Surgical Anatomy of the Microscopic and Endoscopic Transorbital Approach to the Middle Fossa and Cavernous Sinus: Anatomo-Radiological Study with Clinical Applications
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphometric Study on Dry Skulls
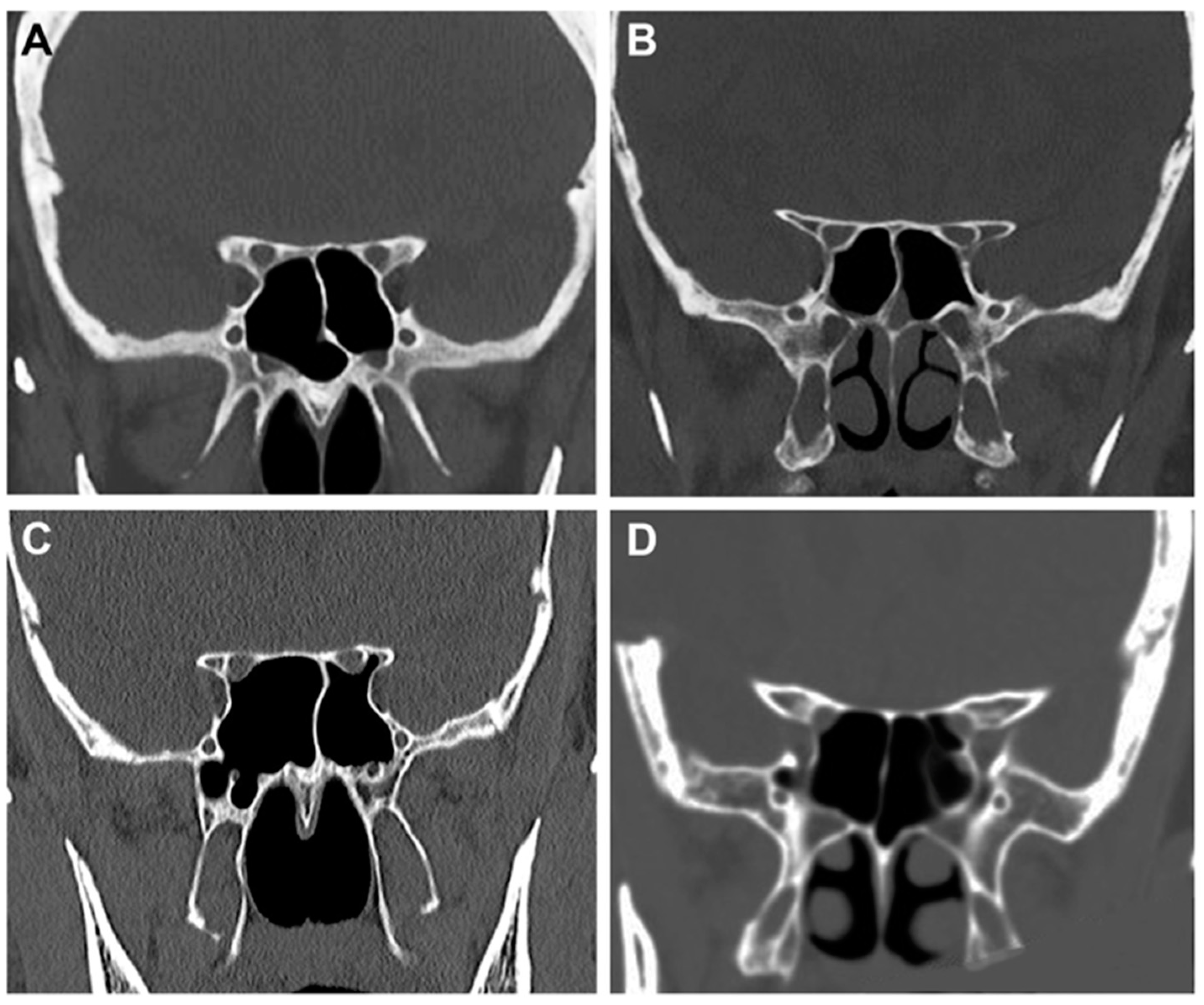
2.2. Radiological Study
- -
- Type 1: the optic canal was adjacent to the sphenoid sinus (SpS) without impression;
- -
- Type 2: the optic canal caused an impression on the SpS;
- -
- Type 3: the optic canal was identified in the SpS;
- -
- Type 4: the optic canal was found lateral to the SpS and the posterior ethmoid sinus (presence of Onodi cell or spheno-ethmoidal air cell).
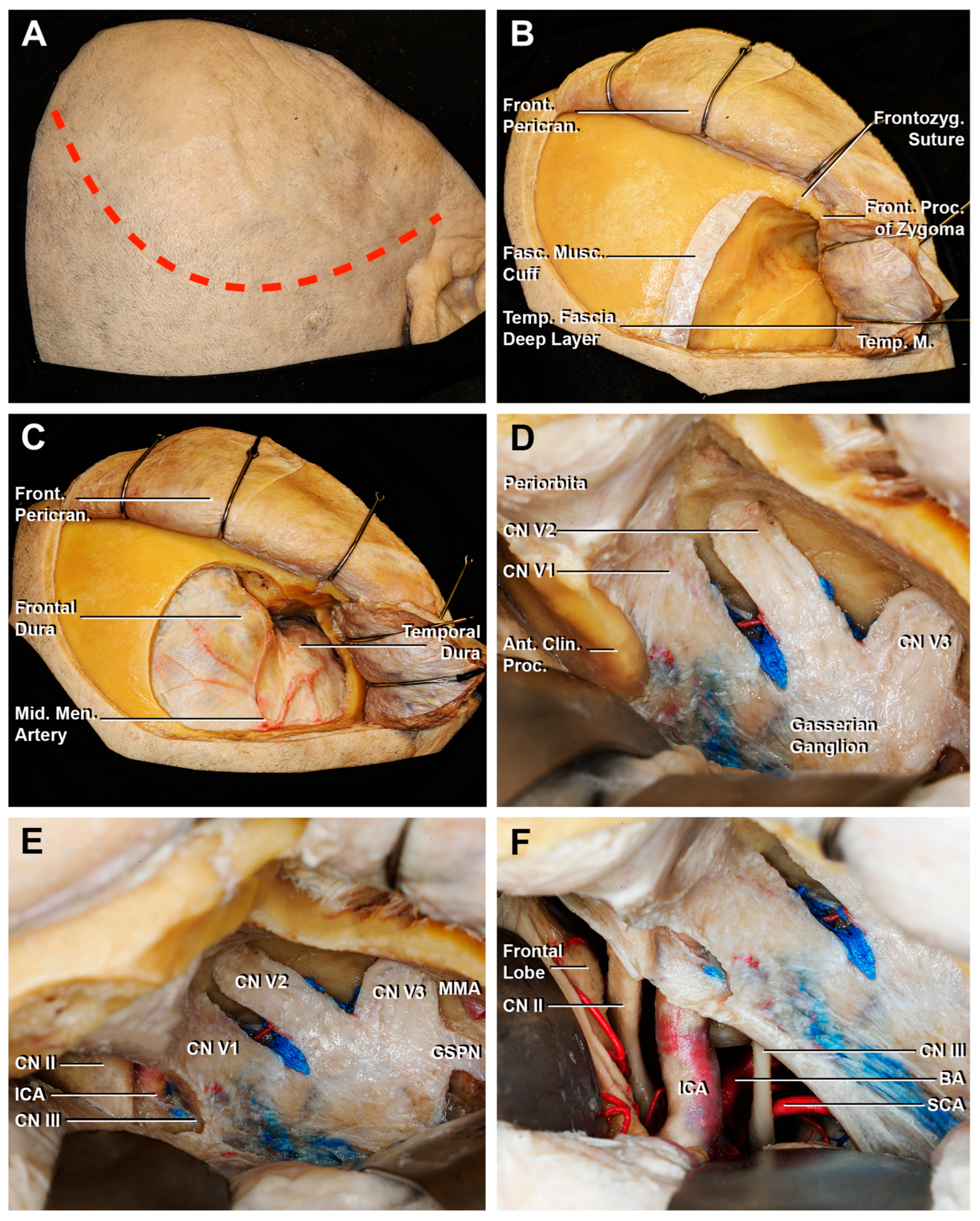
2.3. Anatomical Dissections
3. Results
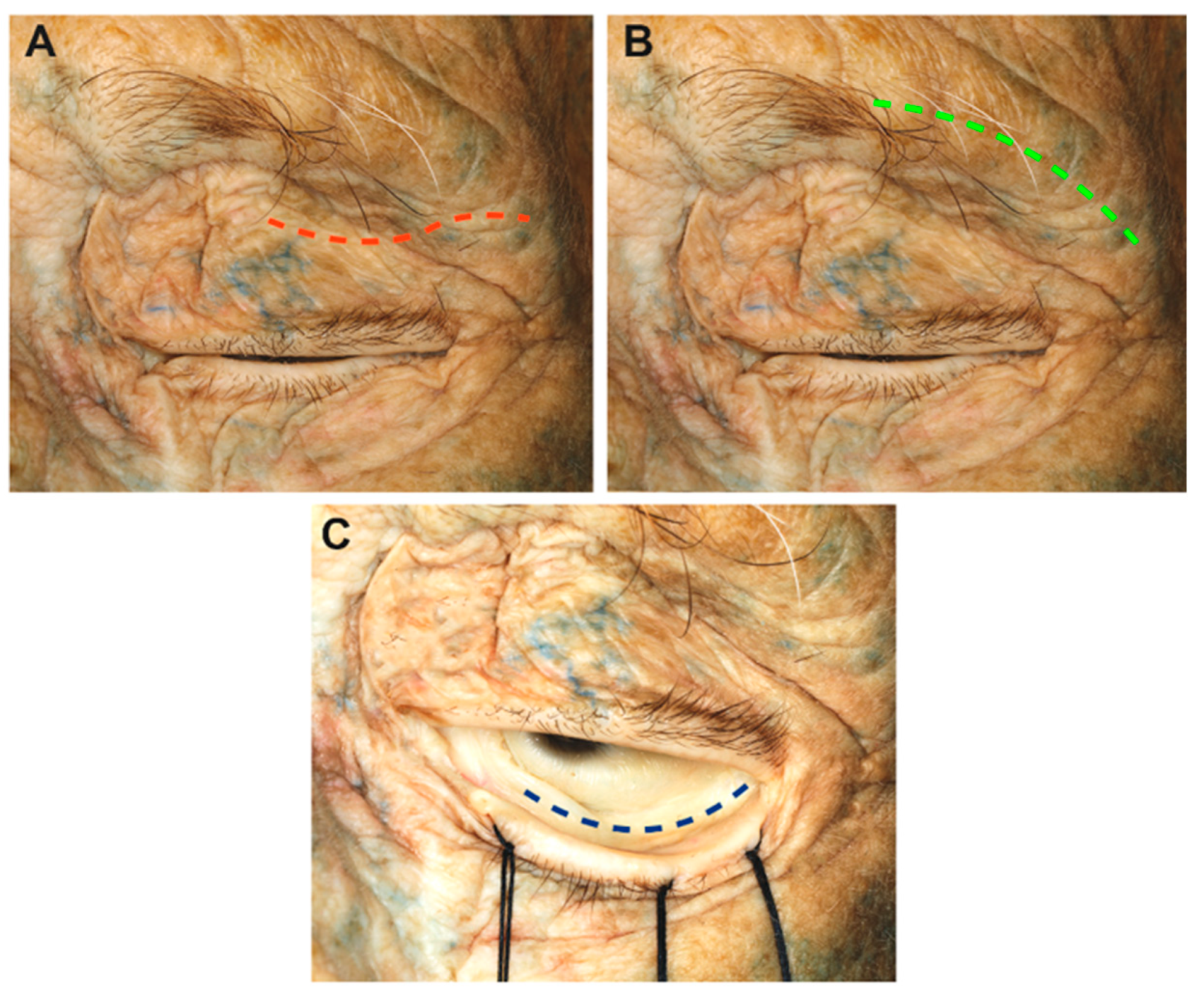
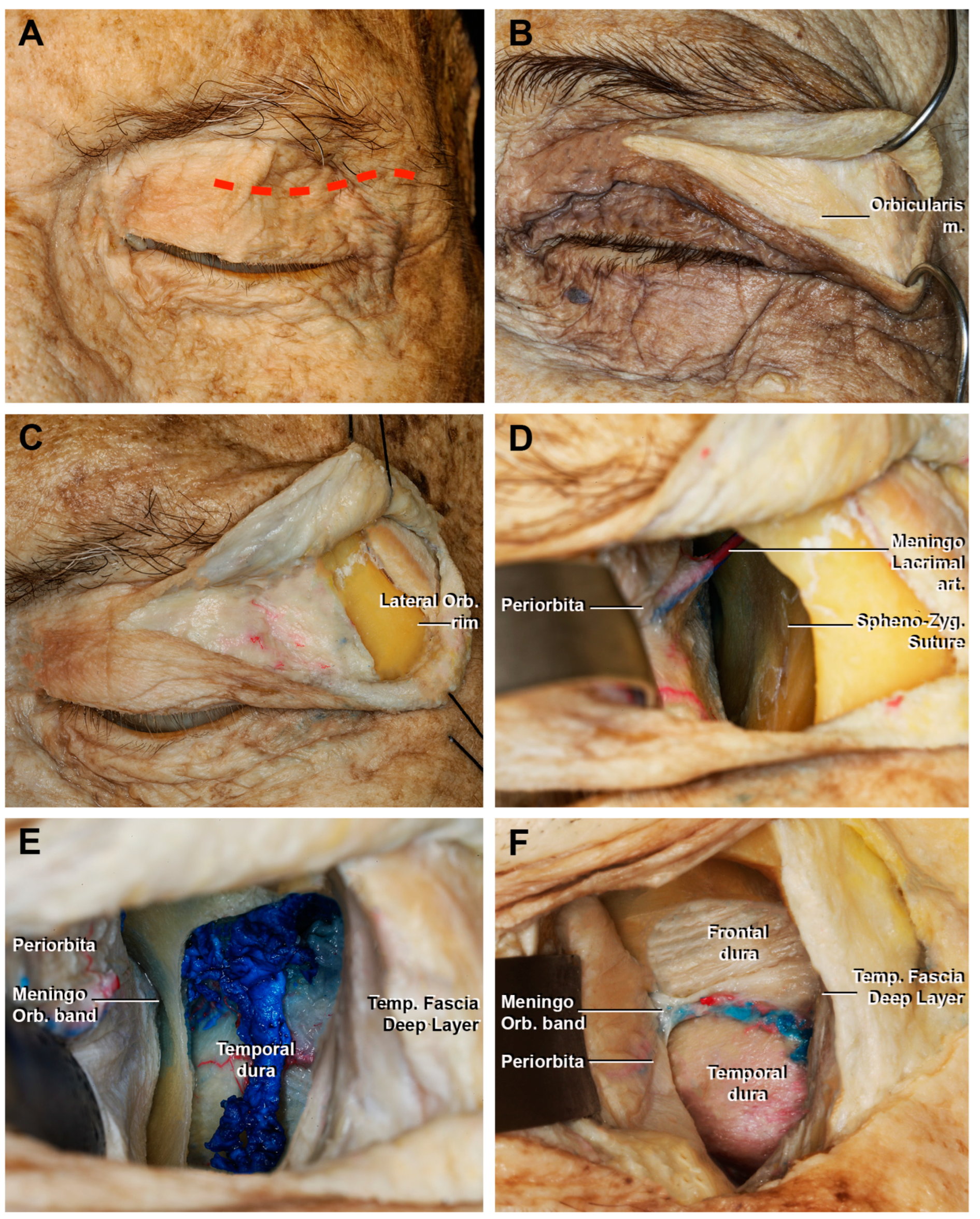
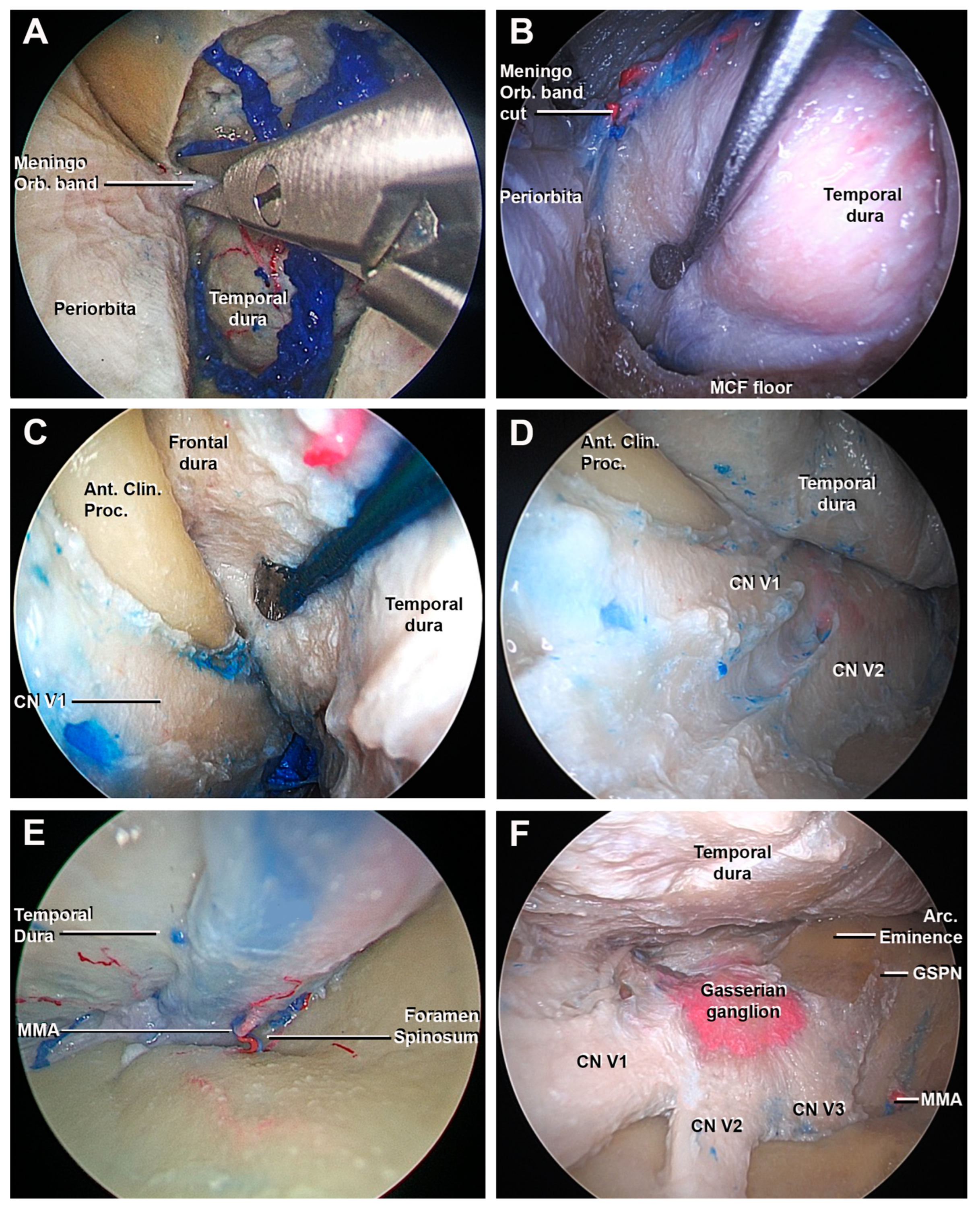
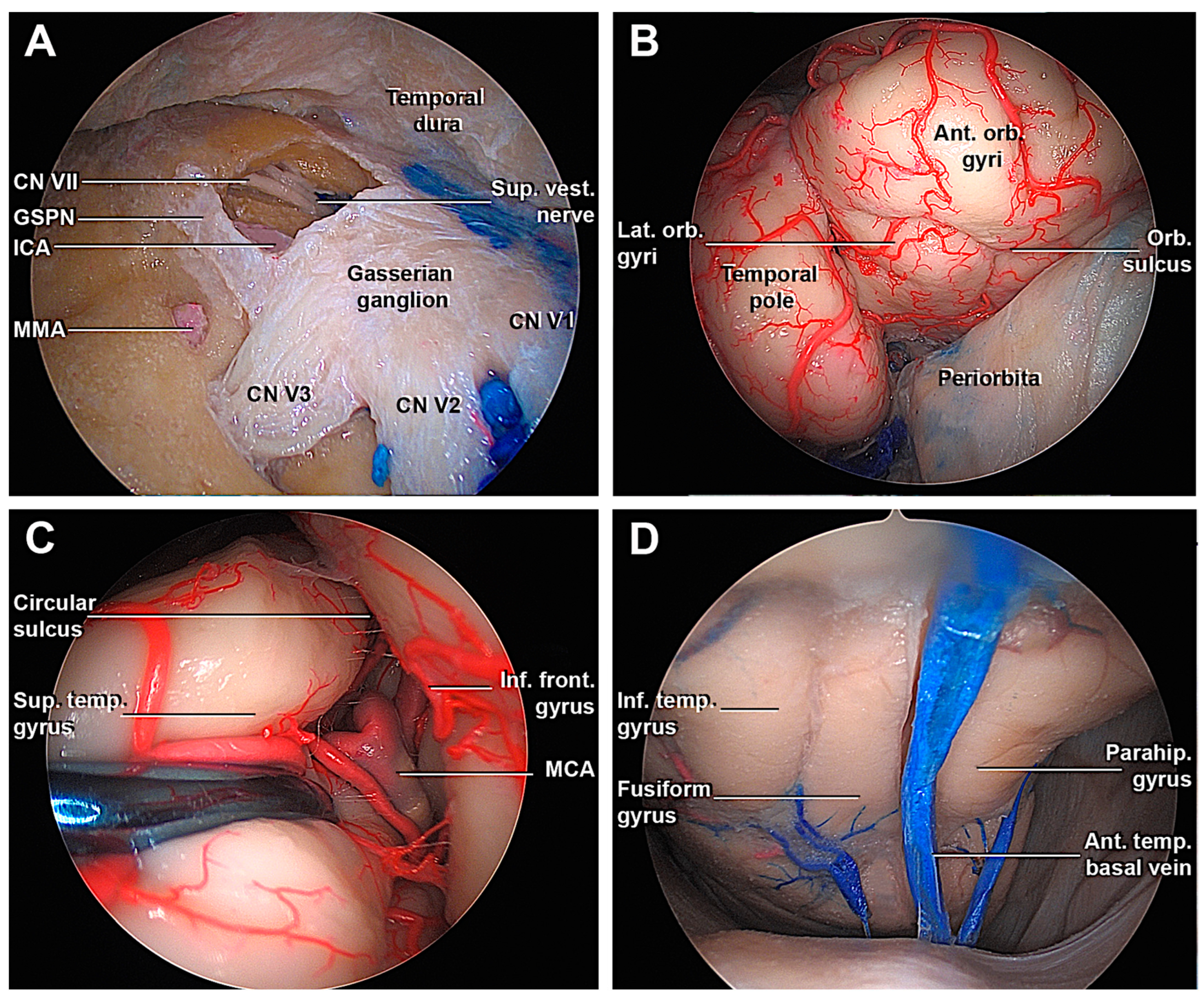
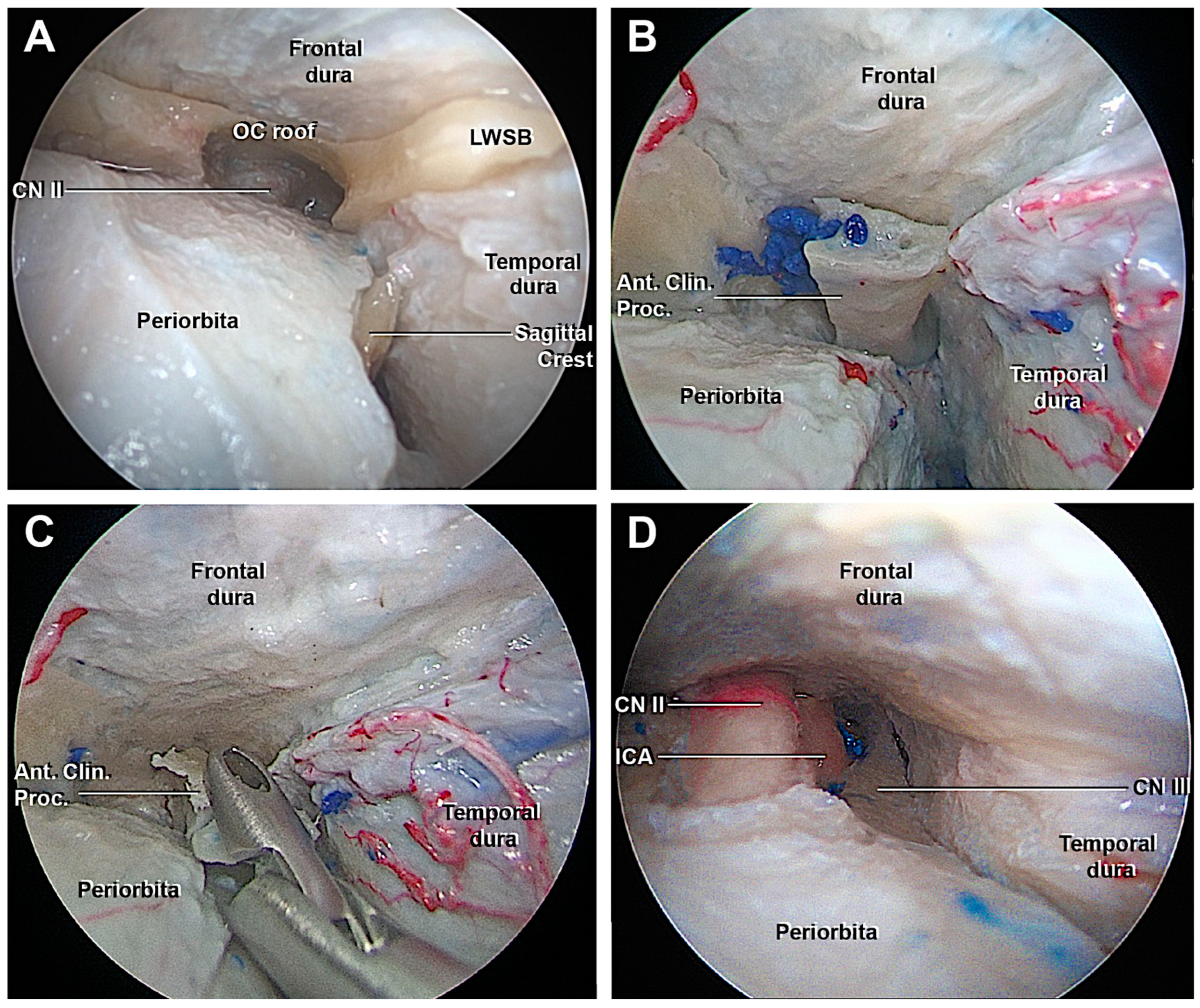
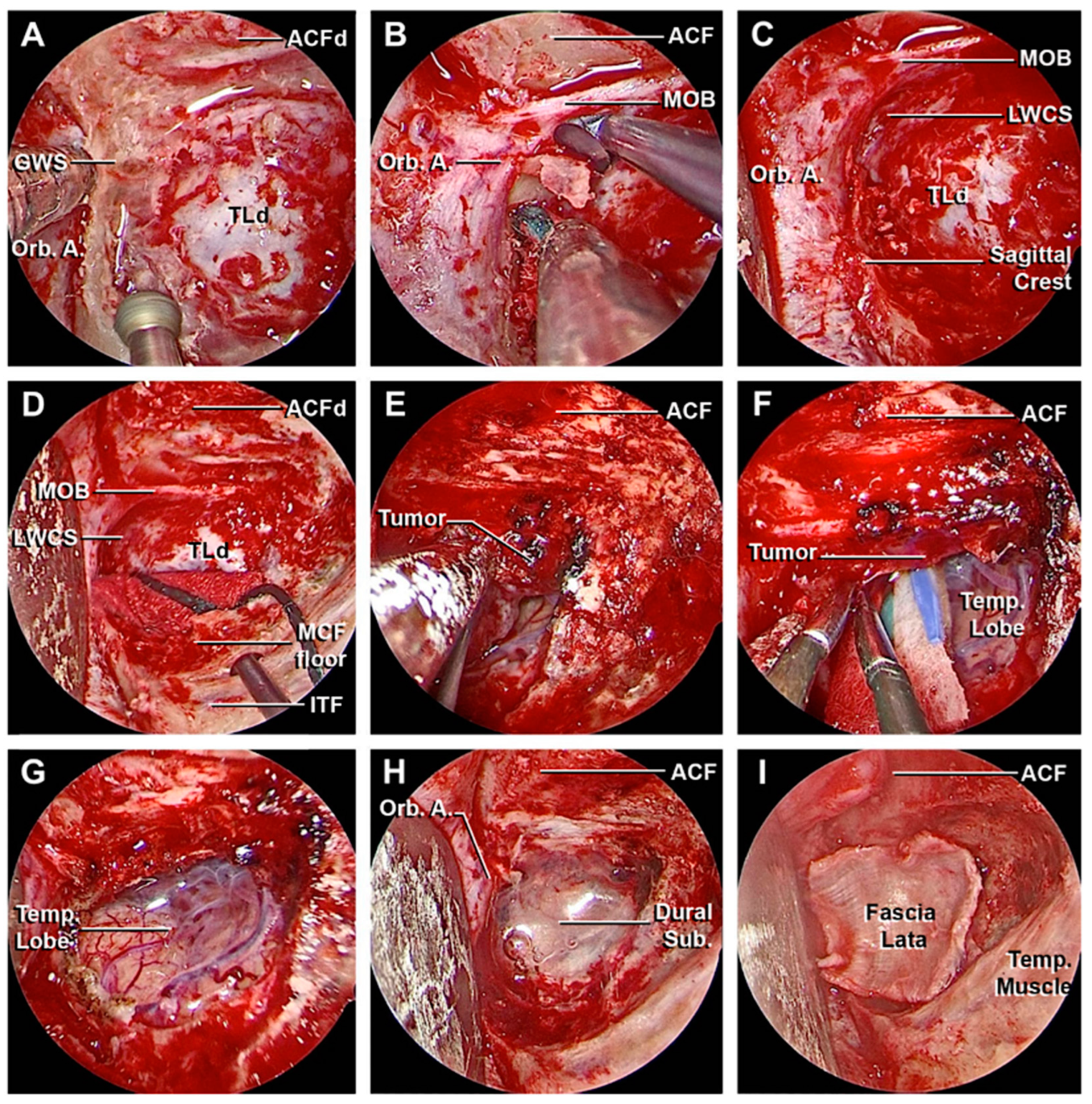
3.1. Step-by-Step Dissection
3.1.1. Craniectomy
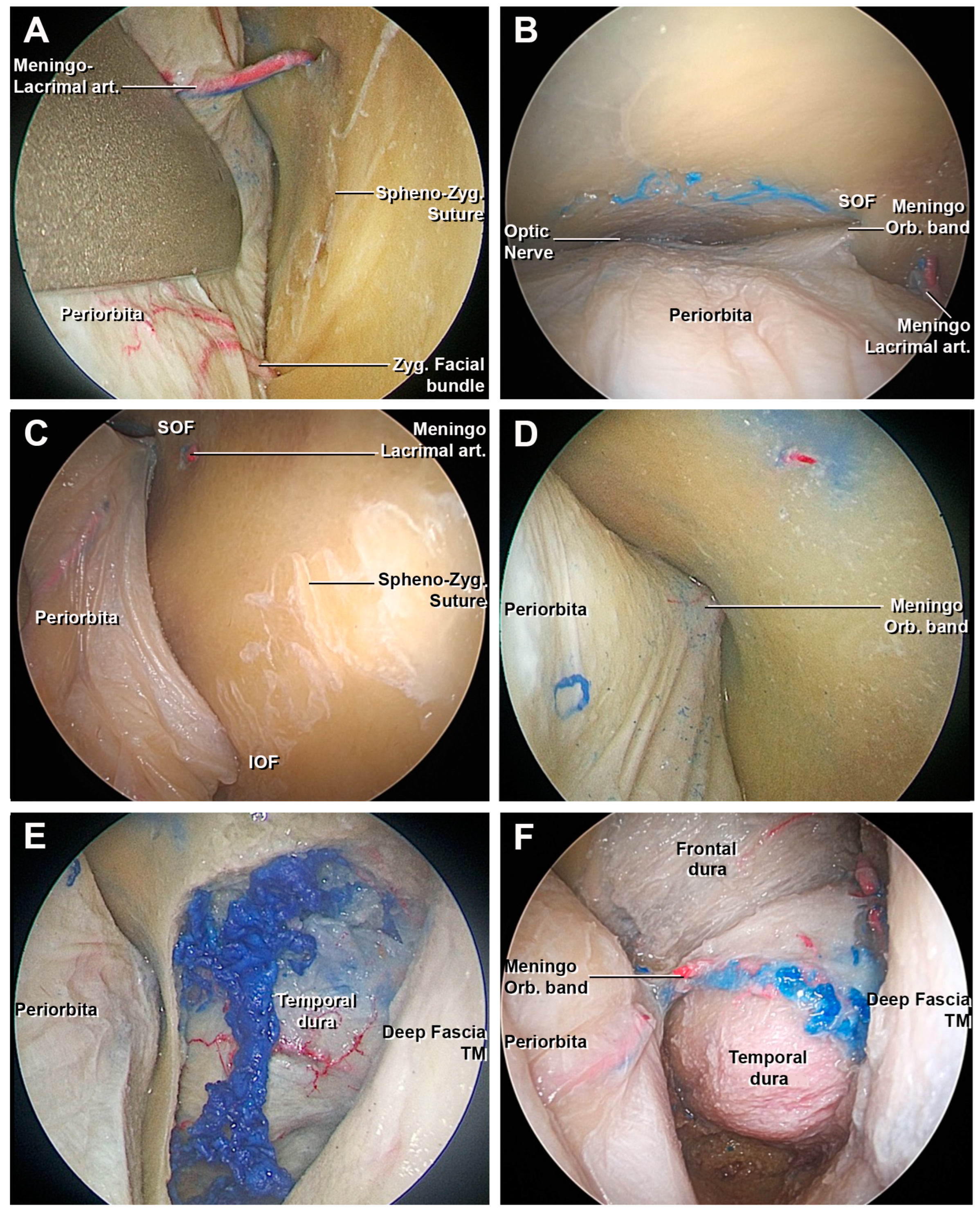
3.1.2. Access to the Middle Fossa and Petrous Apex
3.1.3. Access to the Lateral Wall of the Cavernous Sinus and Anterior Clinoidectomy
3.2. Anatomo-Radiological Measurements
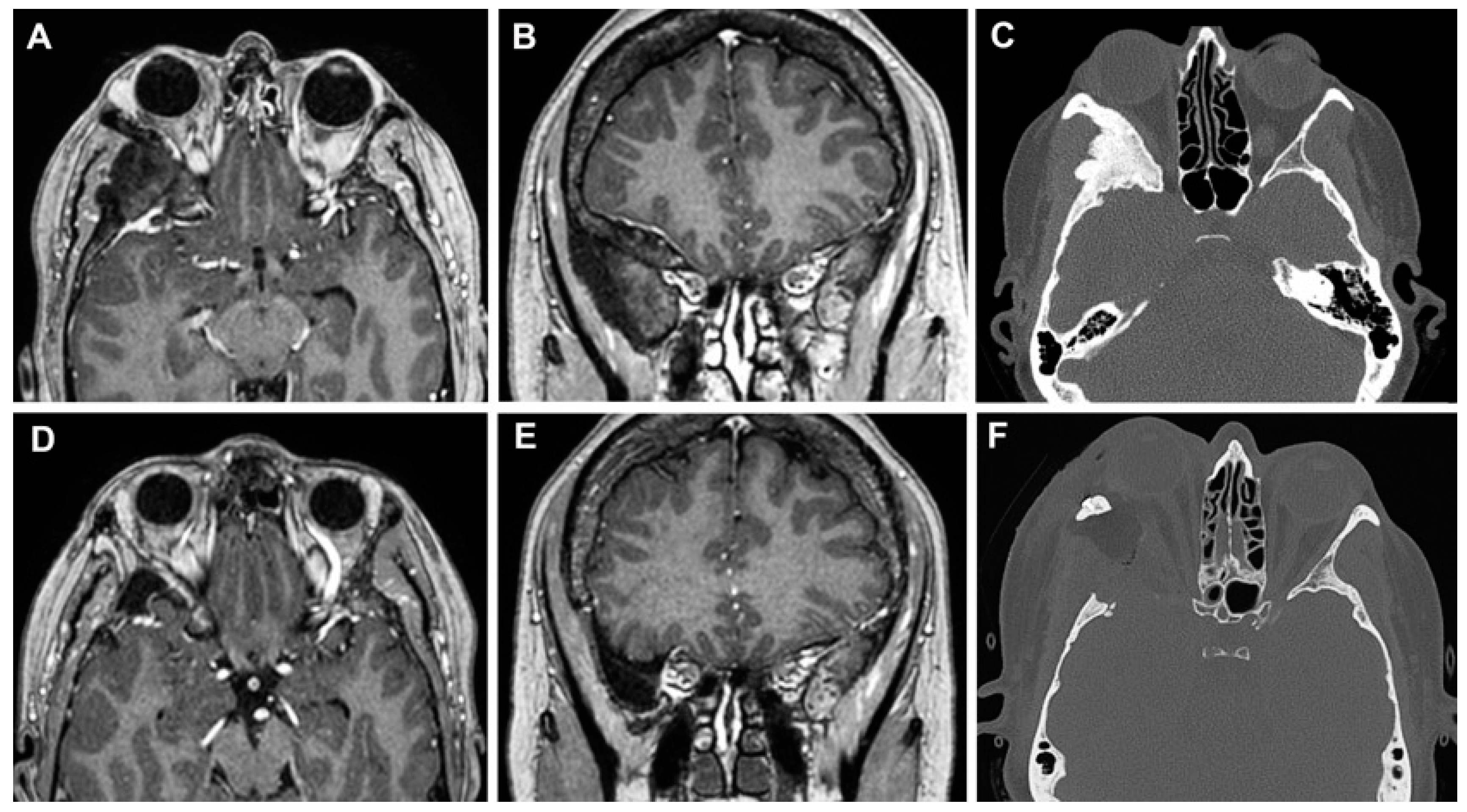
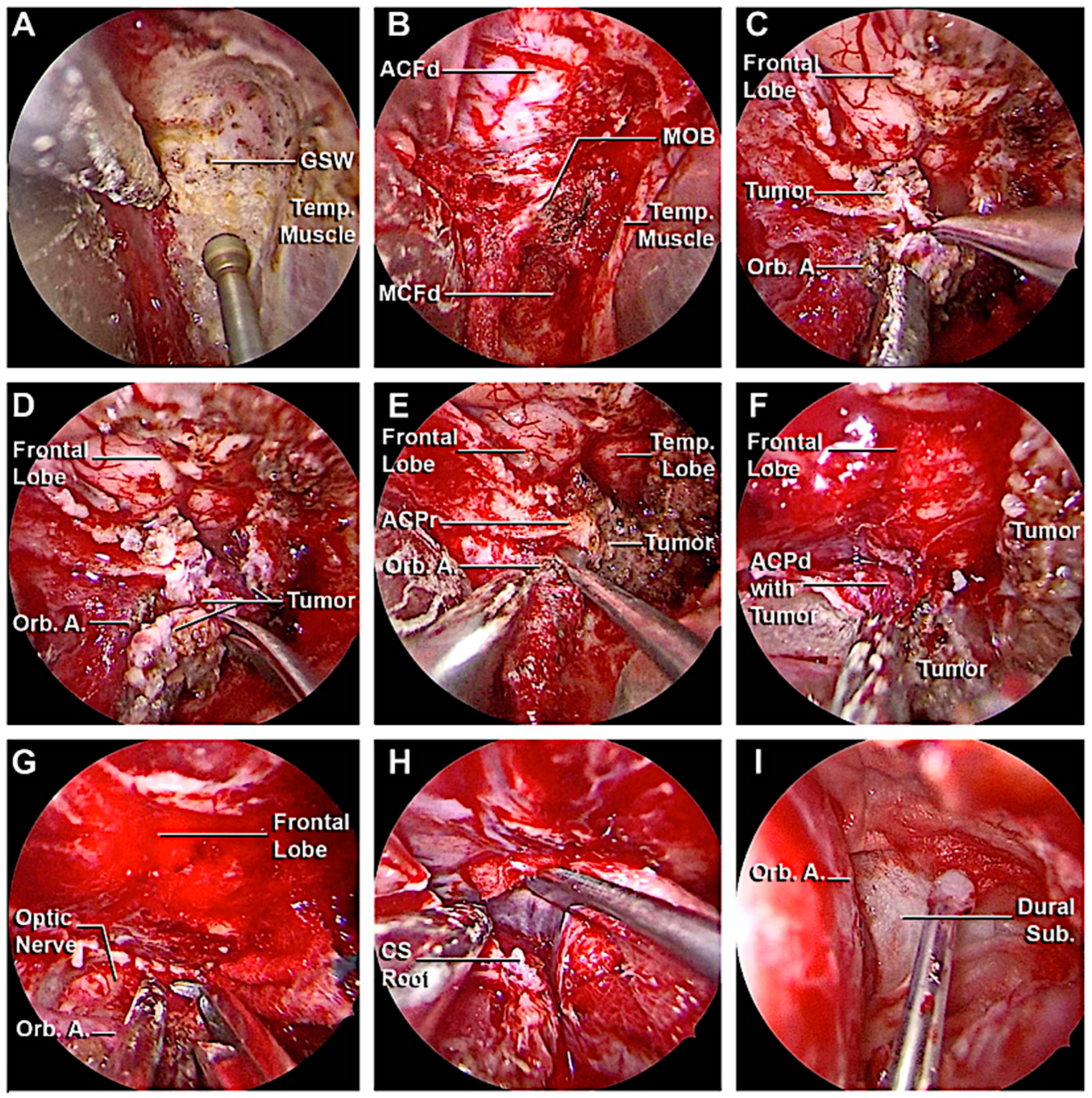
3.3. Clinical Cases
3.3.1. Case 1
3.3.2. Case 2
3.3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andaluz, N.; Romano, A.; Reddy, L.V.; Zuccarello, M. Eyelid approach to the anterior cranial base. J. Neurosurg. 2008, 109, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.M.; Boahene, K.D.; Quinones-Hinojosa, A. The transpalpebral incision: Its use in keyhole approaches to cranial base brain tumors. Expert. Rev. Neurother. 2010, 10, 1629–1632. [Google Scholar] [CrossRef][Green Version]
- Kung, D.S.; Kaban, L.B. Supratarsal fold incision for approach to the superior lateral orbit. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.A.; Soneru, C.P.; Tabaee, A.; Kacker, A.; Anand, V.K.; Schwartz, T.H. Technological and Ideological Innovations in Endoscopic Skull Base Surgery. World Neurosurg. 2019, 124, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Prevedello, D.M.; Doglietto, F.; Jane, J.A., Jr.; Jagannathan, J.; Han, J.; Laws, E.R., Jr. History of endoscopic skull base surgery: Its evolution and current reality. J. Neurosurg. 2007, 107, 206–213. [Google Scholar] [CrossRef]
- Rigante, M.; La Rocca, G.; Lauretti, L.; D’Alessandris, G.Q.; Mangiola, A.; Anile, C.; Olivi, A.; Paludetti, G. Preliminary experience with 4K ultra-high definition endoscope: Analysis of pros and cons in skull base surgery. Acta. Otorhinolaryngol. Ital. 2017, 37, 237–241. [Google Scholar] [CrossRef]
