Treatment of Clival Chordomas: A 20-Year Experience and Systematic Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
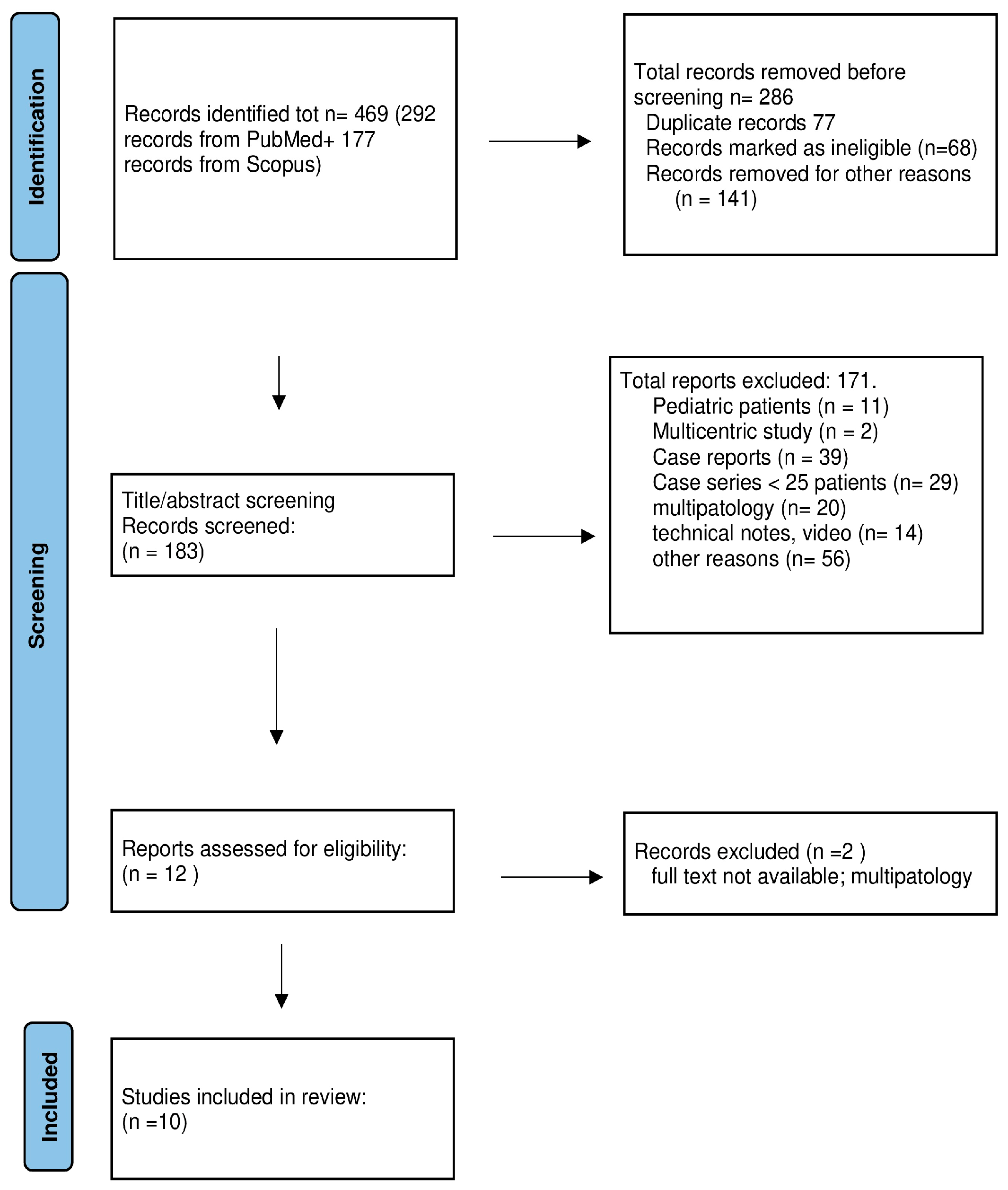
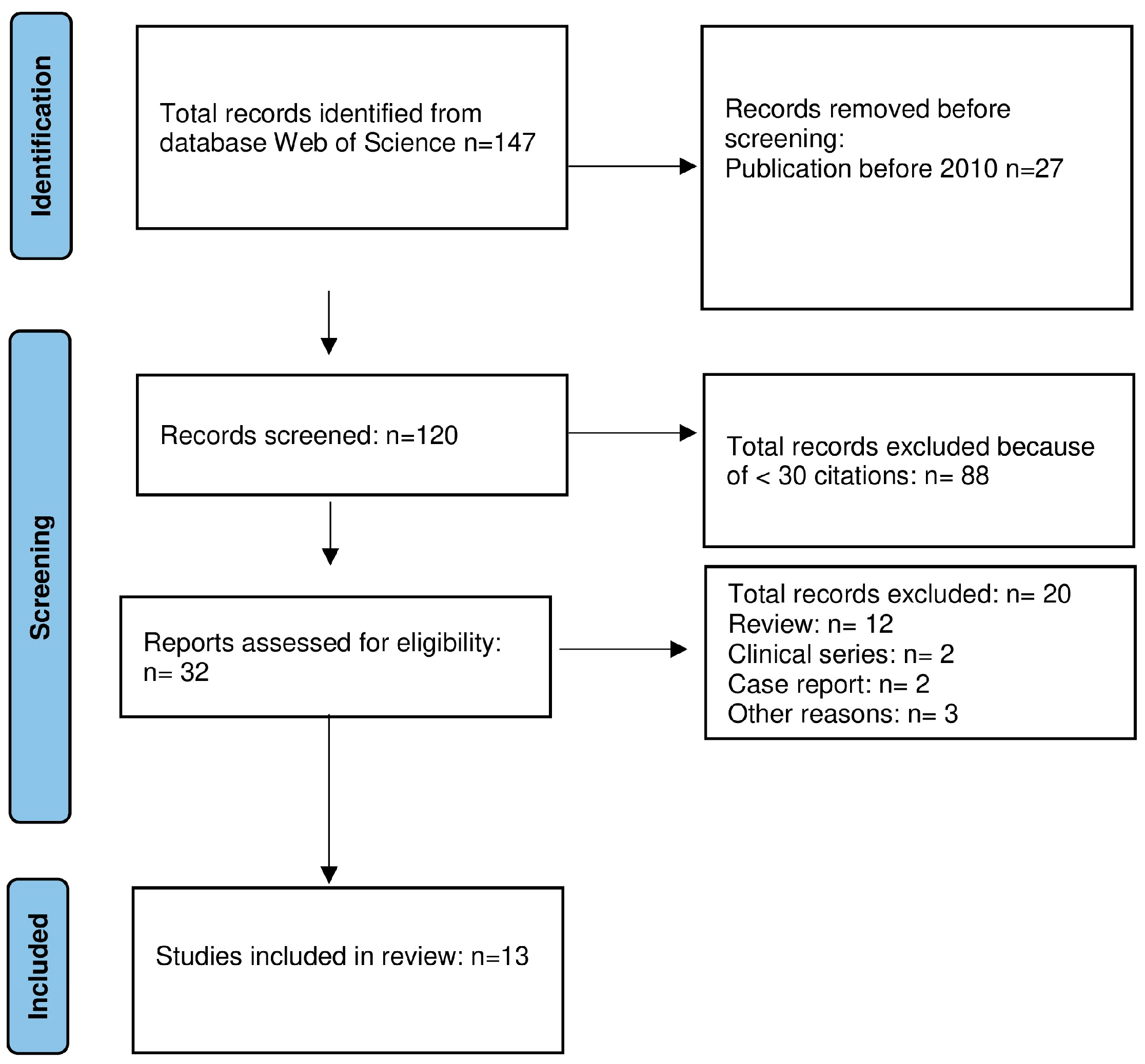
2. Materials and Methods
2.1. Retrospective Case Series
2.2. Literature Review
2.2.1. Surgical Series
2.2.2. Molecular Studies
3. Results
3.1. Review of the Literature
3.1.1. Surgical Series
3.1.2. Molecular Studies
3.2. Institutional Series
4. Discussion
4.1. Not Controversial Issues
4.1.1. Age
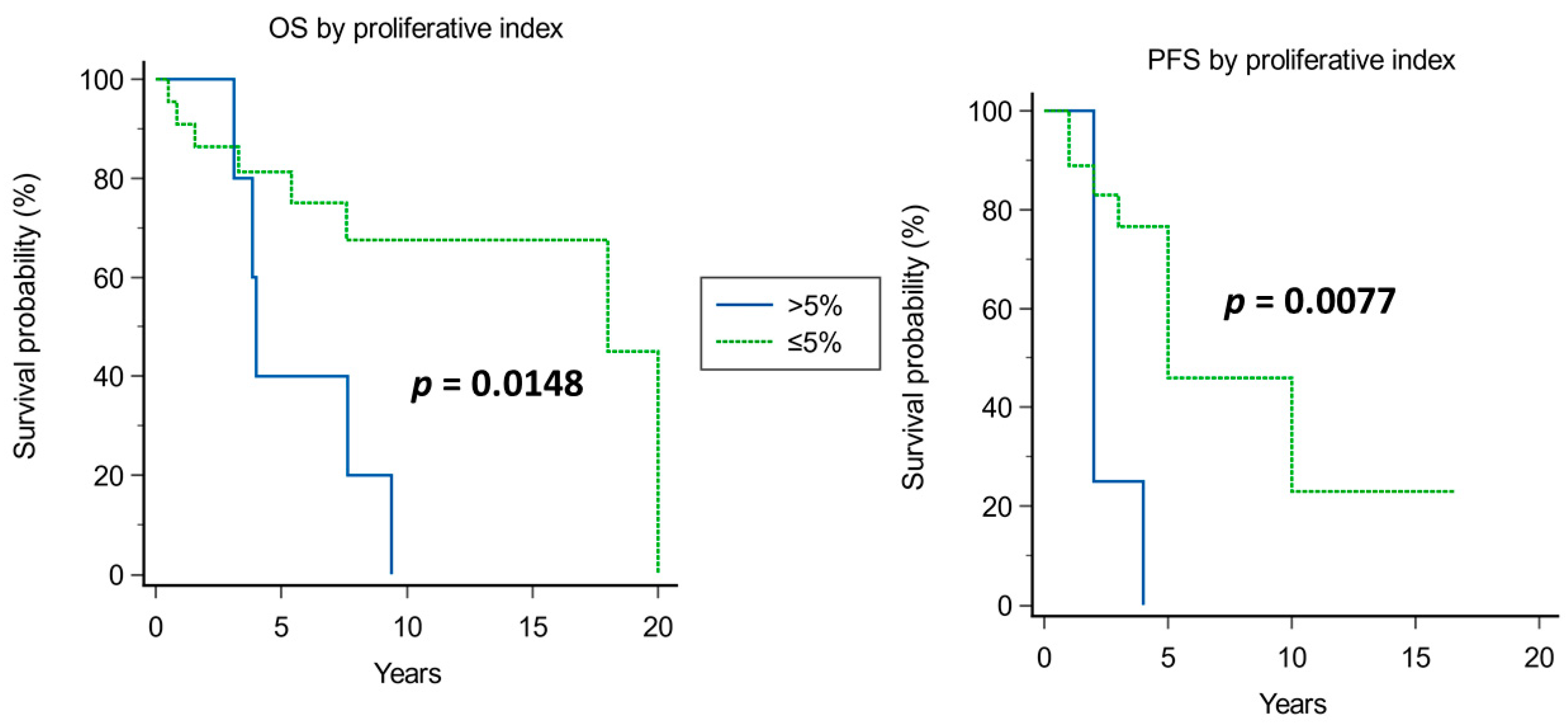
4.1.2. Pathology (Proliferative Index)
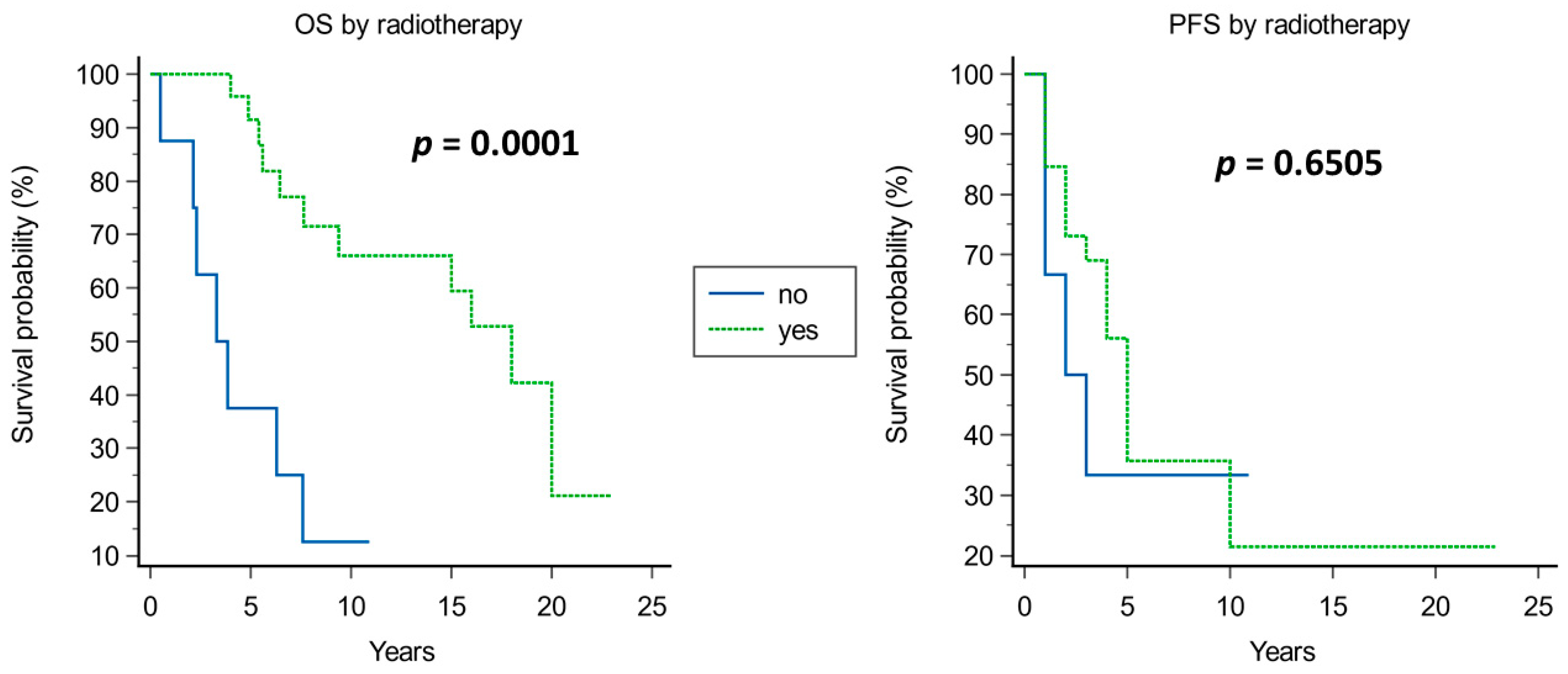
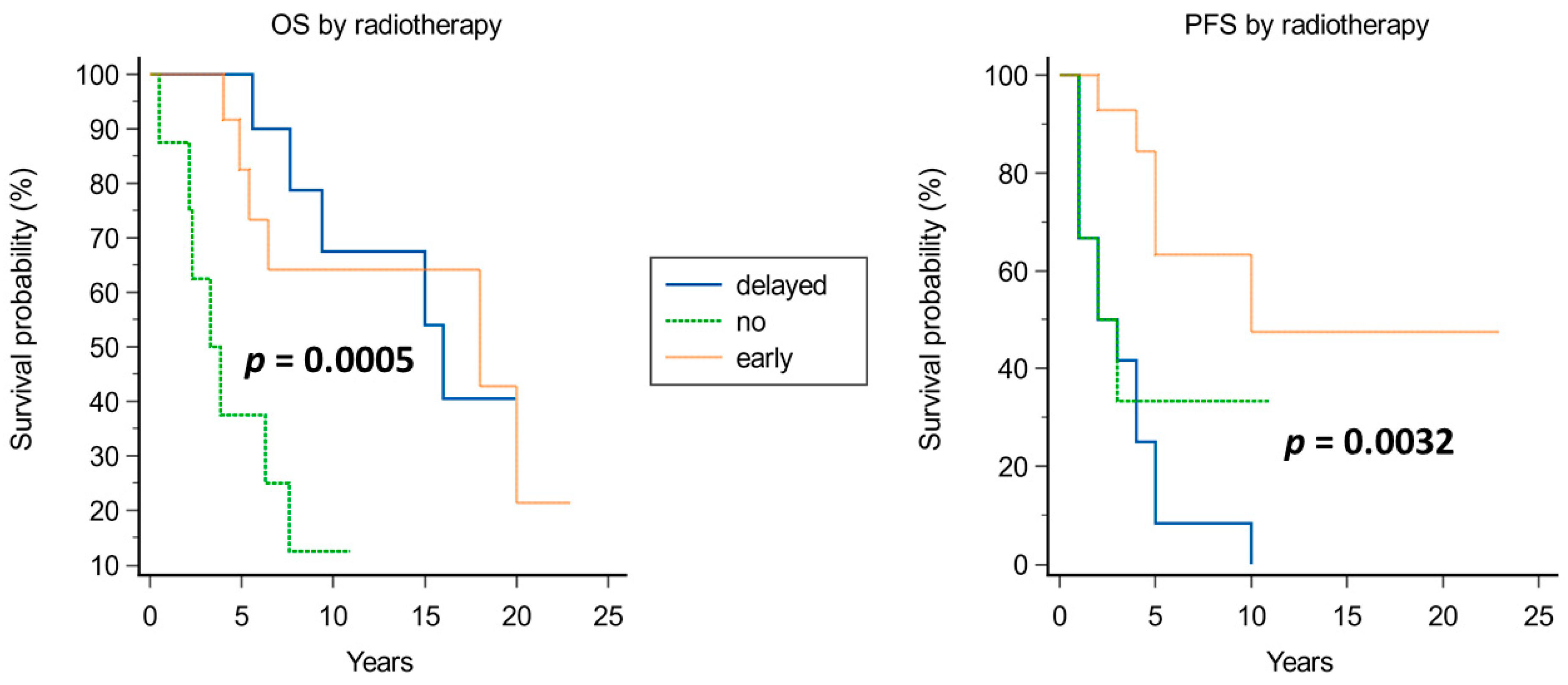
4.1.3. Radiotherapy
4.2. Controversial Issues
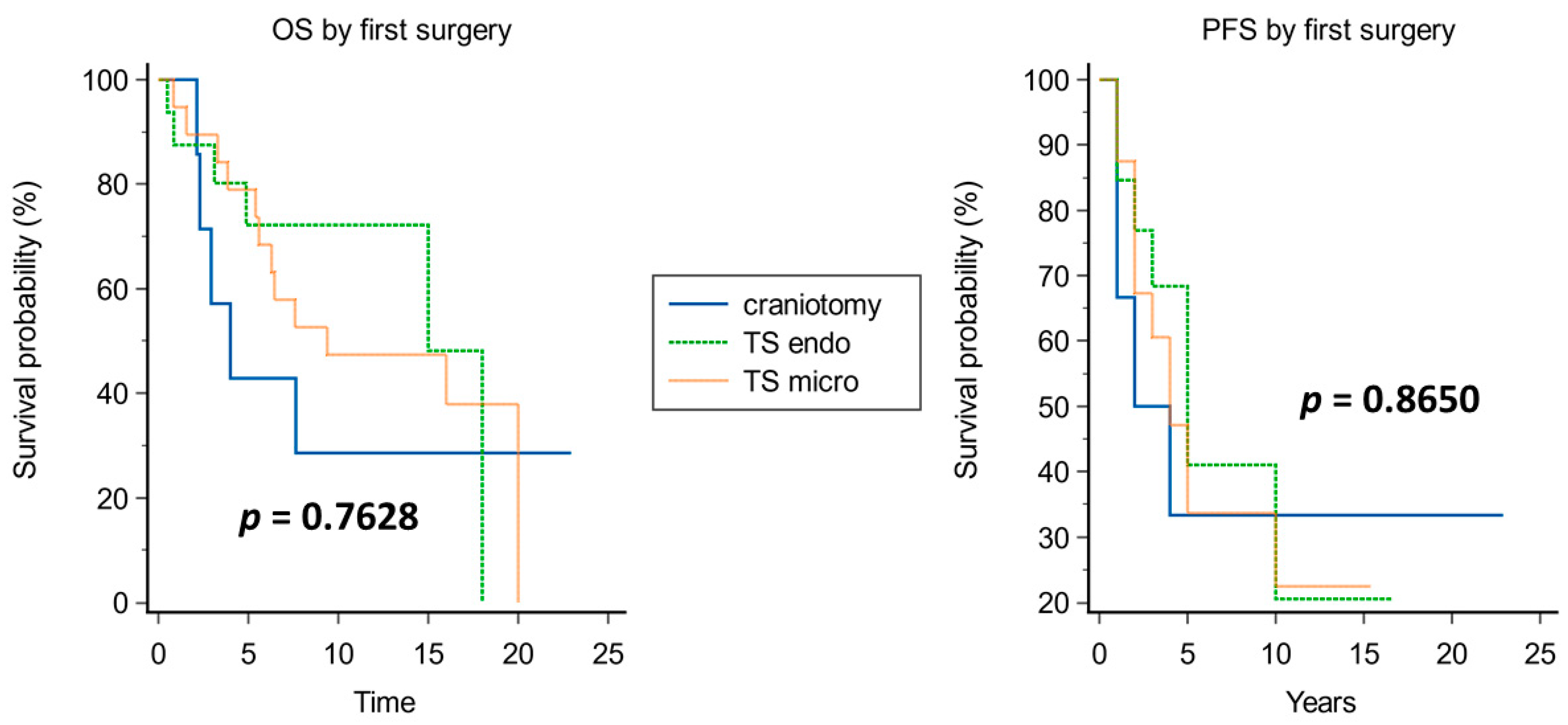
4.2.1. Surgery
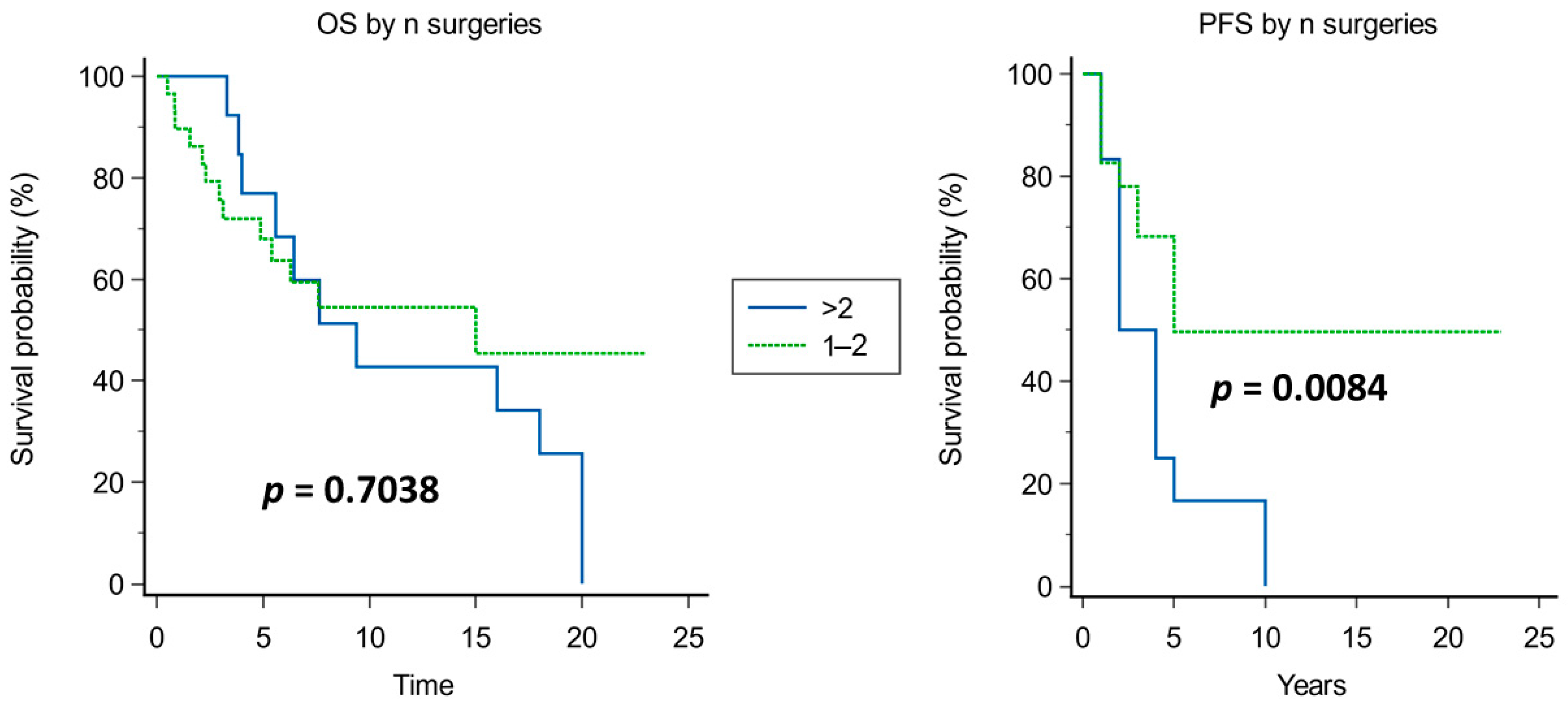
4.2.2. Recurrences and Definition of Cure
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, ISBN 978-92-832-4508-7. [Google Scholar]
- Wang, K.; Xie, S.-N.; Wang, L.; Du, J.; Ma, J.-P.; Huo, X.-L.; Tian, K.-B.; Zhang, L.-W.; Zhang, J.-T.; Wu, Z. Natural Growth Dynamics of Untreated Skull Base Chordomas In Vivo. World Neurosurg. 2020, 136, e310–e321. [Google Scholar] [CrossRef]
- Aydemir, E.; Bayrak, O.F.; Sahin, F.; Atalay, B.; Kose, G.T.; Ozen, M.; Sevli, S.; Dalan, A.B.; Yalvac, M.E.; Dogruluk, T.; et al. Characterization of Cancer Stem-like Cells in Chordoma. J. Neurosurg. 2012, 116, 810–820. [Google Scholar] [CrossRef]
- Owen, J.H.; Komarck, C.M.; Wang, A.C.; Abuzeid, W.M.; Keep, R.F.; McKean, E.L.; Sullivan, S.; Fan, X.; Prince, M.E.P. UM-Chor1: Establishment and Characterization of the First Validated Clival Chordoma Cell Line. J. Neurosurg. 2018, 128, 701–709. [Google Scholar] [CrossRef]
- Das, P.; Soni, P.; Jones, J.; Habboub, G.; Barnholtz-Sloan, J.S.; Recinos, P.F.; Kshettry, V.R. Descriptive Epidemiology of Chordomas in the United States. J. Neurooncol. 2020, 148, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Gronchi, A.; Fossati, P.; Akiyama, T.; Alapetite, C.; Baumann, M.; Blay, J.-Y.; Bolle, S.; Boriani, S.; Bruzzi, P. Best Practices for the Management of Local-Regional Recurrent Chordoma: A Position Paper by the Chordoma Global Consensus Group. Ann. Oncol. 2017, 28, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Chordoma Global Consensus Group. Building a Global Consensus Approach to Chordoma: A Position Paper from the Medical and Patient Community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, M.; Shi, J.; Jing, L.; Zhai, Y.; Zhang, S.; Wang, J.; Zhao, P.; Li, C.; Gui, S.; et al. Mid-Term Follow-Up Surgical Results in 284 Cases of Clival Chordomas: The Risk Factors for Outcome and Tumor Recurrence. Neurosurg. Rev. 2022, 45, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Alvarez-Breckenridge, C.; Akdemir, K.; Conley, A.P.; Bishop, A.J.; Wang, W.-L.; Lazar, A.J.; Rhines, L.D.; De Monte, F.; Raza, S.M. Prognostic Molecular Biomarkers in Chordomas: A Systematic Review and Identification of Clinically Usable Biomarker Panels. Front. Oncol. 2022, 12, 997506. [Google Scholar] [CrossRef]
