COMBI-r: A Prospective, Non-Interventional Study of Dabrafenib Plus Trametinib in Unselected Patients with Unresectable or Metastatic BRAF V600-Mutant Melanoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Objectives
2.3. Study Procedures and Assessments
2.4. Statistical Analysis
2.5. Ethics Committee Approvals and Trial Registration
3. Results
3.1. Patients
3.2. Treatment Exposure
3.3. Clinical Outcomes
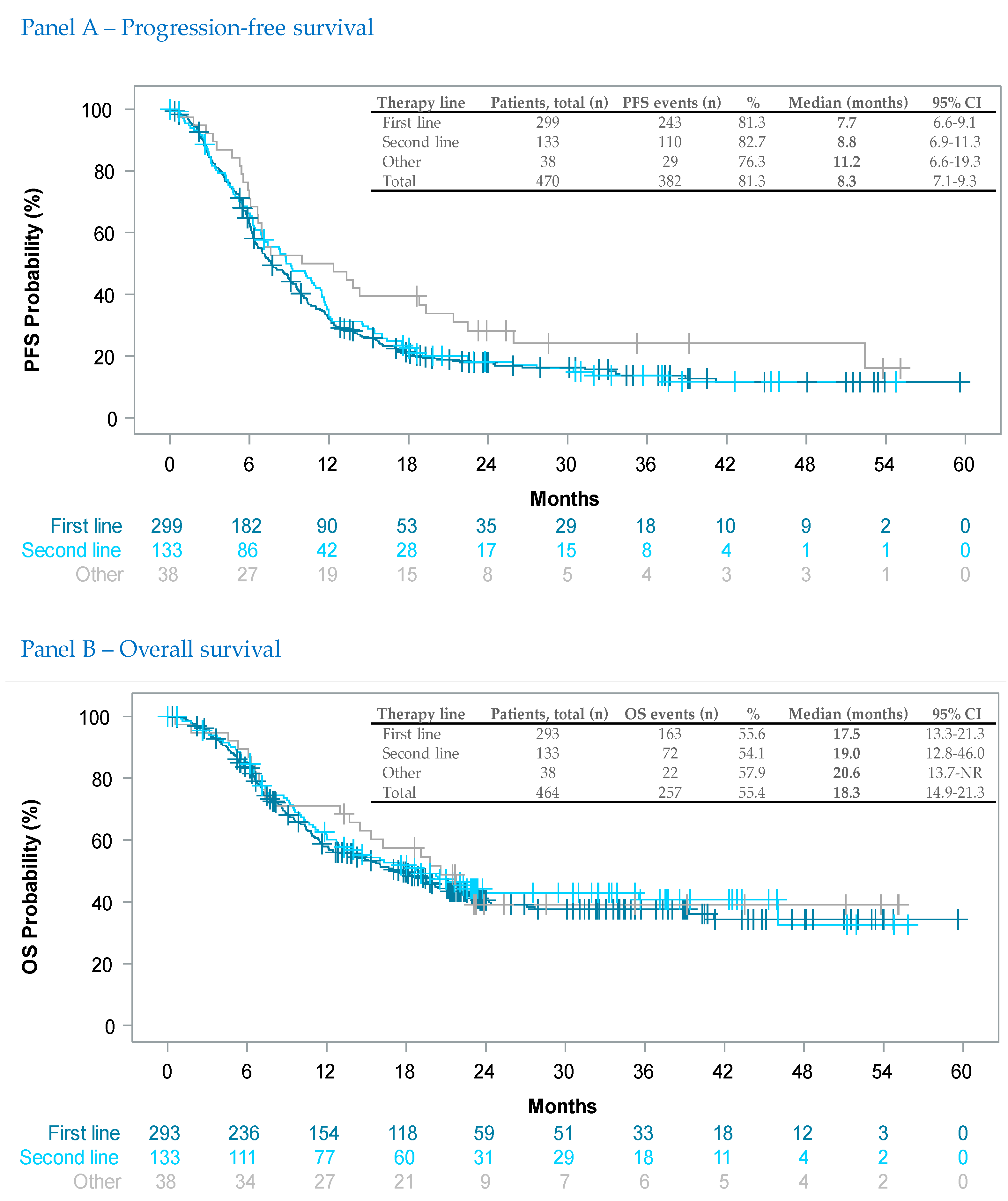
3.3.1. Effectiveness (Per Line of Therapy)
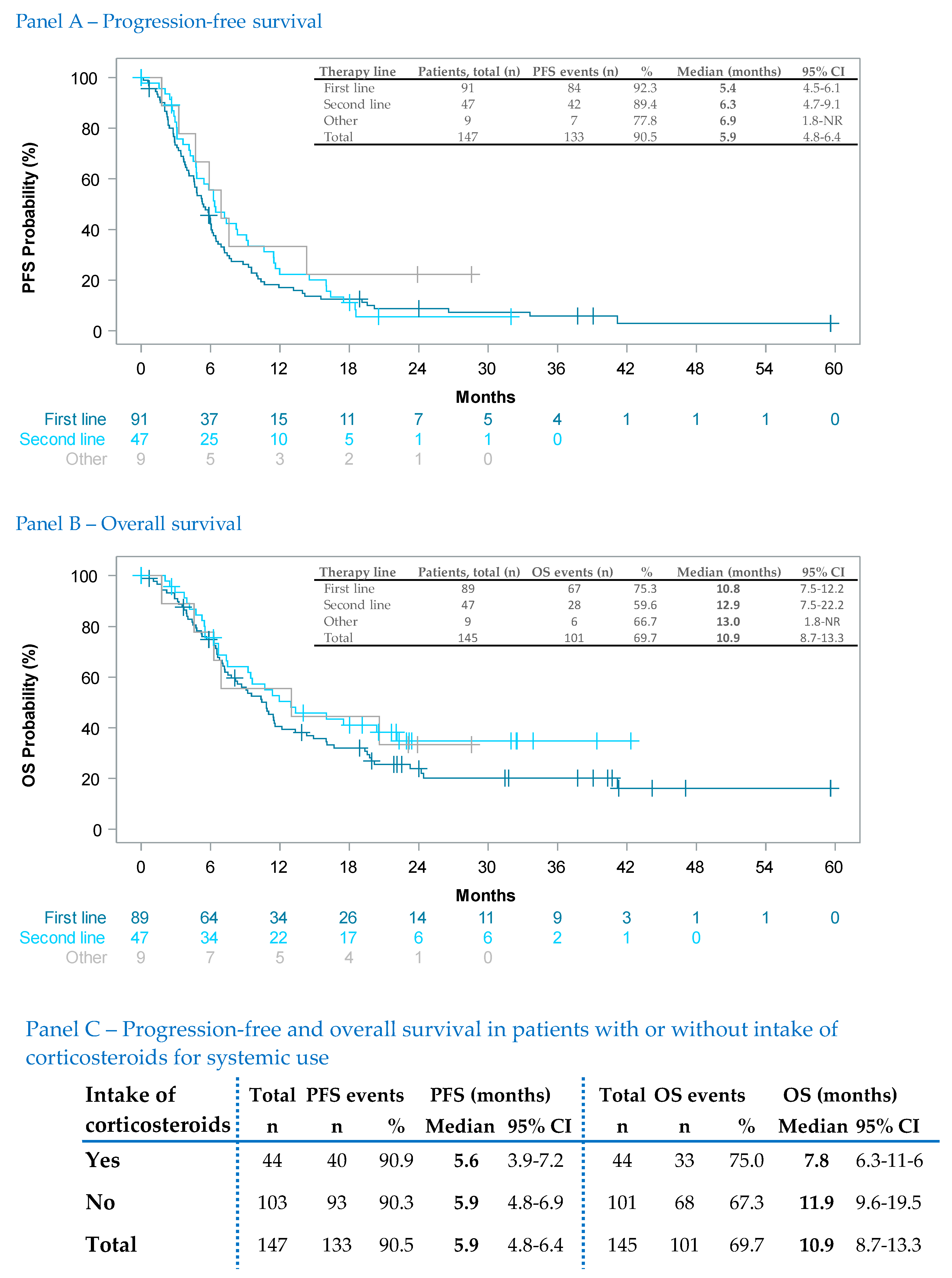
3.3.2. Effectiveness in Selected Subgroups
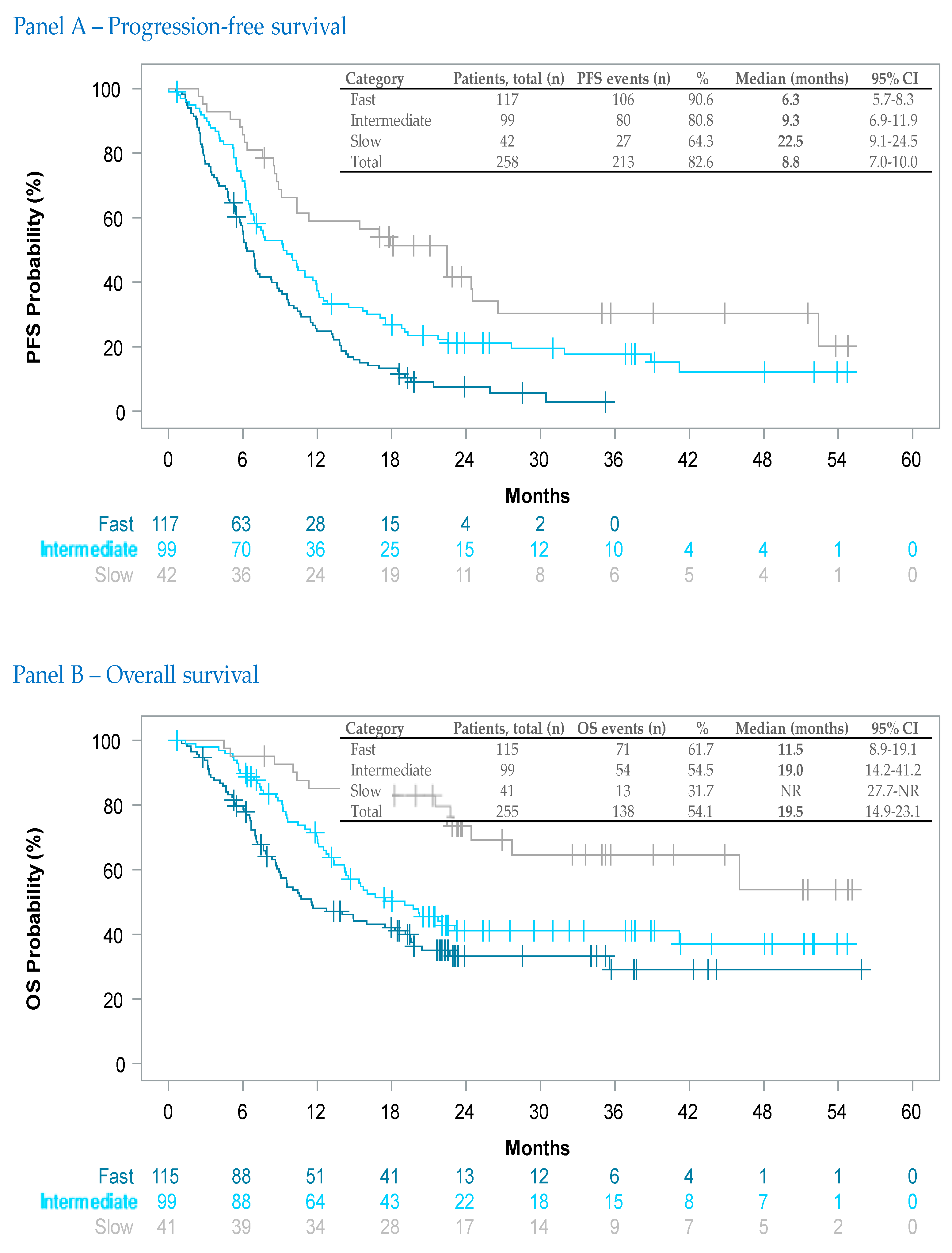
3.3.3. Investigator-Assessed Tumor Dynamics
3.3.4. Therapy-Duration-Dependent Effectiveness
3.4. Tolerability
3.5. Quality-of-Life
4. Discussion
4.1. Real-World Effectiveness of Dabrafenib Plus Trametinib
4.2. Effectiveness in Brain Metastases
4.3. Investigator-Assessed Tumor Dynamics
4.4. Safety Patterns
4.5. Strengths and Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab plus Ipilimumab or Nivolumab Alone versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dréno, B.; Larkin, J.; Ribas, A.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. 5-Year Outcomes with Cobimetinib plus Vemurafenib in BRAFV600 Mutation-Positive Advanced Melanoma: Extended Follow-up of the coBRIM Study. Clin. Cancer Res. 2021, 27, 5225–5235. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Flaherty, K.T.; Robert, C.; Arance, A.; de Groot, J.W.B.; Garbe, C.; Gogas, H.; Gutzmer, R.; Krajsová, I.; Liszkay, G.; et al. COLUMBUS 5-Year Update: A Randomized, Open-Label, Phase III Trial of Encorafenib plus Binimetinib versus Vemurafenib or Encorafenib in Patients with BRAF V600-Mutant Melanoma. J. Clin. Oncol. 2022, 40, 41784188. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.V.; Schneeweiss, S.; Gagne, J.J.; Evers, T.; Gerlinger, C.; Desai, R.; Najafzadeh, M. Using Real-World Data to Extrapolate Evidence from Randomized Controlled Trials. Clin. Pharmacol. Ther. 2019, 105, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Tannock, I.F. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br. J. Cancer 2014, 110, 551–555. