%, Synovial sarcoma 17%, Neurofibrosarcoma 9% | 87% | 87% | NA | NA | ||||
| Cannon et al. [34] | UPS 42%, Liposarcoma 22%, Synovial sarcoma 13% | NA | 62% | 71% | 67% | ||||
| Mullen et al. [30] | UPS 22%, Liposarcoma 16% | 77% (MAID) vs. 42% (Control) | 65% vs. 30% | 80% vs. 48% | 77% vs. 43% | ||||
| Folkert et al. [35] | UPS 37%, Liposarcoma 28%, Synovial sarcoma 9% | 56.8% (51.4–62.8) | NA | NA | NA | ||||
| Roeder et al. [36] | Liposarcoma 31%, UPS 27%, Synovial sarcoma 15% | 61% | 58% | 69% | 66% | ||||
| Cheng et al. [43] | UPS 45%, Liposarcoma 21%, Synovial sarcoma 12% | Pré 56% ± 15%/Post 67 ± 12% | NA | NA | NA | ||||
3.6. Overall Survival
| More Frequent Histologic Subtype in Series | Preoperative RT | Postoperative RT | Prognostic Factors in Predicting Worse OS: | Factors without Significant Influence on OS: | |||
| 5 y OS | 10 y OS | 5 y OS | 10 y OS | ||||
| Tanabe et al. [29] | UPS 41%, Liposarcoma 23%, synovial sarcoma 8% | 66% | NA | High grade, size > 11 cm, and intraoperative tumor violation | Margins status, Local failure, CT | ||
| Wanebo et al. [44] | UPS 20%, Synovial sarcoma 18%, Liposarcoma 17% | 59% | NA | High stage, Extent of surgery. For high-grade tumors: size, locoregional extent. | Site, Age, Gender, Histology | ||
| Dincbas et al. [46] | Synovial sarcoma 35%, liposarcoma 24% UPS 22% | 68.3% | NA | NA | NA | ||
| Pao et al. [47] | Liposarcomas + UPS 60% | NA | NA | Stage IV | Margins status, Site, Size, Gender, Age | ||
| Talber et al. [24] | Synovial sarcoma 32%, UPS 11%, Epithelioid sarcoma 9% | 80% | 69% | NA | NA | ||
| Le Péchoux et al. [45] | Synovial sarcoma 27%, UPS 21%, Liposarcoma 11% | 62% (49–73%) | NA | Size ≥ 5 cm, Margin status | Grade | ||
| Khanfir et al. [40] | UPS 30%, Synovial sarcoma 21% | 5 y: 77% (69–84) | 10 y: 67% (57–76) | High grade (Local and Distant recurrence | Margins status, Use of RT | ||
| Issakov et al. [26] | Liposarcomas 100% | NA | 67% | NA | Margins status, Site, Age, Gender, Type of liposarcoma | ||
| Lee et al. [48] | Liposarcoma 33% Synovial sarcoma 23%, UPS 19% | 69.2% | NA | High grade | |||
| McGee et al. [37] | UPS 51%, Liposarcoma 18% | 79% | 70% | Local control, Age, Gender | Margins status, RT dose (<60/60–66/>66 Gy), Fractionation (hyper vs. conventionnal) | ||
| Felderhof et al. [41] | Myxoid liposarcoma 14%, Leiomyosarcoma 13%, Synovial sarcoma 12% | 69% | 51% | Local recurrence Distant recurrence | Margin status, Site, Size, Grade, Gender, Age, Primary/recurrent, Depth, RT dose (≤56, 60, 66 Gy) | ||
| Beane et al. [39] | NA | NA | 82% (72–90) 71% (59–81) (20 y) | NA | Grade, Use of RT | ||
| Dogan et al. [42] | UPS 26%, Liposarcoma 25%, Synovial sarcoma 13% | 71.8% | 69.1% | NA | Site, CT, RT dose (<60/≥60 Gy) | ||
| More Frequent Histologic Subtype in Series | Preoperative + Postoperative RT | Prognostic Factors in Predicting Worse OS: | Factors without Significant Influence on OS: | ||||
| 5 y OS | 10 y OS | ||||||
| Schoenfeldet al. [25] | UPS 40%, Synovial sarcoma 17%, Neurofibrosarcoma 9% | 96% | 91% | NA | NA | ||
| Cannon et al. [34] | UPS 42%, Liposarcoma 22%, Synovial sarcoma 13% | NA | 62% | NA | NA | ||
| Mullen et al. [30] | UPS 22%, Liposarcoma 16% | 84% MAID vs. 56% control | 66% vs. 38% | No use of neoadjuvant CT | NA | ||
| Folkert et al. [35] | UPS 37%, Liposarcoma 28%, Synovial sarcoma 9% | 71.7% (66.6–77.2) | NA | NA | IMRT | ||
| Kneisl et al. [38] | NA | NA | NA | No use of RT | NA | ||
| Roeder et al. [36] | Liposarcoma 31%, UPS 27%, Synovial sarcoma 15% | 77% | 66% | High grade, metastases at/prior to IOERT | NA | ||