- Nicolai, P.; Battaglia, P.; Bignami, M.; Bolzoni Villaret, A.; Delù, G.; Khrais, T.; Lombardi, D.; Castelnuovo, P. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: A 10-year experience. Am. J. Rhinol. 2008, 22, 308–316. [Google Scholar] [CrossRef]
- Moe Kris, S.; Bergeron Chris, M.; Ellenbogen Richard, G. Transorbital Neuroendoscopic Surgery. Oper. Neurosurg. 2010, 67, ons16–ons28. [Google Scholar] [CrossRef]
- Alqahtani, A.; Padoan, G.; Segnini, G.; Lepera, D.; Fortunato, S.; Dallan, I.; Pistochini, A.; Abdulrahman, S.; Abbate, V.; Hirt, B.; et al. Transorbital transnasal endoscopic combined approach to the anterior and middle skull base: A laboratory investigation. Acta Otorhinolaryngol. Ital. 2015, 35, 173–179. [Google Scholar] [CrossRef]
- Ho, C.L.; Hwang, P.Y. Endoscope-assisted Transorbital Keyhole Surgical Approach to Ruptured Supratentorial Aneurysms. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2015, 76, 376–383. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Moe, K.S. Applications and outcomes of orbital and transorbital endoscopic surgery. Otolaryngol. Head. Neck. Surg. 2011, 144, 815–820. [Google Scholar] [CrossRef]
- Vural, A.; Carobbio, A.L.C.; Ferrari, M.; Rampinelli, V.; Schreiber, A.; Mattavelli, D.; Doglietto, F.; Buffoli, B.; Rodella, L.F.; Taboni, S.; et al. Transorbital endoscopic approaches to the skull base: A systematic literature review and anatomical description. Neurosurg. Rev. 2021, 44, 2857–2878. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Sollini, G.; Rustici, A.; Guaraldi, F.; Asioli, S.; Altavilla, M.V.; Orsatti, A.; Faustini-Fustini, M.; Pasquini, E.; Mazzatenta, D. Endoscopic Transorbital Approach for Spheno-Orbital Tumors: Case Series and Systematic Review of Literature. World Neurosurg. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Park, H.H.; Hong, S.D.; Kim, Y.H.; Hong, C.K.; Woo, K.I.; Yun, I.S.; Kong, D.S. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: A retrospective multicenter analysis (KOSEN-005). J. Neurosurg. 2020, 133, 467–476. [Google Scholar] [CrossRef]
- Di Somma, A.; De Rosa, A.; Ferrés, A.; Mosteiro, A.; Guizzardi, G.; Fassi, J.M.; Topczewski, T.E.; Reyes, L.; Roldán, P.; Torné, R.; et al. Endoscopic Transorbital Approach for the Management of Spheno-Orbital Meningiomas: Literature Review and Preliminary Experience. World Neurosurg. 2023, 176, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Dallan, I.; Sellari-Franceschini, S.; Turri-Zanoni, M.; de Notaris, M.; Fiacchini, G.; Fiorini, F.R.; Battaglia, P.; Locatelli, D.; Castelnuovo, P. Endoscopic Transorbital Superior Eyelid Approach for the Management of Selected Spheno-orbital Meningiomas: Preliminary Experience. Oper. Neurosurg. 2018, 14, 243–251. [Google Scholar] [CrossRef]
