Muscle and Adipose Wasting despite Disease Control: Unaddressed Side Effects of Palliative Chemotherapy for Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
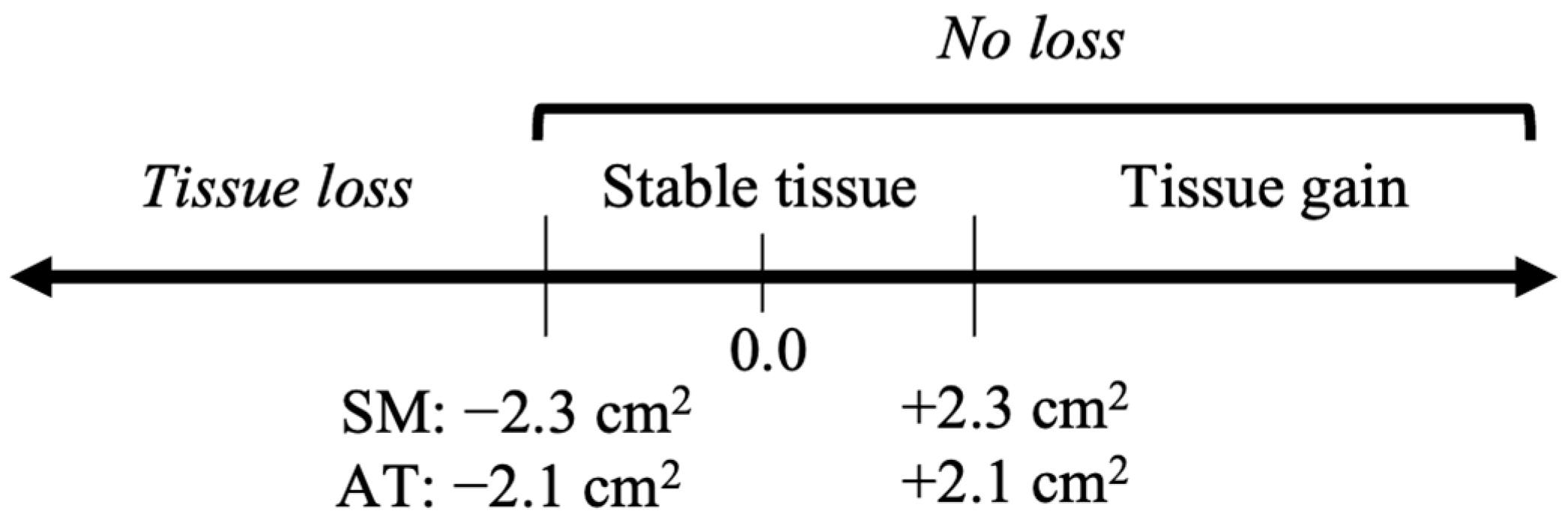
2. Materials and Methods
3. Results
3.1. Participants
3.2. Magnitude and Drivers of Muscle and Adipose Change
3.3. Survival Impact of Muscle and Adipose Losses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019; Canadian Cancer Statistics Advisory Committee: Toronto, ON, Canada, 2019. [Google Scholar]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, S.J.; Plester, C.E.; Richardson, R.A.; Fearon, K.C.H. Changes in Nutritional Status Associated with Unresectable Pancreatic Cancer. Br. J. Cancer 1997, 75, 106–109. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Kasamatsu, E.; Machii, K. Incidence and Frequency of Cancer Cachexia during Chemotherapy for Advanced Pancreatic Ductal Adenocarcinoma. Support. Care Cancer 2020, 28, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Chemotherapy-Induced Sarcopenia. Curr. Treat. Options Oncol. 2020, 21, 7. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A Practical and Precise Approach to Quantification of Body Composition in Cancer Patients Using Computed Tomography Images Acquired during Routine Care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer Cachexia Is Defined by an Ongoing Loss of Skeletal Muscle Mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef]
- Babic, A.; Rosenthal, M.H.; Bamlet, W.R.; Takahashi, N.; Sugimoto, M.; Danai, L.V.; Morales-Oyarvide, V.; Khalaf, N.; Dunne, R.F.; Brais, L.K.; et al. Postdiagnosis Loss of Skeletal Muscle, but Not Adipose Tissue, Is Associated with Shorter Survival of Patients with Advanced Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 2062–2069. [Google Scholar] [CrossRef]
- Salinas-Miranda, E.; Deniffel, D.; Dong, X.; Healy, G.M.; Khalvati, F.; O’Kane, G.M.; Knox, J.; Bathe, O.F.; Baracos, V.E.; Gallinger, S.; et al. Prognostic Value of Early Changes in CT-Measured Body Composition in Patients Receiving Chemotherapy for Unresectable Pancreatic Cancer. Eur. Radiol. 2021, 31, 8662–8670. [Google Scholar] [CrossRef] [PubMed]
- Marusic, U.; Narici, M.; Simunic, B.; Pisot, R.; Ritzmann, R. Nonuniform Loss of Muscle Strength and Atrophy during Bed Rest: A Systematic Review. J. Appl. Physiol. 2021, 131, 194–206. [Google Scholar] [CrossRef]
