Sex Differences in Vestibular Schwannoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Demographics and Patient Characteristics
2.2. Health-Related Quality of Life Questionnaires
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Sex Differences in General and Disease-Related QoL: SF-36 and PANQOL
3.3. Symptom-Specific Sex Differences: HHI, THI, DHI and FDI
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Aboh, I.V.; Chisci, G.; Cascino, F.; Parigi, S.; Gennaro, P.; Gabriele, G.; Iannetti, G. Giant palatal schwannoma. J. Craniofac. Surg. 2014, 25, e418–e420. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Singh, V.; Rohilla, S.; Sharma, B. Trigeminal schwannoma. Natl. J. Maxillofac. Surg. 2017, 8, 149. [Google Scholar] [CrossRef]
- Harazono, Y.; Kayamori, K.; Sakamoto, J.; Akaike, Y.; Kurasawa, Y.; Tsushima, F.; Sasaki, Y.; Harada, H.; Yoda, T. Retrospective analysis of schwannoma in the oral and maxillofacial region: Clinicopathological characteristics and specific pathology of ancient change. Br. J. Oral Maxillofac. Surg. 2022, 60, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, K.S.; Lund-Johansen, M.; Nordahl, S.H.G.; Finnkirk, M.; Goplen, F.K. Long-term Effects of Conservative Management of Vestibular Schwannoma on Dizziness, Balance, and Caloric Function. Otolaryngol.-Head Neck Surg. 2019, 161, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Sughrue, M.E.; Kane, A.J.; Kaur, R.; Barry, J.J.; Rutkowski, M.J.; Pitts, L.H.; Cheung, S.W.; Parsa, A.T. A prospective study of hearing preservation in untreated vestibular schwannomas: Clinical article. J. Neurosurg. 2011, 114, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Sughrue, M.E.; Yang, I.; Aranda, D.; Lobo, K.; Pitts, L.H.; Cheung, S.W.; Parsa, A.T. The natural history of untreated sporadic vestibular schwannomas: A comprehensive review of hearing outcomes—Clinical article. J. Neurosurg. 2010, 112, 163–167. [Google Scholar] [CrossRef]
- Stangerup, S.E.; Caye-Thomasen, P. Epidemiology and Natural History of Vestibular Schwannomas. Otolaryngol. Clin. N. Am. 2012, 45, 257–268. [Google Scholar] [CrossRef]
- Reznitsky, M.; Petersen, M.M.B.S.; West, N.; Stangerup, S.E.; Cayé-Thomasen, P. Epidemiology and Diagnostic Characteristics of Vestibular Schwannomas-Does Gender Matter? Otol. Neurotol. 2020, 41, e1372–e1378. [Google Scholar] [CrossRef]
- Marinelli, J.P.; Lohse, C.M.; Carlson, M.L. Incidence of Vestibular Schwannoma over the Past Half-Century: A Population-Based Study of Olmsted County, Minnesota. Otolaryngol.-Head Neck Surg. 2018, 159, 717–723. [Google Scholar] [CrossRef]
- Brown, C.M.; Ahmad, Z.K.; Ryan, A.F.; Doherty, J.K. Estrogen receptor expression in sporadic vestibular schwannomas. Otol. Neurotol. 2011, 32, 158–162. [Google Scholar] [CrossRef]
- Kachhara, R.; Chandrika Devi, C.G.; Nair, S.; Bhattacharya, R.N.; Radhakrishnan, V.V. Acoustic neurinomas during pregnancy: Report of two cases and review of literature. Acta Neurochir. 2001, 143, 587–591. [Google Scholar] [CrossRef]
- Jaiswal, S.; Agrawal, V.; Jaiswal, A.K.; Pandey, R.; Mahapatra, A.K. Expression of estrogen and progesterone receptors in vestibular schwannomas and their clinical significance. J. Negat. Results Biomed. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Dalgorf, D.M.; Rowsell, C.; Bilbao, J.M.; Chen, J.M. Immunohistochemical investigation of hormone receptors and vascular endothelial growth factor concentration in vestibular schwannoma. Skull Base 2008, 18, 377–384. [Google Scholar] [CrossRef][Green Version]
- Goldbrunner, R.; Weller, M.; Regis, J.; Lund-Johansen, M.; Stavrinou, P.; Reuss, D.; Evans, D.G.; Lefranc, F.; Sallabanda, K.; Falini, A.; et al. Eano guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-oncology 2020, 22, 31–45. [Google Scholar] [CrossRef]