- Locatelli, D.; Restelli, F.; Alfiero, T.; Campione, A.; Pozzi, F.; Balbi, S.; Arosio, A.; Castelnuovo, P. The Role of the Transorbital Superior Eyelid Approach in the Management of Selected Spheno-orbital Meningiomas: In-Depth Analysis of Indications, Technique, and Outcomes from the Study of a Cohort of 35 Patients. J. Neurol. Surg. B Skull Base 2020, 83, 145–158. [Google Scholar] [CrossRef]
- Han, X.; Yang, H.; Wang, Z.; Li, L.; Li, C.; Han, S.; Wu, A. Endoscopic transorbital approach for skull base lesions: A report of 16 clinical cases. Neurosurg. Rev. 2023, 46, 74. [Google Scholar] [CrossRef]
- Jeon, C.; Hong, C.K.; Woo, K.I.; Hong, S.D.; Nam, D.H.; Lee, J.I.; Choi, J.W.; Seol, H.J.; Kong, D.S. Endoscopic transorbital surgery for Meckel’s cave and middle cranial fossa tumors: Surgical technique and early results. J. Neurosurg. 2018, 131, 1126–1135. [Google Scholar] [CrossRef]
- Kong, D.S.; Young, S.M.; Hong, C.K.; Kim, Y.D.; Hong, S.D.; Choi, J.W.; Seol, H.J.; Lee, J.I.; Shin, H.J.; Nam, D.H.; et al. Clinical and ophthalmological outcome of endoscopic transorbital surgery for cranioorbital tumors. J. Neurosurg. 2018, 131, 667–675. [Google Scholar] [CrossRef]
- Moe, K.S.; Kim, L.J.; Bergeron, C.M. Transorbital endoscopic repair of cerebrospinal fluid leaks. Laryngoscope 2011, 121, 13–30. [Google Scholar] [CrossRef]
- Lee, M.H.; Hong, S.D.; Woo, K.I.; Kim, Y.D.; Choi, J.W.; Seol, H.J.; Lee, J.I.; Shin, H.J.; Nam, D.H.; Kong, D.S. Endoscopic endonasal versus transorbital surgery for middle cranial fossa tumors: Comparison of clinical outcomes based on surgical corridors. World Neurosurg. 2019, 122, e1491–e1504. [Google Scholar] [CrossRef] [PubMed]
- Corvino, S.; Sacco, M.; Somma, T.; Berardinelli, J.; Ugga, L.; Colamaria, A.; Corrivetti, F.; Iaconetta, G.; Kong, D.S.; de Notaris, M. Functional and clinical outcomes after superior eyelid transorbital endoscopic approach for spheno-orbital meningiomas: Illustrative case and literature review. Neurosurg. Rev. 2022, 46, 17. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, R.; Kim, L.J.; Bly, R.A.; Moe, K.; Ferreira, M., Jr. Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J. Clin. Neurosci. 2016, 24, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ulutas, M.; Boyacı, S.; Akakın, A.; Kılıç, T.; Aksoy, K. Surgical anatomy of the cavernous sinus, superior orbital fissure, and orbital apex via a lateral orbitotomy approach: A cadaveric anatomical study. Acta Neurochir. 2016, 158, 2135–2148. [Google Scholar] [CrossRef]
- De Rosa, A.; Pineda, J.; Cavallo, L.M.; Di Somma, A.; Romano, A.; Topczewski, T.E.; Somma, T.; Solari, D.; Enseñat, J.; Cappabianca, P.; et al. Endoscopic endo- and extra-orbital corridors for spheno-orbital region: Anatomic study with illustrative case. Acta Neurochir. 2019, 161, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.S.; Diab, A.G.; Ngombu, S.; Prevedello, D.M.; Carrau, R.L. Endoscopic transorbital ligation of the maxillary artery through the inferior orbital fissure. Head Neck 2021, 43, 1830–1837. [Google Scholar] [CrossRef]