- Feijó, P.M.; Rodrigues, V.D.; Viana, M.S.; dos Santos, M.P.; Abdelhay, E.; Viola, J.P.; de Pinho, N.B.; Martucci, R.B. Effects of ω-3 Supplementation on the Nutritional Status, Immune, and Inflammatory Profiles of Gastric Cancer Patients: A Randomized Controlled Trial. Nutrition 2019, 61, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Derksen, J.W.G.; Kurk, S.A.; Peeters, P.H.M.; Dorresteijn, B.; Jourdan, M.; Velden, A.M.T.; Nieboer, P.; Jong, R.S.; Honkoop, A.H.; Punt, C.J.A.; et al. The Association between Changes in Muscle Mass and Quality of Life in Patients with Metastatic Colorectal Cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.E.; Ní Bhuachalla, É.B.; Power, D.G.; Cushen, S.J.; James, K.; Ryan, A.M. Loss of Skeletal Muscle during Systemic Chemotherapy Is Prognostic of Poor Survival in Patients with Foregut Cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Iwashita, T.; Ichikawa, H.; Iwasa, Y.; Mita, N.; Shiraki, M.; Shimizu, M. The Impact of Sarcopenia and Decrease in Skeletal Muscle Mass in Patients with Advanced Pancreatic Cancer during FOLFIRINOX Therapy. Br. J. Nutr. 2021, 125, 1140–1147. [Google Scholar] [CrossRef]
- Basile, D.; Parnofiello, A.; Vitale, M.G.; Cortiula, F.; Gerratana, L.; Fanotto, V.; Lisanti, C.; Pelizzari, G.; Ongaro, E.; Bartoletti, M.; et al. The IMPACT Study: Early Loss of Skeletal Muscle Mass in Advanced Pancreatic Cancer Patients. J. Cachexia Sarcopenia Muscle 2019, 10, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Stene, G.B.; Helbostad, J.L.; Amundsen, T.; Sørhaug, S.; Hjelde, H.; Kaasa, S.; Grønberg, B.H. Changes in Skeletal Muscle Mass during Palliative Chemotherapy in Patients with Advanced Lung Cancer. Acta Oncol. 2015, 54, 340–348. [Google Scholar] [CrossRef]
- Prado, C.M.; Sawyer, M.B.; Ghosh, S.; Lieffers, J.R.; Esfandiari, N.; Antoun, S.; Baracos, V.E. Central Tenet of Cancer Cachexia Therapy: Do Patients with Advanced Cancer Have Exploitable Anabolic Potential? Am. J. Clin. Nutr. 2013, 98, 1012–1019. [Google Scholar] [CrossRef]
- Carnie, L.; Abraham, M.; McNamara, M.G.; Hubner, R.A.; Valle, J.W.; Lamarca, A. Impact on Prognosis of Early Weight Loss during Palliative Chemotherapy in Patients Diagnosed with Advanced Pancreatic Cancer. Pancreatology 2020, 20, 1682–1688. [Google Scholar] [CrossRef]
- Damrauer, J.S.; Stadler, M.E.; Acharyya, S.; Baldwin, A.S.; Couch, M.E.; Guttridge, D.C. Chemotherapy-Induced Muscle Wasting: Association with NF-ΚB and Cancer Cachexia. Eur. J. Transl. Myol. 2018, 28, 158–166. [Google Scholar] [CrossRef]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex Specificity of Pancreatic Cancer Cachexia Phenotypes, Mechanisms, and Treatment in Mice and Humans: Role of Activin. J. Cachexia Sarcopenia Muscle 2022, 13, 2146–2161. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Li, X.; Lin, G.; Ma, Y.; Xiao, R.; Li, H.; Qiu, M.; Yang, F. Skeletal Muscle Wasting during Neoadjuvant Therapy as a Prognosticator in Patients with Esophageal and Esophagogastric Junction Cancer: A Systematic Review and Meta-Analysis. Int. J. Surg. 2022, 97, 106206. [Google Scholar] [CrossRef] [PubMed]