- Sergi, B.; Settimi, S.; Federici, G.; Galloni, C.; Cantaffa, C.; De Corso, E.; Lucidi, D. Factors Influencing Personalized Management of Vestibular Schwannoma: A Systematic Review. J. Pers. Med. 2022, 12, 1616. [Google Scholar] [CrossRef] [PubMed]
- Vorasubin, N.; Alexander, T.H.; Mastrodimos, B.; Cueva, R.A. Factors that affect length of hospital stay after vestibular schwannoma surgery. Otol. Neurotol. 2018, 39, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Rosahl, S.; Bohr, C.; Lell, M.; Hamm, K.; Iro, H. Diagnostics and therapy of vestibular schwannomas—An interdisciplinary challenge. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2017, 16, Doc03. [Google Scholar] [CrossRef]
- Feigl, G.C.; Schebesch, K.M.; Rochon, J.; Warnat, J.; Woertgen, C.; Schmidt, S.; Lange, M.; Schlaier, J.; Brawanski, A.T. Analysis of risk factors influencing the development of severe dizziness in patients with vestibular schwannomas in the immediate postoperative phase. Clin. Neurol. Neurosurg. 2011, 113, 52–56. [Google Scholar] [CrossRef]
- Cao, W.; Hou, Z.; Wang, F.; Jiang, Q.; Shen, W.; Yang, S. Larger tumor size and female gender suggest better tinnitus prognosis after surgical treatment in vestibular schwannoma patients with tinnitus. Acta Otolaryngol. 2020, 140, 373–377. [Google Scholar] [CrossRef]
- Naros, G.; Sandritter, J.; Liebsch, M.; Ofori, A.; Rizk, A.R.; Del Moro, G.; Ebner, F.; Tatagiba, M. Predictors of preoperative tinnitus in unilateral sporadic vestibular schwannoma. Front. Neurol. 2017, 8, 378. [Google Scholar] [CrossRef]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol.-Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
- Erickson, N.J.; Schmalz, P.G.R.; Agee, B.S.; Fort, M.; Walters, B.C.; McGrew, B.M.; Fisher, W.S. Koos Classification of Vestibular Schwannomas: A Reliability Study. Clin. Neurosurg. 2019, 85, 409–414. [Google Scholar] [CrossRef]
- Machetanz, K.; Lee, L.; Wang, S.S.; Tatagiba, M.; Naros, G. Trading mental and physical health in vestibular schwannoma treatment decision. Front. Oncol. 2023, 13, 1152833. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Gandek, B.; Kosinski, M.; Aaronson, N.K.; Apolone, G.; Brazier, J.; Bullinger, M.; Kaasa, S.; Leplège, A.; Prieto, L.; et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: Results from the IQOLA Project. J. Clin. Epidemiol. 1998, 51, 1167–1170. [Google Scholar] [CrossRef]
- Baggio, G.; Corsini, A.; Floreani, A.; Giannini, S.; Zagonel, V. Gender medicine: A task for the third millennium. Clin. Chem. Lab. Med. 2013, 51, 713–727. [Google Scholar] [PubMed]
- Editorial: Putting gender on the agenda. Nature 2010, 465, 665. [CrossRef] [PubMed][Green Version]
- Carlson, M.L.; Tveiten, O.V.; Driscoll, C.L.; Goplen, F.K.; Neff, B.A.; Pollock, B.E.; Tombers, N.M.; Castner, M.L.; Finnkirk, M.K.; Myrseth, E.; et al. Long-term quality of life in patients with vestibular schwannoma: An international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J. Neurosurg. 2015, 122, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Hülse, R.; Biesdorf, A.; Hörmann, K.; Stuck, B.; Erhart, M.; Hülse, M.; Wenzel, A. Peripheral Vestibular Disorders: An Epidemiologic Survey in 70 Million Individuals. Otol. Neurotol. 2019, 40, 88–95. [Google Scholar] [CrossRef]
- Mucci, V.; Hamid, M.; Jacquemyn, Y.; Browne, C.J. Influence of sex hormones on vestibular disorders. Curr. Opin. Neurol. 2022, 35, 135–141. [Google Scholar] [CrossRef]
- Orendorz-Frączkowska, K.; Temporale, H. Organ of hearing and balance in peri- And postmenopausal women. Effects of hormone replacement therapy on hearing and balance in peri- And post-menopausal women- And current state of knowledge. Adv. Clin. Exp. Med. 2020, 29, 751–755. [Google Scholar] [CrossRef]
- Falcioni, M.; Fois, P.; Taibah, A.; Sanna, M. Facial nerve function after vestibular schwannoma surgery: Clinical article. J. Neurosurg. 2011, 115, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Matthies, C.; Samii, M. Management of 1000 vestibular schwannomas (acoustic neuromas): Clinical presentation. Neurosurgery 1997, 40, 1–9; discussion 9–10. [Google Scholar] [PubMed]