| Goertz et al. [27] | UPS 100% | 73.0% (64.5–79.7) | NA | High grade Margin Status Depth Age > 60 No adjuvant RT | Size, Gender | ||
| Cheng et al. [43] | UPS 45%, Liposarcoma 21%, Synovial sarcoma 12% | Pre 75% ± 15% Post 79% ± 11% | NA | NA | High Stage | Timing of RT, Use of RT | |
3.7. Complications
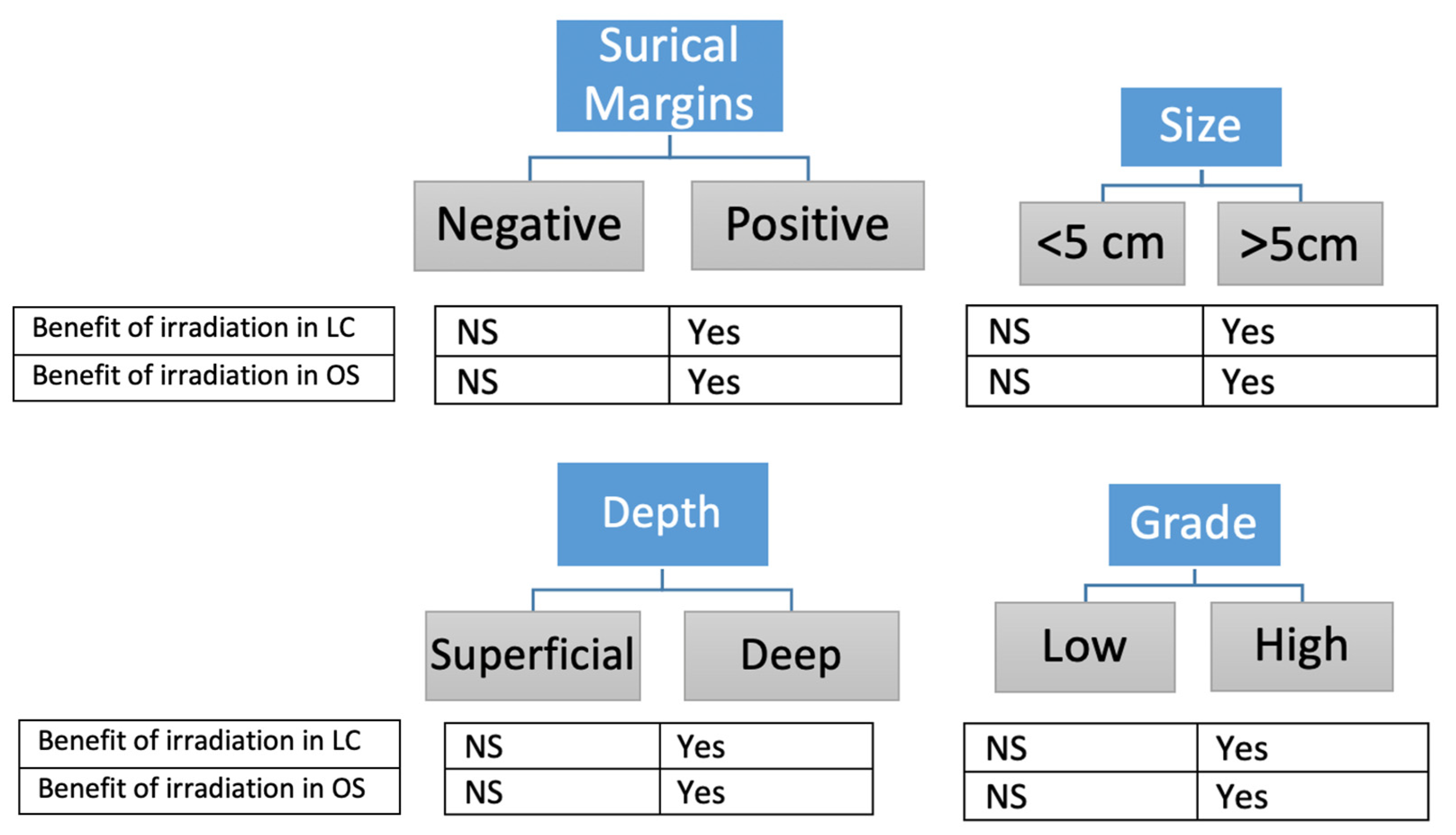
4. Discussion
- Size
- Margins status
- Grade
- Depth
- Histologic subtype
- Localization
- Schedule of RT
- Fractionation
- Chemotherapy
- Complications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2013; Volume 5. [Google Scholar]
- Korah, M.P.; Deyrup, A.T.; Monson, D.K.; Oskouei, S.V.; Weiss, S.W.; Landry, J.; Godette, K.D. Anatomic tumor location influences the success of contemporary limb-sparing surgery and radiation among adults with soft tissue sarcomas of the extremities. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A.; Lewis, J.J.; Brennan, M.F. Soft tissue sarcoma: Prognostic factors and multimodal treatment. Chir. Z Alle. Geb. Oper. Medizen 2000, 71, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Fletcher, C.D. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.; Judson, I.; Peake, D.; Seddon, B. Guidelines for the Management of Soft Tissue Sarcomas. Sarcoma 2010, 2010, 506182. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; Broto, J.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv51–iv67. [Google Scholar] [CrossRef]
- Lindberg, R.D.; Martin, R.G.; Romsdahl, M.M.; Barkley, H.T. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer 1981, 47, 2391–2397. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennam, M.; Demoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef]
- Sadoski, C.; Suit, H.D.; Rosenberg, A.; Mankin, H.; Efird, J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J. Surg. Oncol. 1993, 52, 223–230. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; Delaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Harrison, L.B.; Woodruff, J.M.; Gaynor, J.J.; Brennan, M.F. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J. Clin. Oncol. 1994, 12, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.J.; Leung, D.; Casper, E.S.; Woodruff, J.; Hajdu, S.I.; Brennan, M.F. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch. Surg. 1999, 134, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Suit, H.D.; Mankin, H.J.; Wood, W.C.; Gebhardt, M.C.; Harmon, D.C.; Rosenberg, A.; Tepper, J.E.; Rosenthal, D. Treatment of the patient with stage M0 soft tissue sarcoma. J. Clin. Oncol. 1988, 6, 854–862. [Google Scholar] [CrossRef]
- Huth, J.F.; Eilber, F.R. Patterns of metastatic spread following resection of extremity soft-tissue sarcomas and strategies for treatment. Semin. Surg. Oncol. 1988, 4, 20–26. [Google Scholar] [CrossRef]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003, 97, 2530–2543. [Google Scholar] [CrossRef]
- Baroudi, M.R.; Ferguson, P.C.; Wunder, J.S.; Isler, M.H.; Mottard, S.; Werier, J.A.; Turcotte, R.E. Forearm soft tissue sarcoma: Tumors characteristics and oncologic outcomes following limb salvage surgery. J. Surg. Oncol. 2014, 110, 676–681. [Google Scholar] [CrossRef]
- Singer, S.; Corson, J.M.; Gonin, R.; Labow, B.; Eberlein, T.J. Prognostic Factors Predictive of Survival and Local Recurrence for Extremity Soft Tissue Sarcoma. Ann. Surg. 1994, 219, 165–173. [Google Scholar] [CrossRef]
- Kungwengwe, G.; Clancy, R.; Vass, J.; Slade, R.; Sandhar, S.; Dobbs, T.D.; Bragg, T.W. Preoperative versus Post-operative Radiotherapy for Extremity Soft tissue Sarcoma: A Systematic Review and Meta-analysis of Long-term Survival. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2443–2457. [Google Scholar] [CrossRef]