- Arribas, L.; Sabaté-Llobera, A.; Taberna, M.; Pallarés, N.; Narro Marin, A.; Virgili, N.; Hurtós, L.; Peiró, I.; Vilajosana, E.; Lozano, A.; et al. Adequacy of Nutritional Support Using Computed Tomography (CT) in Patients with Head and Neck Cancer (HNC) during Chemo-Radiotherapy (CRT). Eur. J. Clin. Nutr. 2021, 75, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Oh, D.Y.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Bang, Y.J. Skeletal Muscle Depletion Predicts the Prognosis of Patients with Advanced Pancreatic Cancer Undergoing Palliative Chemotherapy, Independent of Body Mass Index. PLoS ONE 2015, 10, e0139749. [Google Scholar] [CrossRef] [PubMed]
- Kays, J.K.; Shahda, S.; Stanley, M.; Bell, T.M.; O’Neill, B.H.; Kohli, M.D.; Couch, M.E.; Koniaris, L.G.; Zimmers, T.A. Three Cachexia Phenotypes and the Impact of Fat-Only Loss on Survival in FOLFIRINOX Therapy for Pancreatic Cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 673–684. [Google Scholar] [CrossRef]
- Arribas, L.; Sabaté-Llobera, A.; Domingo, M.C.; Taberna, M.; Sospedra, M.; Martin, L.; González-Tampán, A.R.; Pallarés, N.; Mesía, R.; Baracos, V.E. Assessing Dynamic Change in Muscle during Treatment of Patients with Cancer: Precision Testing Standards. Clin. Nutr. 2022, 41, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.W.; Hickey, G.L.; Head, S.J. Statistical Primer: Multivariable Regression Considerations and Pitfalls†. Eur. J. Cardio-Thorac. Surg. 2019, 55, 179–185. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
- Rupert, J.E.; Narasimhan, A.; Jengelley, D.H.A.; Jiang, Y.; Liu, J.; Au, E.; Silverman, L.M.; Sandusky, G.; Bonetto, A.; Cao, S.; et al. Tumor-Derived IL-6 and Trans-Signaling among Tumor, Fat, and Muscle Mediate Pancreatic Cancer Cachexia. J. Exp. Med. 2021, 218, e20190450. [Google Scholar] [CrossRef]
- Witvliet-Van Nierop, J.E.; Lochtenberg-Potjes, C.M.; Wierdsma, N.J.; Scheffer, H.J.; Kazemier, G.; Ottens-Oussoren, K.; Meijerink, M.R.; De Van Der Schueren, M.A.E. Assessment of Nutritional Status, Digestion and Absorption, and Quality of Life in Patients with Locally Advanced Pancreatic Cancer. Gastroenterol. Res. Pract. 2017, 2017, 6193765. [Google Scholar] [CrossRef] [PubMed]