- DeMaria, P.J.; Bilusic, M.; Park, D.M.; Heery, C.R.; Donahue, R.N.; Madan, R.A.; Bagheri, M.H.; Strauss, J.; Shen, V.; Marté, J.L.; et al. Randomized, Double-Blind, Placebo-Controlled Phase II Study of Yeast-Brachyury Vaccine (GI-6301) in Combination with Standard-of-Care Radiotherapy in Locally Advanced, Unresectable Chordoma. Oncologist 2021, 26, e847–e858. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, R.; Yin, H.; Song, D.; Meng, T. Research Hotspots and Trends of Chordoma: A Bibliometric Analysis. Front. Oncol. 2022, 12, 946597. [Google Scholar] [CrossRef]
- Bai, J.; Shi, J.; Li, C.; Wang, S.; Zhang, T.; Hua, X.; Zhu, B.; Koka, H.; Wu, H.-H.; Song, L.; et al. Whole Genome Sequencing of Skull-Base Chordoma Reveals Genomic Alterations Associated with Recurrence and Chordoma-Specific Survival. Nat. Commun. 2021, 12, 757. [Google Scholar] [CrossRef]
- Maira, G.; Pallini, R.; Anile, C.; Fernandez, E.; Salvinelli, F.; La Rocca, L.M.; Rossi, G.F. Surgical Treatment of Clival Chordomas: The Transsphenoidal Approach Revisited. J. Neurosurg. 1996, 85, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Runci, D.; D’Alessandris, Q.G.; Cenci, T.; Martini, M.; Bianchi, F.; Maira, G.; Stancato, L.; De Maria, R.; Larocca, L.M.; et al. Chemotherapy of Skull Base Chordoma Tailored on Responsiveness of Patient-Derived Tumor Cells to Rapamycin. Neoplasia 2013, 15, 773–782. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Della Pepa, G.M.; Offi, M.; Pallini, R.; Olivi, A. Shifting from Targeted Therapies to Personalised Treatment in Chordoma. Lancet Oncol. 2020, 21, e547. [Google Scholar] [CrossRef]
- Samii, A.; Gerganov, V.M.; Herold, C.; Hayashi, N.; Naka, T.; Mirzayan, M.J.; Ostertag, H.; Samii, M. Chordomas of the Skull Base: Surgical Management and Outcome. J. Neurosurg. 2007, 107, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Koutourousiou, M.; Gardner, P.A.; Tormenti, M.J.; Henry, S.L.; Stefko, S.T.; Kassam, A.B.; Fernandez-Miranda, J.C.; Snyderman, C.H. Endoscopic Endonasal Approach for Resection of Cranial Base Chordomas: Outcomes and Learning Curve. Neurosurgery 2012, 71, 614–624; discussion 624–625. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Zhang, N.; Zhang, Y.; Jiao, J.; Ren, J.; Huang, T.; Chen, J. Clinical Characteristics, Immunohistochemistry, and Outcomes of 77 Patients with Skull Base Chordomas. World Neurosurg. 2014, 81, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Chin, A.T.; Wagner, J.R.; Kunwar, S.; Ames, C.; Chou, D.; Barani, I.; Parsa, A.T.; McDermott, M.W.; Benet, A.; et al. Factors Predicting Recurrence after Resection of Clival Chordoma Using Variable Surgical Approaches and Radiation Modalities. Neurosurgery 2015, 76, 179–185; discussion 185–186. [Google Scholar] [CrossRef]
- Zhang, H.-K.; Sun, X.-C.; Hu, L.; Wang, J.-J.; Wang, D.-H. Endonasal Endoscopic Resection and Radiotherapy in Skull Base Chordomas. J. Craniofac. Surg. 2016, 27, e709–e713. [Google Scholar] [CrossRef]
- Raza, S.M.; Bell, D.; Freeman, J.L.; Grosshans, D.R.; Fuller, G.N.; DeMonte, F. Multimodality Management of Recurrent Skull Base Chordomas: Factors Impacting Tumor Control and Disease-Specific Survival. Oper. Neurosurg. 2018, 15, 131–143. [Google Scholar] [CrossRef]