- Slump, J.; Bastiaannet, E.; Halka, A.; Hoekstra, H.J.; Ferguson, P.C.; Wunder, J.S.; Hofer, S.O.; O’Neill, A.C. Risk factors for postoperative wound complications after extremity soft tissue sarcoma resection: A systematic review and meta-analyses. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 89, 105906. [Google Scholar] [CrossRef]
- Talbert, M.L.; Zagars, G.K.; Sherman, N.E.; Romsdahl, M.M. Conservative surgery and radiation therapy for soft tissue sarcoma of the wrist, hand, ankle, and foot. Cancer 1990, 66, 2482–2491. [Google Scholar] [CrossRef]
- Schoenfeld, G.S.; Morris, C.G.; Scarborough, M.T.; Zlotecki, R.A. Adjuvant Radiotherapy in the Management of Soft Tissue Sarcoma Involving the Distal Extremities. Am. J. Clin. Oncol. 2006, 29, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Issakov, J.; Soyfer, V.; Kollender, Y.; Bickels, J.; Meller, I.; Merimsky, O. Liposarcoma in adult limbs treated by limb-sparing surgery and adjuvant radiotherapy. J. Bone Jt. Surg. 2006, 88, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Goertz, O.; Pieper, A.; von der Lohe, L.; Stricker, I.; Dadras, M.; Behr, B.; Lehnhardt, M.; Harati, K. The Impact of Surgical Margins and Adjuvant Radiotherapy in Patients with Undifferentiated Pleomorphic Sarcomas of the Extremities: A Single-Institutional Analysis of 192 Patients. Cancers 2020, 12, 362. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Velasco, J.; Zelefsky, M.; Woodruff, J.; Lewis, J.; Brennan, M. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int. J. Radiat. Oncol. 2000, 48, 1051–1058. [Google Scholar] [CrossRef]
- Tanabe, K.K.; Pollock, R.E.; Ellis, L.M.; Murphy, A.; Sherman, N.; Romsdahl, M.M. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer 1994, 73, 1652–1659. [Google Scholar] [CrossRef]
- Mullen, J.T.; Kobayashi, W.; Wang, J.J.; Harmon, D.C.; Choy, E.; Hornicek, F.J.; Rosenberg, A.E.; Chen, Y.-L.; Spiro, I.J.; DeLaney, T.F. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer 2011, 118, 3758–3765. [Google Scholar] [CrossRef]
- Dickie, C.I.; Parent, A.L.; Griffin, A.M.; Fung, S.; Chung, P.W.; Catton, C.N.; Ferguson, P.C.; Wunder, J.S.; Bell, R.S.; Sharpe, M.B.; et al. Bone fractures following external beam radiotherapy and limb-preservation surgery for lower extremity soft tissue sarcoma: Relationship to irradiated bone length, volume, tumor location and dose. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1119–1124. [Google Scholar] [CrossRef]
- Choong, P.F.; Petersen, I.A.; Nascimento, A.G.; Sim, F.H. Is Radiotherapy Important for Low-Grade Soft Tissue Sarcoma of the Extremity? Clin. Orthop. Relat. Res. 2001, 387, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Blaes, A.H.; Lindgren, B.; Mulrooney, D.A.; Willson, L.; Cho, L.C. Pathologic femur fractures after limb-sparing treatment of soft-tissue sarcomas. J. Cancer Surviv. Res. Pract. 2010, 4, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Ballo, M.T.; Zagars, G.K.; Mirza, A.N.; Lin, P.P.; Lewis, V.O.; Yasko, A.W.; Benjamin, R.S.; Pisters, P.W. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006, 107, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Folkert, M.R.; Singer, S.; Brennan, M.F.; Kuk, D.; Qin, L.-X.; Kobayashi, W.K.; Crago, A.M.; Alektiar, K.M. Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J. Clin. Oncol. 2014, 32, 3236–3241. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F.; de Paoli, A.; Saleh-Ebrahimi, L.; Alldinger, I.; Bertola, G.; Boz, G.; Navarria, F.; Cuervo, M.; Uhl, M.; Alvarez, A.; et al. Intraoperative Electron Radiation Therapy Combined with External Beam Radiation Therapy after Gross Total Resection in Extremity Soft Tissue Sarcoma: A European Pooled Analysis. Ann. Surg. Oncol. 2018, 25, 3833–3842. [Google Scholar] [CrossRef]