- Kordes, M.; Larsson, L.; Engstrand, L.; Löhr, J.-M. Pancreatic Cancer Cachexia: Three Dimensions of a Complex Syndrome. Br. J. Cancer 2021, 124, 1623–1636. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Petzel, M.Q.B.; Zimmers, T.A.; Denlinger, C.S.; Matrisian, L.M.; Picozzi, V.J.; Rahib, L.; Hendifar, A.; Tuli, R.; Wolpin, B.; et al. Pancreas Cancer-Associated Weight Loss. Oncologist 2019, 24, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Jordhøy, M.S.; Skjegstad, G.; Ledsaak, O.; Iversen, P.O.; Hjermstad, M.J. Symptoms in Advanced Pancreatic Cancer Are of Importance for Energy Intake. Support. Care Cancer 2013, 21, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Campelj, D.G.; Goodman, C.A.; Rybalka, E. Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere. Cancers 2021, 13, 3615. [Google Scholar] [CrossRef] [PubMed]
- Schiessel, D.L.; Baracos, V.E. Barriers to Cancer Nutrition Therapy: Excess Catabolism of Muscle and Adipose Tissues Induced by Tumour Products and Chemotherapy. Proc. Nutr. Soc. 2018, 77, 394–402. [Google Scholar] [CrossRef]
- Halle, J.L.; Counts, B.R.; Zhang, Q.; Carson, J.A. Short Duration Treadmill Exercise Improves Physical Function and Skeletal Muscle Mitochondria Protein Expression after Recovery from <scp>FOLFOX</Scp> Chemotherapy in Male Mice. FASEB J. 2022, 36, e22437. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Cardaci, T.D.; Madero, S.S.; McDonald, S.J.; Bullard, B.M.; Price, R.L.; Carson, J.A.; Fan, D.; Murphy, E.A. 5-Fluorouracil Disrupts Skeletal Muscle Immune Cells and Impairs Skeletal Muscle Repair and Remodeling. J. Appl. Physiol. 2022, 133, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, K.C.; Hamilton-Reeves, J.M.; Baracos, V.E. Current Therapeutic Targets in Cancer Cachexia: A Pathophysiologic Approach. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389942. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Naumann, P.; Eberlein, J.; Farnia, B.; Liermann, J.; Hackert, T.; Debus, J.; Combs, S.E. Cachectic Body Composition and Inflammatory Markers Portend a Poor Prognosis in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers 2019, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Okayama, T.; Aoyama, T.; Ohashi, T.; Masuda, Y.; Kimura, M.; Shiozaki, H.; Murakami, H.; Kenmotsu, H.; Taira, T.; et al. Skeletal Muscle Depletion during Chemotherapy Has a Large Impact on Physical Function in Elderly Japanese Patients with Advanced Non-Small-Cell Lung Cancer. BMC Cancer 2017, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Griffin, O.M.; Duggan, S.N.; Ryan, R.; McDermott, R.; Geoghegan, J.; Conlon, K.C. Characterising the Impact of Body Composition Change during Neoadjuvant Chemotherapy for Pancreatic Cancer. Pancreatology 2019, 19, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Heiligensetzer, M.; Krakowski-Roosen, H.; Büchler, M.W.; Friess, H.; Martignoni, M.E. Cachexia Worsens Prognosis in Patients with Resectable Pancreatic Cancer. J. Gastrointest. Surg. 2008, 12, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the Vicious Circle: Sarcopenia Increases Toxicity, Decreases Response to Chemotherapy and Worsens with Chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar]