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Corrigan-Curay, J.; Sacks, L.; Woodcock, J. Real-World Evidence and Real-World Data for Evaluating Drug Safety and Effectiveness. JAMA 2018, 320, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Khozin, S.; Blumenthal, G.M.; Pazdur, R. Real-world Data for Clinical Evidence Generation in Oncology. J. Natl. Cancer Inst. 2017, 109, djx87. [Google Scholar] [CrossRef] [PubMed]
- Saiag, P.; Robert, C.; Grob, J.J.; Mortier, L.; Dereure, O.; Lebbe, C.; Mansard, S.; Grange, F.; Neidhardt, E.M.; Lesimple, T.; et al. Efficacy, safety and factors associated with disease progression in patients with unresectable (stage III) or distant metastatic (stage IV) BRAF V600-mutant melanoma: An open label, non-randomized, phase IIIb study of trametinib in combination with dabrafenib. Eur. J. Cancer 2021, 154, 57–65. [Google Scholar] [CrossRef]
- Ismail, R.K.; Suijkerbuijk, K.P.M.; de Boer, A.; van Dartel, M.; Hilarius, D.L.; Pasmooij, A.M.G.; van Zeijl, M.C.T.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Blank, C.U.; et al. Long-term survival of patients with advanced melanoma treated with BRAF-MEK inhibitors. Melanoma Res. 2022, 32, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Teshima, Y.; Kizaki, M.; Kurihara, R.; Kano, R.; Harumiya, M. Interim analysis for post-marketing surveillance of dabrafenib and trametinib combination therapy in Japanese patients with unresectable and metastatic melanoma with BRAF V600 mutation. Int. J. Clin. Oncol. 2020, 25, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Pérol, D.; Duval-Modeste, A.B.; El Adaoui, L.; Lelarge, Y.; Niarra, R.; Mateus, C. Survival in adult patients with BRAFV600 mutation-positive advanced melanoma: A noninterventional ambispective study of patients with cobimetinib combined with vemurafenib during the French early access program: MELANIS study. Melanoma Res. 2022, 32, 269–277. [Google Scholar] [CrossRef]