- McGee, L.; Indelicato, D.J.; Dagan, R.; Morris, C.G.; Knapik, J.A.; Reith, J.D.; Scarborough, M.T.; Gibbs, C.P.; Marcus, R.B.; Zlotecki, R.A. Long-Term Results Following Postoperative Radiotherapy for Soft Tissue Sarcomas of the Extremity. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Kneisl, J.S.; Ferguson, C.; Robinson, M.; Crimaldi, A.; Ahrens, W.; Symanowski, J.; Bates, M.; Ersek, J.L.; Livingston, M.; Patt, J.; et al. The effect of radiation therapy in the treatment of adult soft tissue sarcomas of the extremities: A long-term community-based cancer center experience. Cancer Med. 2017, 6, 516–525. [Google Scholar] [CrossRef]
- Beane, J.D.; Yang, J.C.; White, D.; Steinberg, S.M.; Rosenberg, S.A.; Rudloff, U. Efficacy of Adjuvant Radiation Therapy in the Treatment of Soft Tissue Sarcoma of the Extremity: 20-year Follow-Up of a Randomized Prospective Trial. Ann. Surg. Oncol. 2014, 21, 2484–2489. [Google Scholar] [CrossRef]
- Khanfir, K.; Alzieu, L.; Terrier, P.; Le Péchoux, C.; Bonvalot, S.; Vanel, D.; Le Cesne, A. Does adjuvant radiation therapy increase loco-regional control after optimal resection of soft-tissue sarcoma of the extremities? Eur. J. Cancer 2003, 39, 1872–1880. [Google Scholar] [CrossRef]
- Felderhof, J.M.; Creutzberg, C.L.; Putter, H.; Nout, R.A.; Bovée, J.V.M.G.; Dijkstra, P.D.S.; Hartgrink, H.H.; Marijnen, C.A.M. Long-term clinical outcome of patients with soft tissue sarcomas treated with limb-sparing surgery and postoperative radiotherapy. Acta Oncol. 2013, 52, 745–752. [Google Scholar] [CrossRef]
- Dogan, Ö.Y.; Oksuz, D.Ç.; Atalar, B.; Dincbas, F.O. Long-term results of extremity soft tissue sarcomas limb-sparing surgery and radiotherapy. Acta Ortop. Bras. 2019, 27, 207–211. [Google Scholar] [CrossRef]
- Cheng, E.Y.; Dusenbery, K.E.; Winters, M.R.; Thompson, R.C. Soft tissue sarcomas: Preoperative versus postoperative radiotherapy. J. Surg. Oncol. 1996, 61, 90–99. [Google Scholar] [CrossRef]
- Wanebo, H.J.; Temple, W.J.; Popp, M.B.; Constable, W.; Aron, B.; Cunningham, S.L. Preoperative regional therapy for extremity sarcoma. A tricenter update. Cancer 1995, 75, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Le Péchoux, C.; Le Deley, M.-C.; Delaloge, S.; Lartigau, E.; Levy-Piedbois, C.; Bonvalot, S.; Le Cesne, A.; Missenard, G.; Terrier, P.; Vanel, D.; et al. Postoperative radiotherapy in the management of adult soft tissue sarcoma of the extremities: Results with two different total dose, fractionation, and overall treatment time schedules. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Dincbas, F.O.; Oksuz, D.C.; Yetmen, O.; Hiz, M.; Dervisoglu, S.; Turna, H.; Kantarci, F.; Mandel, N.M.; Koca, S. Neoadjuvant Treatment with Preoperative Radiotherapy for Extremity Soft Tissue Sarcomas: Long-Term Results from a Single Institution in Turkey. Asian Pac. J. Cancer Prev. 2014, 15, 1775–1781. [Google Scholar] [CrossRef][Green Version]
- Pao, W.J.; Pilepich, M.V. Postoperative radiotherapy in the treatment of extremity soft tissue sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 907–911. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.J.; Yang, D.S.; Yoon, W.S.; Lee, J.A.; Rim, C.H.; Kim, C.Y. Treatment outcome of conservative surgery plus postoperative radiotherapy for extremity soft tissue sarcoma. Radiat. Oncol. J. 2012, 30, 62–69. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Zelefsky, M.J.; Brennan, M.F. Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1273–1279. [Google Scholar] [CrossRef]