- Nemer, L.; Krishna, S.G.; Shah, Z.K.; Conwell, D.L.; Cruz-Monserrate, Z.; Dillhoff, M.; Guttridge, D.C.; Hinton, A.; Manilchuk, A.; Pawlik, T.M.; et al. Predictors of Pancreatic Cancer-Associated Weight Loss and Nutritional Interventions. Pancreas 2017, 46, 1152–1157. [Google Scholar] [CrossRef]
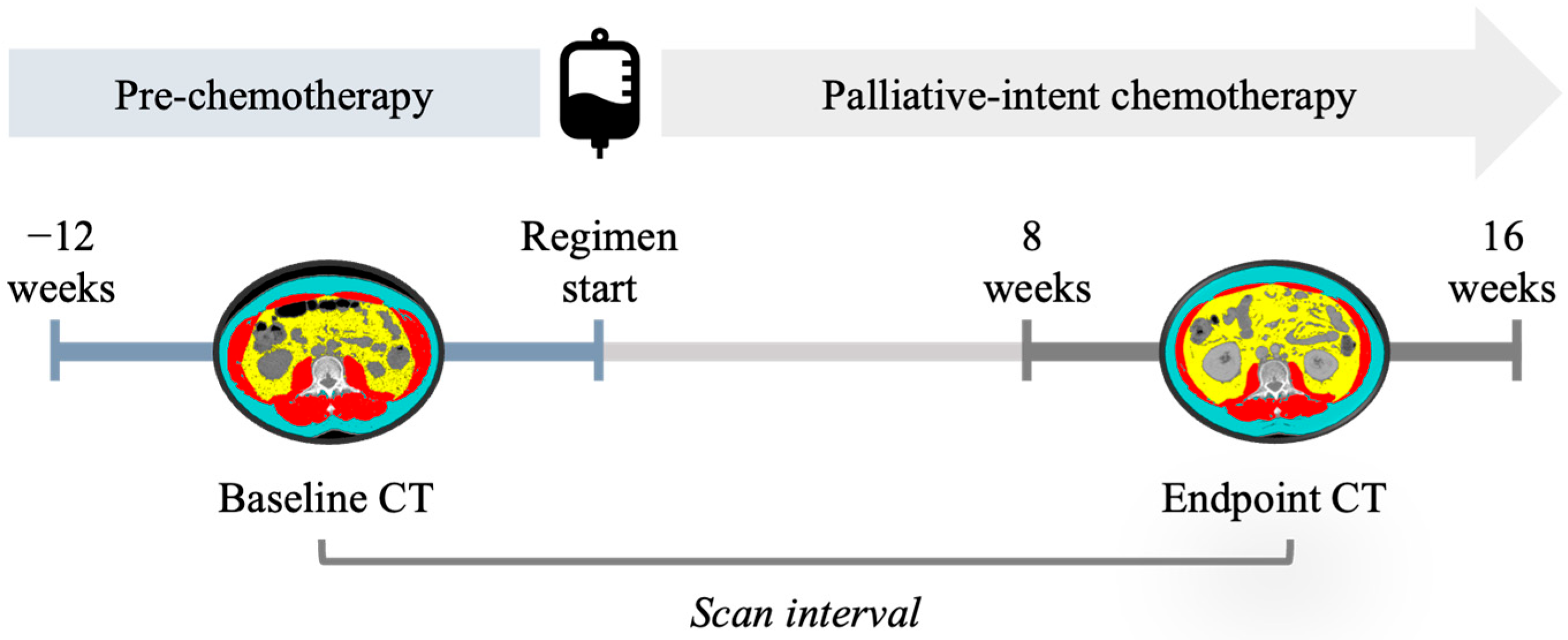

| Overall | FOLFIRINOX | GEM/NAB | |
|---|---|---|---|
| Demographics | |||
| Number of patients, N (% of cohort) | 210 | 90 (42.9) | 120 (57.1) |
| Age, years, median (IQR) | 64 (58, 70) | 61 (57, 66) | 68 (59, 73) * |
| Sex, male | 114 (54.3) | 40 (55.6) | 64 (53.3) |
| Treatment centre, N (%) | |||
| Centre 1 | 106 (50.5) | 44 (48.9) | 62 (51.7) |
| Centre 2 | 104 (49.5) | 46 (51.1) | 58 (48.3) |
| Baseline Disease Characteristics | |||
| Tumour topography, N (%) | |||
| head/neck | 125 (59.5) | 48 (53.3) | 77 (64.2) |
| body/tail | 52 (24.8) | 24 (26.7) | 28 (23.3) |
| overlapping/unspecified | 33 (15.7) | 18 (20.0) | 15 (12.5) |
| Disease stage, N (%) | |||
| Locally advanced, unresectable | 69 (32.9) | 29 (32.2) | 40 (33.3) |
| Metastatic or recurrent | 141 (67.1) | 61 (67.8) | 80 (66.7) |
| Primary tumour, N (%) | |||
| Previously resected | 26 (12.4) | 12 (13.3) | 14 (11.7) |
| Present | 184 (87.6) | 78 (86.7) | 106 (88.3) |
| Baseline Body Composition | |||
| Skeletal muscle index (cm2/m2) | |||
| Male | 49.4 (8.2) | 50.5 (8.9) | 48.5 (7.6) |
| Female | 39.0 (5.9) | 39.1 (6.8) | 39.0 (5.3) |
| Adipose tissue index (cm2/m2) | |||
| Male | 99.6 (52.8) | 91.4 (44.2) | 106.1 (58.2) |
| Female | 115.5 (65.6) | 104.1 (55.0) | 123.7 (71.5) |
| Body mass index (kg/m2) | |||
| Male | 26.2 (4.1) | 25.8 (3.6) | 26.6 (4.4) |
| Female | 25.4 (4.9) | 25.1 (4.7) | 25.6 (5.2) |
| BMI WHO classification, N (%) | |||
| underweight, <18.5 | 6 (2.9) | 2 (2.2) | 4 (3.3) |
| normal weight, 18.5–24.9 | 88 (41.9) | 41 (45.6) | 47 (39.2) |
| overweight, 25.0–29.9 | 80 (38.1) | 35 (38.9) | 45 (37.5) |
| obesity, ≥30.0 | 36 (17.1) | 12 (13.3) | 24 (20.0) |
| Clinical Outcomes | |||
| Tumour response at endpoint CT, N (%) | |||
| Tumour control | 137 (65.2) | 57 (63.3) | 80 (66.7) |
| Progression | 73 (34.8) | 33 (36.7) | 40 (33.3) |
| Treatment after endpoint CT, N (%) | |||
| No further treatment | 68 (32.4) | 19 (21.1) * | 49 (40.8) |
| Ongoing palliative chemotherapy | 131 (62.4) | 63 (70.0) * | 68 (56.7) |
| Curative resection | 11 (5.2) | 8 (8.9) * | 3 (2.5) |
| Overall survival | |||
| Days (median, 95% CI) | 377 (335, 418) | 409 (342, 476) | 349 (304, 394) |