- Arosio, A.D.; Coden, E.; Valentini, M.; Czaczkes, C.; Battaglia, P.; Bignami, M.; Castelnuovo, P.; Karligkiotis, A. Combined Endonasal-Transorbital Approach to Manage the Far Lateral Frontal Sinus: Surgical Technique. World Neurosurg. 2021, 151, 5. [Google Scholar] [CrossRef] [PubMed]
- Caklili, M.; Emengen, A.; Cabuk, B.; Anik, I.; Ceylan, S. Endoscopic Transorbital Approach to the Cavernous Sinus Lateral Compartment (Anatomical Cadaver Study). Turk. Neurosurg. 2021, 31, 813–819. [Google Scholar] [CrossRef]
- Noiphithak, R.; Yanez-Siller, J.C.; Revuelta Barbero, J.M.; Cho, R.I.; Otto, B.A.; Carrau, R.L.; Prevedello, D.M. Comparative Analysis of the Exposure and Surgical Freedom of the Endoscopic Extended Minipterional Craniotomy and the Transorbital Endoscopic Approach to the Anterior and Middle Cranial Fossae. Oper. Neurosurg. 2019, 17, 174–181. [Google Scholar] [CrossRef]
- Dallan, I.; Castelnuovo, P.; Locatelli, D.; Turri-Zanoni, M.; AlQahtani, A.; Battaglia, P.; Hirt, B.; Sellari-Franceschini, S. Multiportal Combined Transorbital Transnasal Endoscopic Approach for the Management of Selected Skull Base Lesions: Preliminary Experience. World Neurosurg. 2015, 84, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Andaluz, N.; Cavallo, L.M.; Keller, J.T.; Solari, D.; Zimmer, L.A.; de Notaris, M.; Zuccarello, M.; Cappabianca, P. Supraorbital vs Endo-Orbital Routes to the Lateral Skull Base: A Quantitative and Qualitative Anatomic Study. Oper. Neurosurg. 2018, 15, 567–576. [Google Scholar] [CrossRef]
- Guizzardi, G.; Mosteiro, A.; Hoyos, J.; Ferres, A.; Topczewski, T.; Reyes, L.; Alobid, I.; Matas, J.; Cavallo, L.M.; Cappabianca, P.; et al. Endoscopic Transorbital Approach to the Middle Fossa: Qualitative and Quantitative Anatomic Study. Oper. Neurosurg. 2022, 23, e267–e275. [Google Scholar] [CrossRef]
- Di Somma, A.; Andaluz, N.; Cavallo, L.M.; de Notaris, M.; Dallan, I.; Solari, D.; Zimmer, L.A.; Keller, J.T.; Zuccarello, M.; Prats-Galino, A.; et al. Endoscopic transorbital superior eyelid approach: Anatomical study from a neurosurgical perspective. J. Neurosurg. 2018, 129, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- López, C.B.; Di Somma, A.; Cepeda, S.; Arrese, I.; Sarabia, R.; Agustín, J.H.; Topczewski, T.E.; Enseñat, J.; Prats-Galino, A. Extradural anterior clinoidectomy through endoscopic transorbital approach: Laboratory investigation for surgical perspective. Acta Neurochir. 2021, 163, 2177–2188. [Google Scholar] [CrossRef]