- Zoli, M.; Milanese, L.; Bonfatti, R.; Faustini-Fustini, M.; Marucci, G.; Tallini, G.; Zenesini, C.; Sturiale, C.; Frank, G.; Pasquini, E.; et al. Clival Chordomas: Considerations after 16 Years of Endoscopic Endonasal Surgery. J. Neurosurg. 2018, 128, 329–338. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, J.; Wang, Y. Clinical Classification of Clival Chordomas for Transnasal Approaches. Neurosurg. Rev. 2020, 43, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Passeri, T.; Champagne, P.-O.; Giammattei, L.; Abbritti, R.; Cartailler, J.; Calugaru, V.; Feuvret, L.; Guichard, J.-P.; Polivka, M.; Adle-Biassette, H.; et al. Management Strategies in Clival and Craniovertebral Junction Chordomas: A 29-Year Experience. J. Neurosurg. 2022, 138, 1640–1652. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, E.; Virdis, E.; Negri, T.; Orsenigo, M.; Brich, S.; Conca, E.; Gronchi, A.; Stacchiotti, S.; Manenti, G.; Casali, P.G.; et al. Analysis of Receptor Tyrosine Kinases (RTKs) and Downstream Pathways in Chordomas. Neuro Oncol. 2010, 12, 776–789. [Google Scholar] [CrossRef]
- Horbinski, C.; Oakley, G.J.; Cieply, K.; Mantha, G.S.; Nikiforova, M.N.; Dacic, S.; Seethala, R.R. The Prognostic Value of Ki-67, P53, Epidermal Growth Factor Receptor, 1p36, 9p21, 10q23, and 17p13 in Skull Base Chordomas. Arch. Pathol. Lab. Med. 2010, 134, 1170–1176. [Google Scholar] [CrossRef]
- Le, L.P.; Nielsen, G.P.; Rosenberg, A.E.; Thomas, D.; Batten, J.M.; Deshpande, V.; Schwab, J.; Duan, Z.; Xavier, R.J.; Hornicek, F.J.; et al. Recurrent Chromosomal Copy Number Alterations in Sporadic Chordomas. PLoS ONE 2011, 6, e18846. [Google Scholar] [CrossRef]
- Shalaby, A.; Presneau, N.; Ye, H.; Halai, D.; Berisha, F.; Idowu, B.; Leithner, A.; Liegl, B.; Briggs, T.R.W.; Bacsi, K.; et al. The Role of Epidermal Growth Factor Receptor in Chordoma Pathogenesis: A Potential Therapeutic Target. J. Pathol. 2011, 223, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, O.F.; Gulluoglu, S.; Aydemir, E.; Ture, U.; Acar, H.; Atalay, B.; Demir, Z.; Sevli, S.; Creighton, C.J.; Ittmann, M.; et al. MicroRNA Expression Profiling Reveals the Potential Function of MicroRNA-31 in Chordomas. J. Neurooncol. 2013, 115, 143–151. [Google Scholar] [CrossRef]
- Kitamura, Y.; Sasaki, H.; Kimura, T.; Miwa, T.; Takahashi, S.; Kawase, T.; Yoshida, K. Molecular and Clinical Risk Factors for Recurrence of Skull Base Chordomas: Gain on Chromosome 2p, Expression of Brachyury, and Lack of Irradiation Negatively Correlate with Patient Prognosis. J. Neuropathol. Exp. Neurol. 2013, 72, 816–823. [Google Scholar] [CrossRef]
- Choy, E.; MacConaill, L.E.; Cote, G.M.; Le, L.P.; Shen, J.K.; Nielsen, G.P.; Iafrate, A.J.; Garraway, L.A.; Hornicek, F.J.; Duan, Z. Genotyping Cancer-Associated Genes in Chordoma Identifies Mutations in Oncogenes and Areas of Chromosomal Loss Involving CDKN2A, PTEN, and SMARCB1. PLoS ONE 2014, 9, e101283. [Google Scholar] [CrossRef]