- Dickie, C.I.; Parent, A.; Griffin, A.; Craig, T.; Catton, C.; Chung, P.; Panzarella, T.; O’Sullivan, B.; Sharpe, M. A Device and Procedure for Immobilization of Patients Receiving Limb-Preserving Radiotherapy for Soft Tissue Sarcoma. Med. Dosim. 2009, 34, 243–249. [Google Scholar] [CrossRef]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef]
- Pisters, P.W.; Leung, D.H.; Woodruff, J.; Shi, W.; Brennan, M.F. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcomas of the extremities. J. Clin. Oncol. 1996, 14, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Coindre, J.M.; Terrier, P.; Bui, N.B.; Bonichon, F.; Collin, F.; Le Doussal, V.; Mandard, A.M.; O Vilain, M.; Jacquemier, J.; Duplay, H.; et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J. Clin. Oncol. 1996, 14, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, D.; Rineer, J.; Katsoulakis, E.; Sroufe, R.L.; Lange, C.S.; Nwokedi, E.; Schwartz, D.; Choi, K.; Rotman, M. Impact of Postoperative Radiation on Survival for High-grade Soft Tissue Sarcoma of the Extremities After Limb Sparing Radical Resection. Am. J. Clin. Oncol. 2012, 35, 13–17. [Google Scholar] [CrossRef]
- Geer, R.J.; Woodruff, J.; Casper, E.S.; Brennan, M.F. Management of Small Soft-Tissue Sarcoma of the Extremity in Adults. Arch. Surg. 1992, 127, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Gadd, M.A.; Casper, E.S.; Woodruff, J.M.; McCormack, P.M.; Brennan, M.F. Development and Treatment of Pulmonary Metastases in Adult Patients with Extremity Soft Tissue Sarcoma. Ann. Surg. 1993, 218, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Eilber, F.C.; Rosen, G.; Nelson, S.D.; Selch, M.; Dorey, F.; Eckardt, J.; Eilber, F.R. High-Grade Extremity Soft Tissue Sarcomas. Ann. Surg. 2003, 237, 218–226. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Leung, D.H.Y.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the Prognostic Significance of Microscopic Margins in 2084 Localized Primary Adult Soft Tissue Sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef]
- Lehnhardt, M.; Daigeler, A.; Homann, H.H.; Schwaiberger, V.; Goertz, O.; Kuhnen, C.; Steinau, H.U. MFH revisited: Outcome after surgical treatment of undifferentiated pleomorphic or not otherwise specified (NOS) sarcomas of the extremities—An analysis of 140 patients. Langenbeck’s Arch. Surg. 2009, 394, 313–320. [Google Scholar] [CrossRef]
- Callegaro, D.; Miceli, R.; Bonvalot, S.; Ferguson, P.; Strauss, D.C.; Levy, A.; Griffin, A.; Hayes, A.J.; Stacchiotti, S.; Le Pechoux, C.; et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: A retrospective analysis. Lancet Oncol. 2016, 17, 671–680. [Google Scholar] [CrossRef]
- Vodanovich, D.A.; Spelman, T.; May, D.; Slavin, J.; Choong, P.F.M. Predicting the prognosis of undifferentiated pleomorphic soft tissue sarcoma: A 20-year experience of 266 cases. ANZ J. Surg. 2019, 89, 1045–1050. [Google Scholar] [CrossRef]
- Kamat, N.V.; Million, L.; Yao, D.-H.; Donaldson, S.S.; Mohler, D.G.; van de Rijn, M.; Avedian, R.S.; Kapp, D.S.; Ganjoo, K.N. The Outcome of Patients with Localized Undifferentiated Pleomorphic Sarcoma of the Lower Extremity Treated at Stanford University. Am. J. Clin. Oncol. 2019, 42, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Vasileios, K.A.; Eward, W.C.; Brigman, B.E. Surgical treatment and prognosis in patients with high-grade soft tissue malignant fibrous histiocytoma of the extremities. Arch. Orthop. Trauma Surg. 2012, 132, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Le Doussal, V.; Coindre, J.M.; Leroux, A.; Hacene, K.; Terrier, P.; Bui, N.B.; Bonichon, F.; Collin, F.; Mandard, A.-M.; Contesso, G. Prognostic factors for patients with localized primary malignant fibrous histiocytoma: A multicenter study of 216 patients with multivariate analysis. Cancer 1996, 77, 1823–1830. [Google Scholar] [CrossRef]