- Lim, J.; Sung, K.S.; Yoo, J.; Oh, J.; Moon, J.H. Endoscopic transorbital extradural anterior clinoidectomy: A stepwise surgical technique and case series study [SevEN-013]. Front. Oncol. 2022, 12, 991065. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, C.K.; Shin, H.J.; Kong, D.S. Feasibility and efficacy of endoscopic transorbital optic canal decompression for meningiomas causing compressive optic neuropathy. J. Neurosurg. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Priddy, B.H.; Nunes, C.F.; Beer-Furlan, A.; Carrau, R.; Dallan, I.; Prevedello, D.M. A Side Door to Meckel’s Cave: Anatomic Feasibility Study for the Lateral Transorbital Approach. Oper. Neurosurg. 2017, 13, 614–621, Erratum in Oper. Neurosurg. 2017, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Dallan, I.; Cristofani-Mencacci, L.; Fiacchini, G.; Caniglia, M.; Sellari-Franceschini, S.; Berrettini, S. When multidisciplinary surgical trans-orbital approaches should be considered to reach the skull base. Acta Otorhinolaryngol. Ital. 2021, 41 (Suppl. S1), S59–S66. [Google Scholar] [CrossRef]
- Dallan, I.; Di Somma, A.; Prats-Galino, A.; Solari, D.; Alobid, I.; Turri-Zanoni, M.; Fiacchini, G.; Castelnuovo, P.; Catapano, G.; de Notaris, M. Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: A descriptive anatomical study. J. Neurosurg. 2017, 127, 622–629. [Google Scholar] [CrossRef]
- De Rosa, A.; Di Somma, A.; Mosteiro, A.; Ferrés, A.; Reyes, L.A.; Roldan, P.; Torné, R.; Torales, J.; Solari, D.; Cavallo, L.M.; et al. Superior eyelid endoscopic transorbital approach to the tentorial area: A qualitative and quantitative anatomic study. Front. Surg. 2022, 9, 1007447. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.J.; Hong, K.T.; Chung, T.T.; Liu, W.H.; Hueng, D.Y.; Chen, Y.H.; Ju, D.T.; Ma, H.I.; Liu, M.Y.; Hung, H.C.; et al. Endoscopic transorbital transtentorial approach to middle incisural space: Preclinical cadaveric study. Acta Neurochir. 2019, 161, 831–839. [Google Scholar] [CrossRef]
- Di Somma, A.; Andaluz, N.; Cavallo, L.M.; Topczewski, T.E.; Frio, F.; Gerardi, R.M.; Pineda, J.; Solari, D.; Enseñat, J.; Prats-Galino, A.; et al. Endoscopic transorbital route to the petrous apex: A feasibility anatomic study. Acta Neurochir. 2018, 160, 707–720. [Google Scholar] [CrossRef]
- Noiphithak, R.; Yanez-Siller, J.C.; Revuelta Barbero, J.M.; Otto, B.A.; Carrau, R.L.; Prevedello, D.M. Quantitative analysis of the surgical exposure and surgical freedom between transcranial and transorbital endoscopic anterior petrosectomies to the posterior fossa. J. Neurosurg. 2018, 131, 569–577, Erratum in J. Neurosurg. 2018, 131, 635–636. [Google Scholar] [CrossRef]
- Lee, W.J.; Hong, S.D.; Woo, K.I.; Seol, H.J.; Choi, J.W.; Lee, J.I.; Nam, D.H.; Kong, D.S. Endoscopic endonasal and transorbital approaches to petrous apex lesions. J. Neurosurg. 2021, 136, 431–440. [Google Scholar] [CrossRef]