- Scheil-Bertram, S.; Kappler, R.; von Baer, A.; Hartwig, E.; Sarkar, M.; Serra, M.; Brüderlein, S.; Westhoff, B.; Melzner, I.; Bassaly, B.; et al. Molecular Profiling of Chordoma. Int. J. Oncol. 2014, 44, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schiff, D.; Park, D.; Abounader, R. MicroRNA-608 and MicroRNA-34a Regulate Chordoma Malignancy by Targeting EGFR, Bcl-XL and MET. PLoS ONE 2014, 9, e91546. [Google Scholar] [CrossRef]
- von Witzleben, A.; Goerttler, L.T.; Marienfeld, R.; Barth, H.; Lechel, A.; Mellert, K.; Böhm, M.; Kornmann, M.; Mayer-Steinacker, R.; von Baer, A.; et al. Preclinical Characterization of Novel Chordoma Cell Systems and Their Targeting by Pharmocological Inhibitors of the CDK4/6 Cell-Cycle Pathway. Cancer Res. 2015, 75, 3823–3831. [Google Scholar] [CrossRef]
- Wang, L.; Zehir, A.; Nafa, K.; Zhou, N.; Berger, M.F.; Casanova, J.; Sadowska, J.; Lu, C.; Allis, C.D.; Gounder, M.; et al. Genomic Aberrations Frequently Alter Chromatin Regulatory Genes in Chordoma. Genes. Chromosomes Cancer 2016, 55, 591–600. [Google Scholar] [CrossRef]
- Bai, J.; Zhai, Y.; Wang, S.; Li, M.; Zhang, S.; Li, C.; Gui, S.; Li, Q.; Zhang, Y. LncRNA and MRNA Expression Profiles Reveal the Potential Roles of LncRNA Contributing to Regulating Dural Penetration in Clival Chordoma. Aging 2020, 12, 10809–10826. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Pierconti, F.; Falchetti, M.L.; Petrucci, G.; Maira, G.; De Maria, R.; Larocca, L.M.; Pallini, R. Establishing Tumor Cell Lines from Aggressive Telomerase-Positive Chordomas of the Skull Base. Technical Note. J. Neurosurg. 2006, 105, 482–484. [Google Scholar] [CrossRef]
- Pallini, R.; Maira, G.; Pierconti, F.; Falchetti, M.L.; Alvino, E.; Cimino-Reale, G.; Fernandez, E.; D’Ambrosio, E.; Larocca, L.M. Chordoma of the Skull Base: Predictors of Tumor Recurrence. J. Neurosurg. 2003, 98, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Cote, G.M.; He, J.; Choy, E. Next-Generation Sequencing for Patients with Sarcoma: A Single Center Experience. Oncologist 2018, 23, 234–242. [Google Scholar] [CrossRef]
- Zenonos, G.A.; Fernandez-Miranda, J.C.; Mukherjee, D.; Chang, Y.-F.; Panayidou, K.; Snyderman, C.H.; Wang, E.W.; Seethala, R.R.; Gardner, P.A. Prospective Validation of a Molecular Prognostication Panel for Clival Chordoma. J. Neurosurg. 2018, 130, 1528–1537. [Google Scholar] [CrossRef]
- Suit, H.D.; Goitein, M.; Munzenrider, J.; Verhey, L.; Davis, K.R.; Koehler, A.; Linggood, R.; Ojemann, R.G. Definitive Radiation Therapy for Chordoma and Chondrosarcoma of Base of Skull and Cervical Spine. J. Neurosurg. 1982, 56, 377–385. [Google Scholar] [CrossRef]
- Austin-Seymour, M.; Munzenrider, J.E.; Goitein, M.; Gentry, R.; Gragoudas, E.; Koehler, A.M.; McNulty, P.; Osborne, E.; Ryugo, D.K.; Seddon, J. Progress in Low-LET Heavy Particle Therapy: Intracranial and Paracranial Tumors and Uveal Melanomas. Radiat. Res. Suppl. 1985, 8, S219–S226. [Google Scholar] [CrossRef]
- Sen, C.; Shrivastava, R.; Anwar, S.; Triana, A. Lateral Transcondylar Approach for Tumors at the Anterior Aspect of the Craniovertebral Junction. Neurosurgery 2010, 66, 104–112. [Google Scholar] [CrossRef]