- Atkinson, V.G.; Quaglino, P.; Aglietta, M.; Del Vecchio, M.; Depenni, R.; Consoli, F.; Bafaloukos, D.; Ferrucci, P.F.; Tulyte, S.; Krajsová, I.; et al. A Retrospective Analysis of Dabrafenib and/or Dabrafenib plus Trametinib Combination in Patients with Metastatic Melanoma to Characterize Patients with Long-Term Benefit in the Individual Patient Program (DESCRIBE III). Cancers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Aglietta, M.; Chiarion-Sileni, V.; Fava, P.; Guidoboni, M.; Depenni, R.; Minisini, A.; Consoli, F.; Ascierto, P.; Rinaldi, G.; Banzi, M.; et al. Retrospective Chart Review of Dabrafenib plus Trametinib in Patients with Metastatic BRAF V600-Mutant Melanoma Treated in the Individual Patient Program (DESCRIBE Italy). Target. Oncol. 2021, 16, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, V.G.; Sandhu, S.; Hospers, G.; Long, G.V.; Aglietta, M.; Ferrucci, P.F.; Tulyte, S.; Cappellini, G.C.A.; Soriano, V.; Ali, S.; et al. Dabrafenib plus trametinib is effective in the treatment of BRAF V600-mutated metastatic melanoma patients: Analysis of patients from the dabrafenib plus trametinib Named Patient Program (DESCRIBE II). Melanoma Res. 2020, 30, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Yang, Z.; Algazi, A.P.; Lomeli, S.H.; Wang, Y.; Othus, M.; Hong, A.; Wang, X.; Randolph, C.E.; et al. Anti-PD-1/L1 lead-in before MAPK inhibitor combination maximizes antitumor immunity and efficacy. Cancer Cell 2021, 39, 1375–1387.e6. [Google Scholar] [CrossRef]
- Cybulska-Stopa, B.; Czarnecka, A.M.; Ostaszewski, K.; Piejko, K.; Zietek, M.; Dziura, R.; Rutkowska, E.; Galus, L.; Ziolkowska, B.; Kempa-Kamińska, N.; et al. Sequential treatment with targeted and immune checkpoint inhibitor therapies in patients with BRAF positive metastatic melanoma: Real-world data. J. Clin. Oncol. 2022, 40, e21539. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab plus Nivolumab and Encorafenib plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. 2022, 41, 212–221. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Loong, H.H.; Summers, Y.; Thomas, Z.M.; French, P.; Lin, B.K.; Sashegyi, A.; Wolf, J.; Yang, J.C.; Drilon, A. Correlation between treatment effects on response rate and progression-free survival and overall survival in trials of targeted therapies in molecularly enriched populations. ESMO Open 2022, 7, 100398. [Google Scholar] [CrossRef]