- Park, H.H.; Roh, T.H.; Choi, S.; Yoo, J.; Kim, W.H.; Jung, I.H.; Yun, I.S.; Hong, C.K. Endoscopic Transorbital Approach to Mesial Temporal Lobe for Intra-Axial Lesions: Cadaveric Study and Case Series (SevEN-008). Oper. Neurosurg. 2021, 21, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.W.; Miller, T.A.; Liu, H.Y.; Abel, T.J.; McDowell, M.M. Utility of minimally invasive endoscopic skull base approaches for the treatment of drug-resistant mesial temporal lobe epilepsy: A review of current techniques and trends. J. Neurosurg. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- DeLano, M.C.; Fun, F.Y.; Zinreich, S.J. Relationship of the optic nerve to the posterior paranasal sinuses: A CT anatomic study. Am. J. Neuroradiol. 1996, 17, 669. [Google Scholar] [CrossRef]
- Chibbaro, S.; Ganau, M.; Scibilia, A.; Todeschi, J.; Zaed, I.; Bozzi, M.T.; Ollivier, I.; Cebula, H.; Santin, M.D.N.; Djennaoui, I.; et al. Endoscopic Transorbital Approaches to Anterior and Middle Cranial Fossa: Exploring the Potentialities of a Modified Lateral Retrocanthal Approach. World Neurosurg. 2021, 150, e74–e80. [Google Scholar] [CrossRef]
- Corrivetti, F.; de Notaris, M.; Di Somma, A.; Dallan, I.; Enseñat, J.; Topczewski, T.; Solari, D.; Cavallo, L.M.; Cappabianca, P.; Prats-Galino, A. “Sagittal Crest”: Definition, Stepwise Dissection, and Clinical Implications from a Transorbital Perspective. Oper. Neurosurg. 2022, 22, e206–e212. [Google Scholar] [CrossRef]
- Bly, R.A.; Ramakrishna, R.; Ferreira, M.; Moe, K.S. Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. J. Neurol. Surg. Part B Skull Base 2014, 75, 011–017. [Google Scholar]
- Bander, E.D.; Carnevale, J.A.; Tosi, U.; Godfrey, K.J.; Schwartz, T.H. Lateral Transorbital Endoscope-Assisted Approach to the Cavernous Sinus. Oper. Neurosurg. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- In Woo, K.; Kong, D.S.; Park, J.W.; Kim, M.; Kim, Y.D. Orbital decompressive effect of endoscopic transorbital surgery for sphenoorbital meningioma. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 259, 1015–1024. [Google Scholar] [CrossRef]
- Park, H.H.; Yoo, J.; Yun, I.S.; Hong, C.K. Comparative Analysis of Endoscopic Transorbital Approach and Extended Mini-Pterional Approach for Sphenoid Wing Meningiomas with Osseous Involvement: Preliminary Surgical Results. World Neurosurg. 2020, 139, e1–e12. [Google Scholar] [CrossRef]
- Di Somma, A.; Sanchez España, J.C.; Alobid, I.; Enseñat, J. Endoscopic superior eyelid transorbital approach: How I do it. Acta Neurochir. 2022, 164, 1953–1959. [Google Scholar] [CrossRef]