- Ln, S.; Vl, S.; Nf, J. Subtemporal-Preauricular Infratemporal Fossa Approach to Large Lateral and Posterior Cranial Base Neoplasms. J. Neurosurg. 1987, 67, 488–499. [Google Scholar] [CrossRef]
- Jankowski, R.; Auque, J.; Simon, C.; Marchal, J.C.; Hepner, H.; Wayoff, M. Endoscopic Pituitary Tumor Surgery. Laryngoscope 1992, 102, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, S.; Emengen, A.; Caklili, M.; Ergen, A.; Yılmaz, E.; Uzuner, A.; Icli, D.; Cabuk, B.; Anik, I. Operative Nuances and Surgical Limits of the Endoscopic Approach to Clival Chordomas and Chondrosarcomas: A Single-Center Experience of 72 Patients. Clin. Neurol. Neurosurg. 2021, 208, 106875. [Google Scholar] [CrossRef]
- Gui, S.; Zong, X.; Wang, X.; Li, C.; Zhao, P.; Cao, L.; Zhang, Y. Classification and Surgical Approaches for Transnasal Endoscopic Skull Base Chordoma Resection: A 6-Year Experience with 161 Cases. Neurosurg. Rev. 2016, 39, 321–332; discussion 332–333. [Google Scholar] [CrossRef]
- Yumiko, O.; Tamura, R.; Takahashi, S.; Morimoto, Y.; Sato, M.; Horikoshi, T.; Hassaan, S.; Yoshida, K.; Toda, M. A Comparative Study Between Traditional Microscopic Surgeries and Endoscopic Endonasal Surgery for Skull Base Chordomas. World Neurosurg. 2020, 134, e1099–e1107. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | N. of Patients | Surgical Approach | Complications | GTR | Follow-Up (Months) | Recurrence | Outcome |
|---|---|---|---|---|---|---|---|
| Samii, 2007 [16] | 49 | Open (49) | CSF leak 5.4%, meningitis 5.4% | 49% | 63 | NA | 5-year OS 65% |
| Koutourousiou, 2012 [17] | 60 | Endoscopic (NA), open (NA) | CSF leak 20%, CN injury 6.7% | 67% | 17.8 | 20% | NA |
| Ouyang, 2014 [18] | 77 | Endoscopic (NA), open (NA) | Overall 27.8%, CN injury 18.2% | 33% | 60 | NA | 3-year PFS 92.0% |
| Jahangiri, 2015 [19] | 50 | Endoscopic (34), open (9), combined (7) | CSF leak 12%, CN injury 6%, meningitis 12% | 52% | 41 | 51% | NA |
| Zhang, 2016 [20] | 32 | Endoscopic (32) | CSF leak 12% | 28% | 20 | NA | 5-year PFS 16.5% 5-year OS 69.5% |
| Raza, 2018 [21] | 29 | NA | NA | 41% | 28 | NA | Disease-specific survival 44.4 months |
| Zoli, 2018 [22] | 65 | Endoscopic (65) | CSF leak 2.5% | 59% | 52 | 40% | NA |
| Wang, 2020 [23] | 49 | Endoscopic (49) | CSF leak 30.1%, CN injury 5.5% | 64–85% | 41.5 | 14% | Mortality 12% |
| Bai, 2022 [8] | 284 | Endoscopic (349), open (31) | CSF leak 3.9% | 40% | 43.9 | 55% | 5-year disease-specific survival 71.0% |
| Passeri, 2022 [24] | 210 | Endoscopic (142), open (123) | CSF leak 12.1%; CN injury 17.7% | 44% | 59.2 | 42% | 5-year PFS 52.1% 5-year OS 75.1% |
| Author, Year | Material | Main Findings | |
|---|---|---|---|
| Bank Cells | Patients’ Tissues | ||
| Tamborini et al., 2010 [25] | Sacrum, spine, and clivus chordomas | The existence of an autocrine/paracrine loop involving some imatinib-related RTKs | |
| Horbinski et al., 2010 [26] | Skull base chordomas | Identification of biomarkers with a prognostic role | |
| Le et al., 2011 [27] | Spine and skull base chordomas | High genomic instability (large copy number losses) | |