| Overall | FOLFIRINOX | GEM/NAB | |
|---|---|---|---|
| Baseline CT (days from regimen start) | −33 (22) | −33 (22) | −33 (23) |
| Endpoint CT (days from regimen start) | 83 (16) | 80 (16) | 85 (15) |
| Scan Interval (days) | 116 (27) | 113 (28) | 118 (26) |
| Skeletal Muscle Index Change (cm2/m2) | |||
| Male | −3.9 (5.3) | −5.1 * (4.9) | −3.0 (5.4) |
| Female | −2.3 (3.9) | −2.4 (3.6) | −2.2 (4.1) |
| Skeletal Muscle Relative Change (%) | |||
| Male | −7.8 (10.1) | −10.2 * (9.3) | −5.9 (10.5) |
| Female | −5.4 (9.5) | −5.7 (8.8) | −5.2 (10.1) |
| Adipose Tissue Index Change (cm2/m2) | |||
| Male | −20.4 (28.2) | −12.3 (28.3) | −26.8 * (26.6) |
| Female | −22.8 (26.9) | −15.7 (25.9) | −27.8 * (26.7) |
| Adipose Tissue Relative Change (%) | |||
| Male | −17.5 (35.1) | −11.0 (30.0) | −22.7 (38.1) |
| Female | −18.8 (26.6) | −13.6 (25.8) | −22.6 (26.8) |
| Weight Relative Change (%) | |||
| Male | −3.7 (6.6) | −4.8 (6.8) | −2.7 (6.3) |
| Female | −3.7 (6.3) | −2.6 (5.6) | −4.5 (6.7) |
| (a) Association with Skeletal Muscle Index (SMI) Change | (b) Association with Adipose Tissue Index (ATI) Change | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| Characteristic | β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value |
| Male sex (vs. female) | −2.02 | −3.34, −0.69 | 0.003 | −1.28 | −2.53, −0.03 | 0.044 | 1.35 | −6.02, 8.72 | 0.719 | 3.63 | −3.26, 10.52 | 0.300 |
| Metastatic at baseline (vs. locally advanced) | −1.28 | −2.71, −0.16 | 0.080 | −0.39 | −1.74, 0.95 | 0.562 | −0.92 | −8.76, 6.92 | 0.817 | - | - | - |
| Primary tumour present (vs. previously resected) | −0.89 | −2.94, 1.17 | 0.396 | - | - | - | −10.43 | −21.47, 0.61 | 0.064 | −10.14 | −20.49, 0.20 | 0.055 |
| Head/neck topography (vs. body/tail/unknown) | 0.93 | −0.45, 2.31 | 0.185 | 0.71 | −0.55, 1.98 | 0.267 | -6.24 | −13.67, 1.20 | 0.100 | −5.64 | −12.62, 1.34 | 0.112 |
| FOLFIRINOX (vs. GEM/NAB) | −1.64 | −2.99, −0.29 | <0.001 | −1.58 | −2.82, −0.34 | 0.013 | 12.01 | 5.35, 19.78 | 0.001 | 11.19 | 4.32, 18.06 | 0.002 |
| Tumour progression (vs. control) | −3.57 | −4.91, −2.23 | <0.001 | −3.22 | −4.53, −1.92 | <0.001 | −11.70 | −18.85, −3.75 | 0.001 | −12.39 | −19.55, −5.23 | 0.001 |
| Baseline BMI (per 5 kg/m2) | −1.19 | −1.93, −0.45 | 0.002 | −1.21 | −1.89, −0.52 | 0.001 | −7.39 | −11.35, −3.24 | <0.001 | −6.85 | −10.63, −3.07 | <0.001 |
| Characteristic | (a) Univariable | (b) Multivariable Model 1 | (c) Multivariable Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (per year) | 0.99 | 0.98, 1.01 | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
| Male sex (vs. female) | 1.10 | 0.90, 1.59 | 0.212 | 0.79 | 0.61, 1.13 | 0.132 | 0.88 | 0.65, 1.19 | 0.880 |