- Herbert, S.H.; Corn, B.W.; Solin, L.J.; Lanciano, R.M.; Schultz, D.J.; McKenna, W.G.; Coia, L.R. Limb-preserving treatment for soft tissue sarcomas of the extremities. The significance of surgical margins. Cancer 1993, 72, 1230–1238. [Google Scholar] [CrossRef]
- Lansu, J.; Bovée, J.V.M.G.; Braam, P.; van Boven, H.; Flucke, U.; Bonenkamp, J.J.; Miah, A.B.; Zaidi, S.H.; Thway, K.; Bruland, Ø.S.; et al. Dose Reduction of Preoperative Radiotherapy in Myxoid Liposarcoma: A Nonrandomized Controlled Trial. JAMA Oncol. 2021, 7, e205865. [Google Scholar] [CrossRef] [PubMed]
- Koseła-Paterczyk, H.; Szacht, M.; Morysiński, T.; Ługowska, I.; Dziewirski, W.; Falkowski, S.; Zdzienicki, M.; Pieńkowski, A.; Szamotulska, K.; Świtaj, T.; et al. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur. J. Surg. Oncol. 2014, 40, 1641–1647. [Google Scholar] [CrossRef]
- Kirilova, M.; Klein, A.; Lindner, L.H.; Nachbichler, S.; Knösel, T.; Birkenmaier, C.; Baur-Melnyk, A.; Dürr, H.R. Amputation for Extremity Sarcoma: Indications and Outcomes. Cancers 2021, 13, 5125. [Google Scholar] [CrossRef]
- Gronchi, A.; Vullo, S.L.; Colombo, C.; Collini, P.; Stacchiotti, S.; Mariani, L.; Fiore, M.; Casali, P.G. Extremity Soft Tissue Sarcoma in a Series of Patients Treated at a Single Institution: Local Control Directly Impacts Survival. Ann. Surg. 2010, 251, 506–511. [Google Scholar] [CrossRef]
- Bonvalot, S.; Levy, A.; Terrier, P.; Tzanis, D.; Bellefqih, S.; Le Cesne, A.; Le Péchoux, C. Primary Extremity Soft Tissue Sarcomas: Does Local Control Impact Survival? Ann. Surg. Oncol. 2017, 24, 194–201. [Google Scholar] [CrossRef]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003, 97, 2544–2553. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Bateni, S.B.; Monjazeb, A.M.; Darrow, M.A.; Thorpe, S.W.; Kirane, A.R.; Bold, R.J.; Canter, R.J. Neoadjuvant Radiotherapy is Associated with R0 Resection and Improved Survival in Extremity Soft Tissue Sarcoma Patients Undergoing Surgery: An NCDB Analysis. Ann. Surg. Oncol. 2017, 24, 3252–3263. [Google Scholar] [CrossRef]
- Potter, D.A.; Glenn, J.; Kinsella, T.; Glatstein, E.; Lack, E.E.; Restrepo, C.; White, D.E.; A Seipp, C.; Wesley, R.; A Rosenberg, S. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J. Clin. Oncol. 1985, 3, 353–366. [Google Scholar] [CrossRef]
- Baldini, E.H.; Le Cesne, A.; Trent, J.C. Neoadjuvant Chemotherapy, Concurrent Chemoradiation, and Adjuvant Chemotherapy for High-Risk Extremity Soft Tissue Sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 1997, 350, 1647–1654. [Google Scholar] [CrossRef]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; Van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.; et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Fisher, C.; Al-Muderis, O.; Judson, I.R. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur. J. Cancer 2005, 41, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); U.S. Department of Health and Human Services: Washington, DC, USA, 2017. [Google Scholar]
- Stewart, A.J.; Lee, Y.K.; Saran, F.H. Comparison of conventional radiotherapy and intensity-modulated radiotherapy for post-operative radiotherapy for primary extremity soft tissue sarcoma. Radiother. Oncol. 2009, 93, 125–130. [Google Scholar] [CrossRef]