| Shalaby et al., 2011 [28] | U-CH1 | Sacrum, spine, and skull base chordomas | The importance of molecular studies for targeted therapies (like EGFR antagonists) |
| Aydemir et al., 2012 [3] | U-CH1 | Chordomas, nucleus pulposum | Characterization of chordoma stem cells |
| Bayrak et al., 2013 [29] | U-CH1 | Chordomas, nucleus pulposum | Identification of down and upregulated miRNAs |
| Kitamura et al., 2013 [30] | Skull base chordomas | Identification of specific genetic/molecular and clinical prognostic factors | |
| Choy et al., 2014 [31] | Sacrum, spine, and skull base chordomas | Point mutations in tumor suppressor genes | |
| Scheil-Bertram et al., 2014 [32] | U-CH1, 2 | Chordomas, nucleus pulposum | Identification and validation of genes involved in chordomas genesis |
| Zhang et al., 2014 [33] | U-CH1, U-CH2 | Clival chordomas | Identification of down-regulated miRNAs as a potential therapeutic tool |
| von Witzleben et al., 2015 [34] | U-CH1, 2, 3, 6, 7, 11, 12 | Sacrum | Development of new cell lines and evaluation of CDK4/6 inhibitors |
| Wang et al., 2016 [35] | Sacrum and spine chordomas | Alterations of chromatin regulatory genes (SETD2) | |
| Bai et al., 2020 [36] | Clival chordomas | Role of LncRNA in dural penetration | |
| Pt N# | Age | Sex | Symptom at Onset | N° of Surgeries | Surgical Approach | EOR | Complications | Adj. RxTp after First Surgery | Histology | Proliferative Index at First Surgery | Recurrence | Treatment of Recurrence | Pt Outcome (Yrs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | F | diplopia | 1 | EEA | GTR | - | yes | chordoma pan-CK+/S100+/vimentin+ | 1–2% | no | AWD (4) | |
| 2 | 23 | M | diplopia | 1 | EEA | GTR | - | yes | chordoma s100+/EMA+/hTERT+/p53- | 1.5% | no | AFD (17) | |
| 3 | 57 | F | incidental | 1 | EEA | GTR | - | yes | chordoma pan-CK+/S100+/vimentin+/EMA+ | 5% | no | AWD (3) | |
| 4 | 29 | M | diplopia | 1 | EEA | GTR | - | yes | chordoma pan-CK+/S100+/vimentin+/EMA+ | 1% | no | AWD (3) | |
| 5 | 32 | M | incidental | 1 | EEA | STR | - | NA | chordoma pan-CK+/S100+/vimentin+ | 2% | NA | NA | |
| 6 | 41 | M | diplopia, loss of vision | 1 | EEA | GTR | - | yes | chordoma S100+/vimentin+/brachyury+ | NA | no | AFD (10) | |
| 7 | 80 | F | diplopia | 2 | EEA; STA | STR | - | no | chordoma pan-CK+/S100+/vimentin+/EMA+ | 5% | Yes @ 3 yrs | EEA + RT | AWD (6) |
| 8 | 59 | M | nystagmus, diplopia | 1 | EEA | GTR | CSF leak | no | chordoma pan-CK+/S100+/vimentin+ | 3% | no | DD (1) | |
| 9 | 40 | M | diplopia, headache | 1 | EEA | STR | - | yes | chordoma pan-CK+/S100+/vimentin+ | 3% | yes @ 5 yrs | RT | AWD (7) |
| 10 | 53 | M | diplopia | 1 | EEA | GTR | - | yes | chordoma pan-CK+/vimentin+ | NA | no | DOD (5) | |
| 11 | 76 | F | dysphonia | 1 | CR | GTR | - | yes | - | <2% | no | DOD (6) | |
| 12 | 54 | F | diplopia | 4 | EEA | PR | hearing loss; bilateral trigeminal pain | yes | chordoma pan-CK+/vimentin+ | NA | yes @ 4 yrs | STA + RT+ sirolimus (7 mL/die) and erlotinib (150 mg/die); @3 yrs STA; EEA | DD (7) |