- Franken, M.G.; Leeneman, B.; Gheorghe, M.; Uyl-de Groot, C.A.; Haanen, J.B.A.G.; van Baal, P.H.M. A systematic literature review and network meta-analysis of effectiveness and safety outcomes in advanced melanoma. Eur. J. Cancer 2019, 123, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Vordermark, D.; Hassel, J.C.; Krex, D.; Wendl, C.; Schadendorf, D.; Sickmann, T.; Rieken, S.; Pukrop, T.; Höller, C.; et al. Melanoma brain metastases—Interdisciplinary management recommendations 2020. Cancer Treat. Rev. 2020, 89, 102083. [Google Scholar] [CrossRef]
- Dutriaux, C.; Robert, C.; Grob, J.J.; Mortier, L.; Dereure, O.; Lebbe, C.; Mansard, S.; Grange, F.; Neidhardt, E.M.; Lesimple, T.; et al. An open label, non-randomised, phase IIIb study of trametinib in combination with dabrafenib in patients with unresectable (stage III) or distant metastatic (stage IV) BRAF V600-mutant melanoma: A subgroup analysis of patients with brain metastases. Eur. J. Cancer 2022, 175, 254–262. [Google Scholar] [CrossRef]
- Wilmott, J.S.; Tawbi, H.; Engh, J.A.; Amankulor, N.M.; Shivalingam, B.; Banerjee, H.; Vergara, I.A.; Lee, H.; Johansson, P.A.; Ferguson, P.M.; et al. Clinical Features Associated with Outcomes and Biomarker Analysis of Dabrafenib plus Trametinib Treatment in Patients with BRAF-Mutant Melanoma Brain Metastases. Clin. Cancer Res. 2023, 29, 521–531. [Google Scholar] [CrossRef]
- Bruno, R.; Bottino, D.; de Alwis, D.P.; Fojo, A.T.; Guedj, J.; Liu, C.; Swanson, K.R.; Zheng, J.; Zheng, Y.; Jin, J.Y. Progress and Opportunities to Advance Clinical Cancer Therapeutics Using Tumor Dynamic Models. Clin. Cancer Res. 2020, 26, 1787–1795. [Google Scholar] [CrossRef]
- Borcoman, E.; Kanjanapan, Y.; Champiat, S.; Kato, S.; Servois, V.; Kurzrock, R.; Goel, S.; Bedard, P.; Le Tourneau, C. Novel patterns of response under immunotherapy. Ann. Oncol. 2019, 30, 385–396. [Google Scholar] [CrossRef]