- Baldini, E.H.; Lapidus, M.R.; Wang, Q.; Manola, J.; Orgill, D.P.; Pomahac, B.; Marcus, K.J.; Bertagnolli, M.M.; Devlin, P.M.; George, S.; et al. Predictors for Major Wound Complications Following Preoperative Radiotherapy and Surgery for Soft-Tissue Sarcoma of the Extremities and Trunk: Importance of Tumor Proximity to Skin Surface. Ann. Surg. Oncol. 2013, 20, 1494–1499. [Google Scholar] [CrossRef]
| More Frequent Histologic Subtype in Series | Preoperative RT | Postoperative RT | Prognostic Factors in Predicting Worse LC: | Factors without Significant Influence on LC: | |||
| 5 y LC | 10 y LC | 5 y LC | 10 y LC | ||||
| Tanabe et al. [29] | UPS 41%, Liposarcoma 23%, synovial sarcoma 8% | 83% | NA | High grade tumors, Intraoperative tumor violation, Positive margins | Size, CT, Site | ||
| Wanebo et al. [44] | UPS 20%, Synovial sarcoma 18%, Liposarcoma 17% | 98.5 (7 y) | NA | NA | NA | ||
| Dincbas et al. [46] | Synovial sarcoma 35%, liposarcoma 24% UPS 22% | 81% | NA | Conventionnal fractionation vs. hyprofractionation | Margins status, Size, RT | ||
| Pao et al. [47] | Liposarcomas + UPS 60% | NA | 78% | NA | Margins status, Grade, Stage, Site | ||
| Talber et al. [24] | Synovial sarcoma 32%, UPS 11%, Epithelioid sarcoma 9% | 80% | 74% | NA | Size, Site, Type of RT, RT dose | ||
| Le Péchoux et al. [45] | Synovial sarcoma 27%, UPS 21%, Liposarcoma 11% | 75% (61–85) | NA | NA | Margins status, Size, Grade | ||
| Alektiar et al. [28] | NA | 82% (76–88) | NA | Age > 50, Central tumor location (shoulder/groin) | Site, Depth, RT, type of RT (BRT vs. EBRT) | ||
| Khanfir et al. [40] | UPS 30%, Synovial sarcoma 21% | 78% (70–84) | 71% (61–79) | No adjuvant RT, UPS histological type | Margins status, Size, Grade, Depth, Age, Treatment era, Re-excision, CT | ||
| Issakov et al. [26] | Liposarcomas 100% | NA | 83% | NA | Margins status, Site, Age, Gender, Type of liposarcoma, | ||
| Lee et al. [48] | Liposarcoma 33% Synovial sarcoma 23%, UPS 19% | 90.7% | NA | NA | Margins status, Grade | ||
| McGee et al. [37] | UPS 51%, Liposarcoma 18% | 89% | 87% | Age > 55 years, Recurrent presentation | Margin status, Stage, Site, RT fractionation, treatment era, RT dose for positive margin | ||
| Felderhof et al. [41] | Myxoid liposarcoma 14%, Leiomyosarcoma 13%, Synovial sarcoma 12% | 91% | 88% | NA | NA | ||
| Beane et al. [39] | NA | NA | 100% (RT arm) | No RT | NA | ||
| Dogan et al. [42] | UPS 26%, Liposarcoma 25%, Synovial sarcoma 13% | 77% | 70.4% | RT dose, CT | Site, Grade, Stage, Gender | ||
| More Frequent Histologic Subtype in Series | Preoperative + Postoperative RT | Prognostic Factors in Predicting Worse LC: | Factors without Significant Influence on LC: | ||||
| 5 y LC | 10 y LC | ||||||
| Choong et al. [32] | UPS 35%, Liposarcoma 34%, Fibrosarcoma 15% | 91.7% (4.4 y) | NA | No RT Size > 5 cm | Margin status, Grade, Depth | ||
| Schoenfeldet al. [25] | UPS 40%, Synovial sarcoma 17%, Neurofibrosarcoma 9% | 91% | 91% | NA | NA | ||
| Cannon et al. [34] | UPS 42%, Liposarcoma 22%, Synovial sarcoma 13% | 89% | 88% | NA | NA | ||
| Mullen et al. [30] | UPS 22%, Liposarcoma 16% | NA | MAID:90% (11.2 y) Control:83% | NA | Preoperative CT | ||
| Folkert et al. [35] | UPS 37%, Liposarcoma 28%, Synovial sarcoma 9% | 92.4% (IMRT) 84.9% (EBRT) | Size > 10 cm, Age > 50 years, IMRT | Margin status, Grade, Depth, Tumor histology, Timing of RT, CT | |||
| Roeder et al. [36] | Liposarcoma 31%, UPS 27%, Synovial sarcoma 15% | 86% | 85% | Positive margins | Site, Grade, Age, Gender, Histology, RT dose, RT timing, primary vs. recurrent, CT | ||
| Goertz et al. [27] | UPS 100% | 67.6% (RT arm) | NA | No adjuvant RT, Positive margins | Size, Site, Grade, Age, Gender, Depth, Preoperative RT | ||