| 13 | 43 | F | diplopia | 5 | EEA | STR | ophthalmoplegia, hypovisus | no, not indicated due to rapid residual growth | chordoma pan-CK+/S100+/vimentin+ | 2% | Yes @ <1 yrs | EEA + RT; EEA + RT; NA (other hospital), NA (other hospital) | AWD (6) |
| 14 | 85 | M | incidental | 2 | EEA | STR | - | NA | chordoma pan-CK+/S100+/vimentin+ | 5–10% | yes @ 2 yrs | EEA | DOD (3) |
| 15 | 23 | M | diplopia | 3 | EEA | PR | - | yes, proton + Imatinib | chordoma pan-CK+/S100+/vimentin+ | 2% | Yes @<1 yrs | EEA + C0 − C4 fixation, sirolimus (7 mL/die) and erlotinib (150 mg/die); CR | DOD (18) |
| 16 | 69 | M | diplopia | 2 | EEA | GTR | - | no | chordoma pan-CK+/vimentin+/S100+ | 2–3% | yes @5 yrs | EEA + RT | AWD (7) |
| 17 | 23 | M | incidental | 1 | CR | GTR | - | yes | chordoma CAM 5.2+/Vimentin+/S100+ | NA | NA | AFD (17) | |
| 18 | 82 | F | diplopia | 2 | STA | STR | visual impairment bilaterally, right VI CN palsy, ipsilateral hearing loss | no | chordoma | NA | yes @1 yr | STA | DD (2) |
| 19 | 41 | M | diplopia | 1 | CR | GTR | - | yes | chordoma | NA | no | AFD (24) | |
| 20 | 42 | M | V2 hypoaesthesia, dysphagia | 1 | CR | GTR | right VI CN palsy; obstructive hydrocephalus requiring CSF shunting | no | chordoma pan-CK+/S100+/vimentin+ | NA | no | DD (2) | |
| 21 | 40 | M | diplopia | 3 | CR | STR | left VII and VIII CN palsy and dysphagia; hemiplegia | yes + Cyber knife | chordoma pan-CK+/S100+/vimentin+/p53+ 30% | 8–10% | yes @ 2 yrs | CR; @<1 yr CR | DD (4) |
| 22 | 43 | M | left tinnitus | 1 | STA | GTR | CSF leak | NA | chordoma pan-CK+/S100+/ p53 +/brachyury+ | 3–4% | no | - | AFD (11) |
| 23 | 60 | F | rhinosinusitis | 1 | STA | GTR | CSF leak | NA | chordoma pan-CK+/S100+/vimentin+ | NA | no | AFD (16) | |
| 24 | 39 | F | diplopia | 4 | STA | GTR | - | no | chordoma | NA | yes @4 yrs | CR; @5 yrs STA+ RT; @3 yrs CR | DOD (30) |
| 25 | 72 | M | incidental | 1 | STA | GTR | seizure | no | chordoma pan-CK+/vimentin+ | 2% | no | DOD (7) | |
| 26 | 68 | F | diplopia | 1 | STA | GTR | - | NA | chordoma pan-CK+/S100+/vimentin+/p53 15% | 1% | no | DOD (2) | |
| 27 | 31 | M | diplopia | 4 | STA | GTR | obstructive hydrocephalus requiring CSF shunting; hemorrhagic infarct | no | chordoma p53+ | NA | yes @ 2 yrs | STA+RT; @4 yrs EEA+ Cyber Knife; @2 yrs CR | AFD (20) |
| 28 | 51 | M | V2 hypoaesthesia | 5 | STA | GTR | hypopituitarism, meningitis | no | chordoma pan-CK+/S100+/vimentin+ | 3% | yes @ 5 yrs | CR+ RT; @ 12 y STA + RT; @1 yrs EEA; @1 yr EEA + Sirolimus | DD (20) |
| 29 | 60 | M | diplopia | 3 | STA | STR | hypovisus | no | chordoma pan-CK+/S100+/vimentin+ | 2% | yes @ 2 yrs | EEA; @1 yr EEA | DOD (3) |
| 30 | 71 | F | diplopia | 2 | STA | STR | NA | no | chordoma | NA | yes @ 3 yrs | STA | DD (4) |
| 31 | 48 | F | diplopia | 2 | STA | STR | - | no | chordoma pan-CK+/vimentin+ | 4–5% | yes @ <1 yr | STA + RT | AFD (11) |
| 32 | 66 | F | diplopia | 2 | STA | GTR | - | no | chordoma pan-CK+/S100+/vimentin+/h-TERT+/p53+ 6–8% | 2% | yes @ 5 yrs | STA | AFD (17) |
| 33 | 55 | F | diplopia | 4 | STA | STR | bilateral VI CN palsy | yes | chordoma pan-CK+/S100+; p53+; hTERT+; | 1–12% | yes @ 2 yrs | STA; @ 2 yrs STA + RT; STA + chemo | DD (10) |