- Carnevale, J.A.; Pandey, A.; Ramirez-Loera, C.; Goldberg, J.L.; Bander, E.D.; Henderson, F.; Niogi, S.N.; Tabaee, A.; Kacker, A.; Anand, V.K.; et al. Endonasal, supraorbital, and transorbital approaches: Minimal access endoscope-assisted surgical approaches for meningiomas in the anterior and middle cranial fossae. J. Neurosurg. 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Di Somma, A.; Guizzardi, G.; Sanchez España, J.C.; Matas Fassi, J.; Topczewski, T.E.; Ferres, A.; Mosteiro, A.; Reyes, L.; Tercero, J.; Lopez, M.; et al. Complications of the Superior Eyelid Endoscopic Transorbital Approach to the Skull Base: Preliminary Experience with Specific Focus on Orbital Outcome. J. Neuroophthalmol. 2023. [Google Scholar] [CrossRef]
- Gonçalves Pacheco Junior, M.; de Melo Junior, J.O.; André Acioly, M.; Mansilla Cabrera Rodrigues, R.; Lima Pessôa, B.; Fernandes, R.A.; Landeiro, J.A. Tailored Anterior Clinoidectomy: Beyond the Intradural and Extradural Concepts. Cureus 2021, 13, e14874. [Google Scholar] [CrossRef]
- Gallardo, F.C.; Bustamante, J.L.; Martin, C.; Targa Garcia, A.A.; Feldman, S.E.; Pastor, F.; Orellana, M.C.; Rubino, P.A.; Quilis Quesada, V. Intra- and extradural anterior clinoidectomy: Anatomy review and surgical technique step by step. Surg. Radiol. Anat. 2021, 43, 1291–1303. [Google Scholar] [CrossRef]
- Kong, D.S.; Kim, Y.H.; Hong, C.K. Optimal indications and limitations of endoscopic transorbital superior eyelid surgery for spheno-orbital meningiomas. J. Neurosurg. 2020, 134, 1472–1479. [Google Scholar] [CrossRef]
- Di Somma, A.; Andaluz, N.; Gogela, S.L.; Cavallo, L.M.; Keller, J.T.; Prats-Galino, A.; Cappabianca, P. Surgical Freedom Evaluation During Optic Nerve Decompression: Laboratory Investigation. World Neurosurg. 2017, 101, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Erdogmus, S.; Govsa, F. Importance of the anatomic features of the lacrimal artery for orbital approaches. J. Craniofac. Surg. 2005, 16, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, S.; Bojanowski, M.; Robert, T. Embryology and anatomical variations of the ophthalmic artery. Neuroradiology 2020, 62, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Kim, Y.H.; Lee, W.J.; Kim, Y.H.; Hong, C.K. Indications and outcomes of endoscopic transorbital surgery for trigeminal schwannoma based on tumor classification: A multicenter study with 50 cases. J. Neurosurg. 2022. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hong, S.D.; Woo, K.I.; Seol, H.J.; Choi, J.W.; Lee, J.I.; Nam, D.H.; Kong, D.S. Combined endoscopic endonasal and transorbital multiportal approach for complex skull base lesions involving multiple compartments. Acta Neurochir. 2022, 164, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
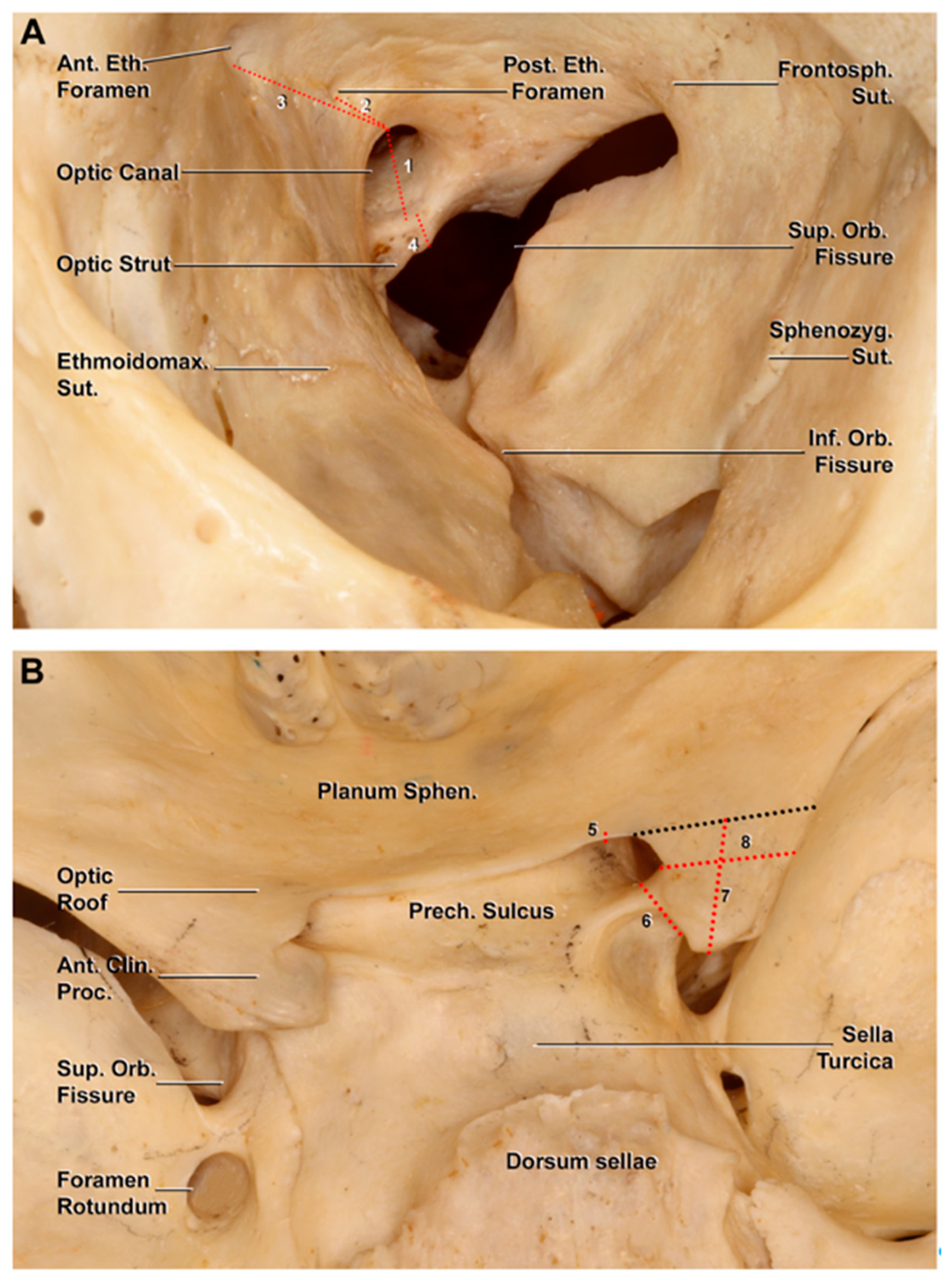


| Measurements on Dry Skulls | |
|---|---|
| Endocranial Surface | |
| Thickness of Optic Strut | 3.6 ± 0.9 mm (range 2–6 mm) |