- Schadendorf, D.; Long, G.V.; Stroiakovski, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion-Sileni, V.; Schachter, J.; Garbe, C.; Dutriaux, C.; et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur. J. Cancer 2017, 82, 45–55. [Google Scholar] [CrossRef]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Schadendorf, D.; Robert, C.; Dummer, R.; Flaherty, K.T.; Tawbi, H.A.; Menzies, A.M.; Banerjee, H.; Lau, M.; Long, G.V. Pyrexia in patients treated with dabrafenib plus trametinib across clinical trials in BRAF-mutant cancers. Eur. J. Cancer 2021, 153, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Schaffner, P. Legal Requirements, Definitions, and Standards for Non-interventional Drug Studies: A Global Picture of Variability-Results and Conclusions from a Single-Institution Survey. Ther. Innov. Regul. Sci. 2013, 47, 684–691. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. Bias and causal associations in observational research. Lancet 2002, 359, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yoshimoto, T.; Nakagawa, S.; Sugitani, Y.; Yamamoto, H.; Asakawa, T. Impact of Tumor Assessment Frequency on Statistical Power in Randomized Cancer Clinical Trials Evaluating Progression-Free Survival. Ther. Innov. Regul. Sci. 2021, 55, 1258–1264. [Google Scholar] [CrossRef]
- Michiels, S.; Saad, E.D.; Buyse, M. Progression-Free Survival as a Surrogate for Overall Survival in Clinical Trials of Targeted Therapy in Advanced Solid Tumors. Drugs 2017, 77, 713–719. [Google Scholar] [CrossRef]
- Kang, J.; Cairns, J. Exploring uncertainty and use of real-world data in the National Institute for Health and Care Excellence single technology appraisals of targeted cancer therapy. BMC Cancer 2022, 22, 1268. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.Z.; Nussbaum, N.C.; Parrinello, C.M.; Bourla, A.B.; Bowser, B.E.; Wagner, S.; Tabano, D.C.; George, D.; Miksad, R.A. Analysis of a Real-World Progression Variable and Related Endpoints for Patients with Five Different Cancer Types. Adv. Ther. 2022, 39, 2831–2849. [Google Scholar] [CrossRef] [PubMed]

| First Line | Second Line | Other Lines | Total | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Total | 300 (100.0) | 134 (100.0) | 38 (100.0) | 472 (100.0) | |