| 34 | 47 | F | headache | 3 | STA | GTR | CSF leakage; hypopituitarism | no | chordoma S100+/vimentin+ | 1% | yes @ 10 yrs | STA; @ 9 yrs EEA + RT | AWD (20) |
| 35 | 33 | M | headache | 1 | CR + c0-c2 fixation | NA | NA | chordoma pan-CK+/vimentin+/S100+ | NA | NA | DD (3) | ||
| 36 | 26 | F | tinnitus, left tongue fasciculation | 7 | CR + c0-c2 fixation | STR | VII to XII CN palsy | RT+oxygen-ozone therapy and Temodal and homeopathic anticancer therapies | chordoma pan-CK+/S100+/vimentin+; p53+ | 5–6% | yes @ 4 yrs | CR; (second surgical time) CR; CR + Sirolimus; EEA and transoral combined; @1 yr CR; EEA; STA + Radiotherapy | DD (8) |
| 37 | 44 | M | V3 hypoaesthesia, ptosis | 5 | STA | STR | hemiparesis, VI CN palsy; obstructive hydrocephalus requiring CSF shunting | no | chondroid chordoma; VEGF+, EGFRvIII+ | NA | yes @ <1 yr | STA; Cyber knife; STA; EEA; EEA + Bevacizumab + Erlotinib | DD (5) |
| 38 | 37 | M | diplopia | 2 | EEA | STR | - | no | chordoma; HTERT+; p53+ | NA | yes @ 1 yr | EEA | DD (13) |
| 39 | 79 | F | hypovisus | 6 | STA | NA | - | no | chordoma ck+/S100+/p53+; vimentin+/hTERT-; | 4–8% | NA | DOD (3) | |
| 40 | 66 | F | NA | 2 | STA | NA | - | NA | chordoma pan-CK+/S100+/vimentin+ | 3% | NA | NA (1) | |
| 41 | 65 | F | NA | 1 | EEA | NA | - | NA | chordoma pan-CK+/S100+/vimentin+ | NA | NA | NA (1) | |
| 42 | 36 | F | headache, diplopia | 1 | STA | NA | - | NA | chordoma pan-CK+/S100+/vimentin+/p53- | 3% | NA | AFD (20) |
| Covariate | B | SE | Wald | p | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Age | −0.9215 | 0.4708 | 3.8307 | 0.0503 | 0.3979 | 0.1581 to 1.0013 |
| Proliferative index | 0.9323 | 0.5355 | 3.0306 | 0.0817 | 2.5402 | 0.8893 to 7.2562 |
| Radiotherapy | 1.1323 | 0.5045 | 5.0372 | 0.0248 | 3.1028 | 1.1543 to 8.3409 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noya, C.; D’Alessandris, Q.G.; Doglietto, F.; Pallini, R.; Rigante, M.; Mattogno, P.P.; Gessi, M.; Montano, N.; Parrilla, C.; Galli, J.; et al. Treatment of Clival Chordomas: A 20-Year Experience and Systematic Literature Review. Cancers 2023, 15, 4493. https://doi.org/10.3390/cancers15184493
Noya C, D’Alessandris QG, Doglietto F, Pallini R, Rigante M, Mattogno PP, Gessi M, Montano N, Parrilla C, Galli J, et al. Treatment of Clival Chordomas: A 20-Year Experience and Systematic Literature Review. Cancers. 2023; 15(18):4493. https://doi.org/10.3390/cancers15184493
Chicago/Turabian StyleNoya, Carolina, Quintino Giorgio D’Alessandris, Francesco Doglietto, Roberto Pallini, Mario Rigante, Pier Paolo Mattogno, Marco Gessi, Nicola Montano, Claudio Parrilla, Jacopo Galli, and et al. 2023. "Treatment of Clival Chordomas: A 20-Year Experience and Systematic Literature Review" Cancers 15, no. 18: 4493. https://doi.org/10.3390/cancers15184493
APA StyleNoya, C., D’Alessandris, Q. G., Doglietto, F., Pallini, R., Rigante, M., Mattogno, P. P., Gessi, M., Montano, N., Parrilla, C., Galli, J., Olivi, A., & Lauretti, L. (2023). Treatment of Clival Chordomas: A 20-Year Experience and Systematic Literature Review. Cancers, 15(18), 4493. https://doi.org/10.3390/cancers15184493