| Cheng et al. [43] | UPS 45%, Liposarcoma 21%, Synovial sarcoma 12% | Pre RT 83% ± 12% | Post RT 91% ± 8% | NA | NA | Timing of RT | |
| Timing of RT/Surgery | Wound Complications | Bone Fractures | Amputations | Chronic Complications | |
|---|---|---|---|---|---|
| Tanabe et al. [29] | Pre | 8% | NA | 1% | NA |
| Wanebo et al. [44] | Pre | 41% | NA | 2.5% | Dysfunction G ≥ 2:4.5% Edema G ≥ 2:7.6% |
| Dincbas et al. [46] | Pre | 20% | 3.3% | 1.7% | 47% Fibrosis: 31.7%, Edema: 13.3% Osteoradionecrosis: 3.3% |
| Pao et al. [47] | Post | NA | NA | 2.5% | Dysfunction G ≥ 2:8% |
| Talbert et al. [24] | Post | NA | NA | 25% | 25% Joint stiffness: 5.1% Edema: 1.3% |
| Le Péchoux et al. [45] | Post | NA | 3.2% | 0 | 3-year complication: 55% (41–68) (dysfunction, edema, sclerosis, pain, skin necrosis nerve damage) |
| Khanfir et al. [40] | Post | NA | NA | 0 | 29% (edema, fibrosis, impairment of joint movement, lymphoedema) |
| Issakov et al. [26] | Post | NA | 5,3% | 2.6% | Pain: 68.4% Neuromotor disturbance: 44.7% Joint stiffness: 16.8% Soft-tissue damage: 65.8% Lymphoedema: 21%. |
| Lee et al. [48] | Post | 14% | NA | NA | 4.7% (lymphedema and skin ulceration) |
| McGee et al. [37] | Post | 3.4% | 6.3% | 2.9% | NA |
| Felderhof et al. [41] | Post | 7% | NA | NA | 71.1% (all grades) Fibrosis: 55% Joint stiffness: 23% |
| Beane et al. [39] | Post | 27% | 2% | 6.7% | Dysfunctions G ≥ 2:12% Edema G ≥ 2:25% |
| Alektiar et al. [28] | Post | 2% | NA | 0 | NA |
| Dogan et al. [42] | Post | NA | 1.1% | NA | Fibrosis: 45.6% Edema: 7.9 |
| Cheng et al. [43] | Pre (43%) + Post (57%) | 18% (13% pre, 5% post) | NA | NA | NA |
| Schoenfeld et al. [25] | Pre (30%) + Post (70%) | NA | 4.8% | 0 | 91.3% (edema, fibrosis, joint function) |
| Cannon et al. [34] | Pre (65%) + Post (35%) | 27% | 1.2% | NA | 10% 20 y radiation-related complication–free survival: 87% |
| Mullen et al. | Pre (50%) + Pre with postoperative boost (40.1%) + Post (9%) | 12.5% | 6.5% | 2.1% | 16.7% (chronic pain, limitations in range of motion, lymphedema) |
| Folkert et al. [30,35] | Pre (12.2%) + Post (87; 8%) | 18.4% | 6.9% | NA | Nerve injuries G ≥ 2:2.6% Joint stiffness G ≥ 2:12.9% Edema G ≥ 2:11.3% |
| Kneisl et al. [38] | Pre (36.9%) + Post (63.1%) | NA | 8% | 4.9% | NA |
| Roeder et al. [36] | Pre (17%) + Post (83%) | NA | NA | 5% | Dysfunction G ≥ 2:19% |
| Blaes et al. [33] | Pre (13%) + Post (67%) + both (20%) | NA | 9% | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebas, A.; Le Fèvre, C.; Waissi, W.; Chambrelant, I.; Brinkert, D.; Noël, G. Prognostic Factors in Extremity Soft Tissue Sarcomas Treated with Radiotherapy: Systematic Review of the Literature. Cancers 2023, 15, 4486. https://doi.org/10.3390/cancers15184486
Lebas A, Le Fèvre C, Waissi W, Chambrelant I, Brinkert D, Noël G. Prognostic Factors in Extremity Soft Tissue Sarcomas Treated with Radiotherapy: Systematic Review of the Literature. Cancers. 2023; 15(18):4486. https://doi.org/10.3390/cancers15184486
Chicago/Turabian StyleLebas, Arthur, Clara Le Fèvre, Waisse Waissi, Isabelle Chambrelant, David Brinkert, and Georges Noël. 2023. "Prognostic Factors in Extremity Soft Tissue Sarcomas Treated with Radiotherapy: Systematic Review of the Literature" Cancers 15, no. 18: 4486. https://doi.org/10.3390/cancers15184486
APA StyleLebas, A., Le Fèvre, C., Waissi, W., Chambrelant, I., Brinkert, D., & Noël, G. (2023). Prognostic Factors in Extremity Soft Tissue Sarcomas Treated with Radiotherapy: Systematic Review of the Literature. Cancers, 15(18), 4486. https://doi.org/10.3390/cancers15184486