| Thickness of Optic Canal Roof | 1.8 ± 0.6 mm (range 0.9–3.2 mm) |
| CC length of ACP | 10.2 ± 3.6 mm (range 5.6–17.4 mm) |
| Transverse Diameter of ACP | 5.5 ± 2.9 mm (range 2.8–8.5 mm) |
| Carotid-Clinoid Foramen | 5 sides |
| Orbital Region | |
| Thickness Optic Strut | 3.1 ± 1.2 mm (range 1–5 mm) |
| Transverse Diameter of OC | 7 ± 0.9 mm (range 5–9 mm) |
| Distance between the OC and AEC | 18.2 ± 3.6 mm (range 8–26 mm) |
| Distance between the OC and PEC | 6.6 ± 2.5 mm (range 3–16 mm) |
| Radiological Measurements | |
|---|---|
| N° of Patients | 200 (105 female, 95 male) |
| Patient Age Distribution | 68 years (range 21–94 ys) |
| CC Length of ACP | 11.7 ± 2.4 mm (range 6.4–18.5 mm) |
| Width of ACP | 6.4 ± 1.4 mm (range 2.6–9.5 mm) |
| Optic Strut | 2.5 ± 0.79 mm (range 0.4–5 mm) |
| Optic Canal Roof | 1.4 ± 0.5 mm (range 0.5–3.5 mm) |
| Pneumatization of ACP | 7.5% (15 patients) |
| DeLano’s classification | |
| Type 1 | 74.5% (149 patients) |
| Type 2 | 17% (34 patients) |
| Type 3 | 7% (14 patients) |
| Type 4 | 1.5% (3 patients) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serioli, S.; Nizzola, M.; Plou, P.; De Bonis, A.; Meyer, J.; Leonel, L.C.P.C.; Tooley, A.A.; Wagner, L.H.; Bradley, E.A.; Van Gompel, J.J.; et al. Surgical Anatomy of the Microscopic and Endoscopic Transorbital Approach to the Middle Fossa and Cavernous Sinus: Anatomo-Radiological Study with Clinical Applications. Cancers 2023, 15, 4435. https://doi.org/10.3390/cancers15184435
Serioli S, Nizzola M, Plou P, De Bonis A, Meyer J, Leonel LCPC, Tooley AA, Wagner LH, Bradley EA, Van Gompel JJ, et al. Surgical Anatomy of the Microscopic and Endoscopic Transorbital Approach to the Middle Fossa and Cavernous Sinus: Anatomo-Radiological Study with Clinical Applications. Cancers. 2023; 15(18):4435. https://doi.org/10.3390/cancers15184435
Chicago/Turabian StyleSerioli, Simona, Mariagrazia Nizzola, Pedro Plou, Alessandro De Bonis, Jenna Meyer, Luciano C. P. C. Leonel, Andrea A. Tooley, Lilly H. Wagner, Elizabeth A. Bradley, Jamie J. Van Gompel, and et al. 2023. "Surgical Anatomy of the Microscopic and Endoscopic Transorbital Approach to the Middle Fossa and Cavernous Sinus: Anatomo-Radiological Study with Clinical Applications" Cancers 15, no. 18: 4435. https://doi.org/10.3390/cancers15184435
APA StyleSerioli, S., Nizzola, M., Plou, P., De Bonis, A., Meyer, J., Leonel, L. C. P. C., Tooley, A. A., Wagner, L. H., Bradley, E. A., Van Gompel, J. J., Benini, M. E., Dallan, I., & Peris-Celda, M. (2023). Surgical Anatomy of the Microscopic and Endoscopic Transorbital Approach to the Middle Fossa and Cavernous Sinus: Anatomo-Radiological Study with Clinical Applications. Cancers, 15(18), 4435. https://doi.org/10.3390/cancers15184435