| Gender | Male | 165 (55.0) | 77 (57.5) | 20 (52.6) | 262 (55.5) |
| Female | 135 (45.0) | 57 (42.5) | 18 (47.4) | 210 (44.5) | |
| Age (at baseline) | Median (years) | 61.0 | 56.5 | 59.7 | 59.2 |
| Range | 24–89 | 21–87 | 28–86 | 21–89 | |
| Missing data | 0 | 0 | 0 | 0 | |
| ECOG PS | 0 | 159 (53.0) | 84 (62.7) | 22 (57.9) | 265 (56.1) |
| 1 | 57 (19.0) | 17 (12.7) | 9 (23.7) | 83 (17.6) | |
| ≥2 | 23 (7.7) | 6 (4.5) | 2 (5.2) | 31 (6.6) | |
| Missing data | 61 (20.3) | 27 (20.1) | 5 (13.2) | 93 (19.7) | |
| Clinical stage | IIIC | 26 (8.7) | 8 (6.0) | 2 (5.3) | 36 (7.6) |
| IV | 268 (89.3) | 125 (93.3) | 36 (94.7) | 429 (90.9) | |
| Missing data * | 6 (2.0) | 1 (0.7) | 0 (0.0) | 7 (1.5) | |
| LDH | Normal/decreased | 102 (34.0) | 44 (32.8) | 17 (44.7) | 163 (34.5) |
| Elevated | 152 (50.7) | 69 (51.5) | 13 (34.2) | 234 (49.6) | |
| Missing data | 46 (15.3) | 21 (15.7) | 8 (21.1) | 75 (15.9) | |
| Distant metastases | M0 | 29 (9.7) | 9 (6.7) | 2 (5.3) | 40 (8.5) |
| M1 | 10 (3.3) | 4 (3.0) | 0 (0.0) | 14 (3.0) | |
| M1a | 27 (9.0) | 12 (9.0) | 3 (7.9) | 42 (8.9) | |
| M1b | 35 (11.7) | 26 (19.4) | 8 (21.0) | 69 (14.6) | |
| M1c ** | 192 (64.0) | 81 (60.4) | 25 (65.8) | 298 (63.1) | |
| Mx | 4 (1.3) | 0 (0.0) | 0 (0.0) | 4 (0.8) | |
| Missing data | 3 (1.0) | 2 (1.5) | 0 (0.0) | 5 (1.1) | |
| Affected organ systems † | 1 | 68 (26.3) | 38 (31.4) | 9 (25.0) | 115 (27.6) |
| 2 | 69 (26.6) | 26 (21.5) | 15 (41.7) | 110 (26.4) | |
| ≥3 | 122 (47.1) | 57 (47.1) | 12 (33.3) | 191 (45.9) | |
| Lung †† | 156 (60.9) | 76 (62.8) | 21 (58.3) | 253 (61.3) | |
| Bone †† | 66 (25.6) | 27 (22.3) | 8 (22.2) | 101 (24.3) | |
| Liver †† | 94 (36.4) | 37 (30.6) | 12 (33.3) | 143 (34.5) | |
| CNS †† | 91 (35.3) | 47 (38.8) | 9 (25.0) | 147 (35.4) | |
| Lymph node †† | 115 (44.4) | 54 (44.6) | 15 (41.7) | 184 (44.2) |
| First Line n (%) | Second Line n (%) | Other Lines n (%) | Total n (%) | |
|---|---|---|---|---|
| Any adverse event | 278 (92.7) | 132 (98.5) | 37 (97.4) | 447 (94.7) |
| Any dabrafenib-related AE | 186 (62.0) | 96 (71.6) | 28 (73.3) | 310 (65.7) |