| Metastatic (vs. locally advanced) | 1.50 | 1.11, 2.02 | 0.008 | 1.29 | 0.94, 1.80 | 0.119 | 1.28 | 0.92, 1.78 | 0.137 |
| FOLFIRINOX (vs. GEM/NAB) | 0.70 | 0.53, 0.93 | 0.014 | 1.01 | 0.742, 1.38 | 0.942 | 0.94 | 0.69, 1.29 | 0.716 |
| Tumour progression (vs. tumour control) | 3.04 | 2.25, 4.13 | <0.001 | 2.20 | 1.58, 3.04 | <0.001 | 2.14 | 1.53, 2.99 | <0.001 |
| Treatment after endpoint CT: | |||||||||
| no further treatment; | ref | ref | ref | ||||||
| ongoing palliative chemotherapy; | 0.29 | 0.29, 0.39 | <0.001 | 0.35 | 0.25, 0.49 | <0.001 | 0.33 | 0.23, 0.47 | <0.001 |
| curative resection | 0.06 | 0.02, 0.14 | <0.001 | 0.07 | 0.02, 0.20 | <0.001 | 0.06 | 0.21, 0.19 | <0.001 |
| SMI change (per −2.0 cm2/m2) | 1.09 | 1.05, 1.13 | <0.001 | 1.10 | 1.04, 1.18 | 0.003 | - | - | - |
| ATI change (per −10.0 cm2/m2) | 1.15 | 1.10, 1.19 | <0.001 | 1.10 | 1.03, 1.17 | 0.003 | - | - | - |
| SMI change tertile (cm2/m2) | |||||||||
| ≥−1.0 (T3, gain/maintenance/mild loss) | ref | - | - | - | ref | ||||
| −1.1 to −4.2 (T2, moderate loss) | 1.26 | 0.88, 1.77 | 0.199 | - | - | - | 1.29 | 0.90, 1.85 | 0.168 |
| ≤−4.3 (T1, severe loss) | 2.20 | 1.56, 3.11 | <0.001 | - | - | - | 1.72 | 1.16, 2.57 | 0.007 |
| ATI change tertile (cm2/m2) | |||||||||
| ≥−7.6 (T3, gain or mild loss) | ref | - | - | - | ref | ||||
| −7.7 to −27.6 (T2, moderate loss) | 1.55 | 1.09, 2.21 | 0.014 | - | - | - | 1.08 | 0.73, 1.59 | 0.696 |
| ≤−27.7 (T1, severe loss) | 3.01 | 2.10, 4.30 | <0.001 | - | - | - | 1.73 | 1.13, 2.66 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klassen, P.N.; Baracos, V.; Ghosh, S.; Martin, L.; Sawyer, M.B.; Mazurak, V.C. Muscle and Adipose Wasting despite Disease Control: Unaddressed Side Effects of Palliative Chemotherapy for Pancreatic Cancer. Cancers 2023, 15, 4368. https://doi.org/10.3390/cancers15174368
Klassen PN, Baracos V, Ghosh S, Martin L, Sawyer MB, Mazurak VC. Muscle and Adipose Wasting despite Disease Control: Unaddressed Side Effects of Palliative Chemotherapy for Pancreatic Cancer. Cancers. 2023; 15(17):4368. https://doi.org/10.3390/cancers15174368
Chicago/Turabian StyleKlassen, Pamela N., Vickie Baracos, Sunita Ghosh, Lisa Martin, Michael B. Sawyer, and Vera C. Mazurak. 2023. "Muscle and Adipose Wasting despite Disease Control: Unaddressed Side Effects of Palliative Chemotherapy for Pancreatic Cancer" Cancers 15, no. 17: 4368. https://doi.org/10.3390/cancers15174368
APA StyleKlassen, P. N., Baracos, V., Ghosh, S., Martin, L., Sawyer, M. B., & Mazurak, V. C. (2023). Muscle and Adipose Wasting despite Disease Control: Unaddressed Side Effects of Palliative Chemotherapy for Pancreatic Cancer. Cancers, 15(17), 4368. https://doi.org/10.3390/cancers15174368