| Any trametinib-related AE | 177 (59.0) | 98 (73.1) | 26 (68.4) | 301 (63.8) |
| Dose interruption * (dabrafenib-related AE) | 95 (31.7) | 51 (38.1) | 16 (42.1) | 162 (34.3) |
| Dose interruption * (trametinib-related AE) | 88 (29.3) | 53 (39.6) | 16 (42.1) | 157 (33.3) |
| Any serious adverse event | 190 (63.3) | 104 (77.6) | 25 (65.8) | 319 (67.6) |
| Death from any cause | 115 (38.3) | 55 (41.0) | 15 (39.5) | 185 (39.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berking, C.; Livingstone, E.; Debus, D.; Loquai, C.; Weichenthal, M.; Leiter, U.; Kiecker, F.; Mohr, P.; Eigentler, T.K.; Remy, J.; et al. COMBI-r: A Prospective, Non-Interventional Study of Dabrafenib Plus Trametinib in Unselected Patients with Unresectable or Metastatic BRAF V600-Mutant Melanoma. Cancers 2023, 15, 4436. https://doi.org/10.3390/cancers15184436
Berking C, Livingstone E, Debus D, Loquai C, Weichenthal M, Leiter U, Kiecker F, Mohr P, Eigentler TK, Remy J, et al. COMBI-r: A Prospective, Non-Interventional Study of Dabrafenib Plus Trametinib in Unselected Patients with Unresectable or Metastatic BRAF V600-Mutant Melanoma. Cancers. 2023; 15(18):4436. https://doi.org/10.3390/cancers15184436
Chicago/Turabian StyleBerking, Carola, Elisabeth Livingstone, Dirk Debus, Carmen Loquai, Michael Weichenthal, Ulrike Leiter, Felix Kiecker, Peter Mohr, Thomas K. Eigentler, Janina Remy, and et al. 2023. "COMBI-r: A Prospective, Non-Interventional Study of Dabrafenib Plus Trametinib in Unselected Patients with Unresectable or Metastatic BRAF V600-Mutant Melanoma" Cancers 15, no. 18: 4436. https://doi.org/10.3390/cancers15184436
APA StyleBerking, C., Livingstone, E., Debus, D., Loquai, C., Weichenthal, M., Leiter, U., Kiecker, F., Mohr, P., Eigentler, T. K., Remy, J., Schober, K., Heppt, M. V., von Wasielewski, I., Schadendorf, D., & Gutzmer, R. (2023). COMBI-r: A Prospective, Non-Interventional Study of Dabrafenib Plus Trametinib in Unselected Patients with Unresectable or Metastatic BRAF V600-Mutant Melanoma. Cancers, 15(18), 4436. https://doi.org/10.3390/cancers15184436