- Ebner, F.H.; Tatagiba, M. Update on diagnostics and microsurgical treatment of vestibular schwannoma. Nervenarzt 2019, 90, 578–586. [Google Scholar]
- Hotton, M.; Huggons, E.; Hamlet, C.; Shore, D.; Johnson, D.; Norris, J.H.; Kilcoyne, S.; Dalton, L. The psychosocial impact of facial palsy: A systematic review. Br. J. Health Psychol. 2020, 25, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Nellis, J.C.; Ishii, M.; Byrne, P.J.; Boahene, K.D.O.; Dey, J.K.; Ishii, L.E. Association among facial paralysis, depression, and quality of life in facial plastic surgery patients. JAMA Facial Plast. Surg. 2017, 19, 190–196. [Google Scholar] [CrossRef]
- Cross, T.; Sheard, C.E.; Garrud, P.; Nikolopoulos, T.P.; O’Donoghue, G.M. Impact of facial paralysis on patients with acoustic neuroma. Laryngoscope 2000, 110, 1539–1542. [Google Scholar] [CrossRef]
- Lazarus, R.S. Stress, Appraisal, and Coping—Richard S. Lazarus, PhD, Susan Folkman, PhD—Google Books. Available online: https://books.google.com.gh/books?hl=en&lr=&id=i-ySQQuUpr8C&oi=fnd&pg=PR5&dq=Stress,+Appraisal+and+Coping&ots=DgIOlnjdU9&sig=sYA22n-S60dvW8x5QjaEeEZ7p1U&redir_esc=y#v=onepage&q=Stress%2C%20Appraisal%20and%20Coping&f=true (accessed on 14 February 2023).
- Matud, M.P. Gender differences in stress and coping styles. Pers. Individ. Differ. 2004, 37, 1401–1415. [Google Scholar] [CrossRef]
- Ben-Zur, H.; Zeidner, M. Gender differences in coping reactions under community crisis and daily routine conditions. Pers. Individ. Differ. 1996, 20, 331–340. [Google Scholar]
- Liang, S.Y.; Liu, H.C.; Lu, Y.Y.; Wu, S.F.; Chien, C.H.; Tsay, S.L. The Influence of Resilience on the Coping Strategies in Patients with Primary Brain Tumors. Asian Nurs. Res. 2020, 14, 50–55. [Google Scholar] [CrossRef]
- Siemann, I.; Kleiss, I.; Beurskens, C.; Custers, J.; Kwakkenbos, L. ‘Everybody is watching me’: A closer look at anxiety in people with facial palsy. J. Plast. Reconstr. Aesthetic Surg. 2023, 77, 408–415. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eshleman, S.; Wittchen, H.U.; Kendler, K.S. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States: Results from the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. Psychopathology in women and men: Focus on female hormones. Am. J. Psychiatry 1997, 154, 1641–1647. [Google Scholar]
- Mainio, A.; Hakko, H.; Niemelä, A.; Tuurinkoski, T.; Koivukangas, J.; Räsänen, P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Niemelä, A.; Koivukangas, J.; Herva, R.; Hakko, H.; Räsänen, P.; Mainio, A. Gender Difference in Quality of Life among Brain Tumor Survivors. J. Neurol. Neurophysiol. 2011, 2, 2155–9562. [Google Scholar] [CrossRef]
- Zimmerberg, B.; Farley, M.J. Sex differences in anxiety behavior in rats: Role of gonadal hormones. Physiol. Behav. 1993, 54, 1119–1124. [Google Scholar] [CrossRef]
- Kalasauskas, D.; Keric, N.; Ajaj, S.A.; von Cube, L.; Ringel, F.; Renovanz, M. Psychological burden in meningioma patients under a wait-and-watch strategy and after complete resection is high—Results of a prospective single center study. Cancers 2020, 12, 3503. [Google Scholar] [CrossRef]
- Goebel, S.; Mehdorn, H.M. Development of anxiety and depression in patients with benign intracranial meningiomas: A prospective long-term study. Support. Care Cancer 2013, 21, 1365–1372. [Google Scholar]
 indicates significant differences between genders. Blue lines: male; orange lines: female.
indicates significant differences between genders. Blue lines: male; orange lines: female.
 indicates significant differences between genders. Blue lines: male; orange lines: female.
indicates significant differences between genders. Blue lines: male; orange lines: female.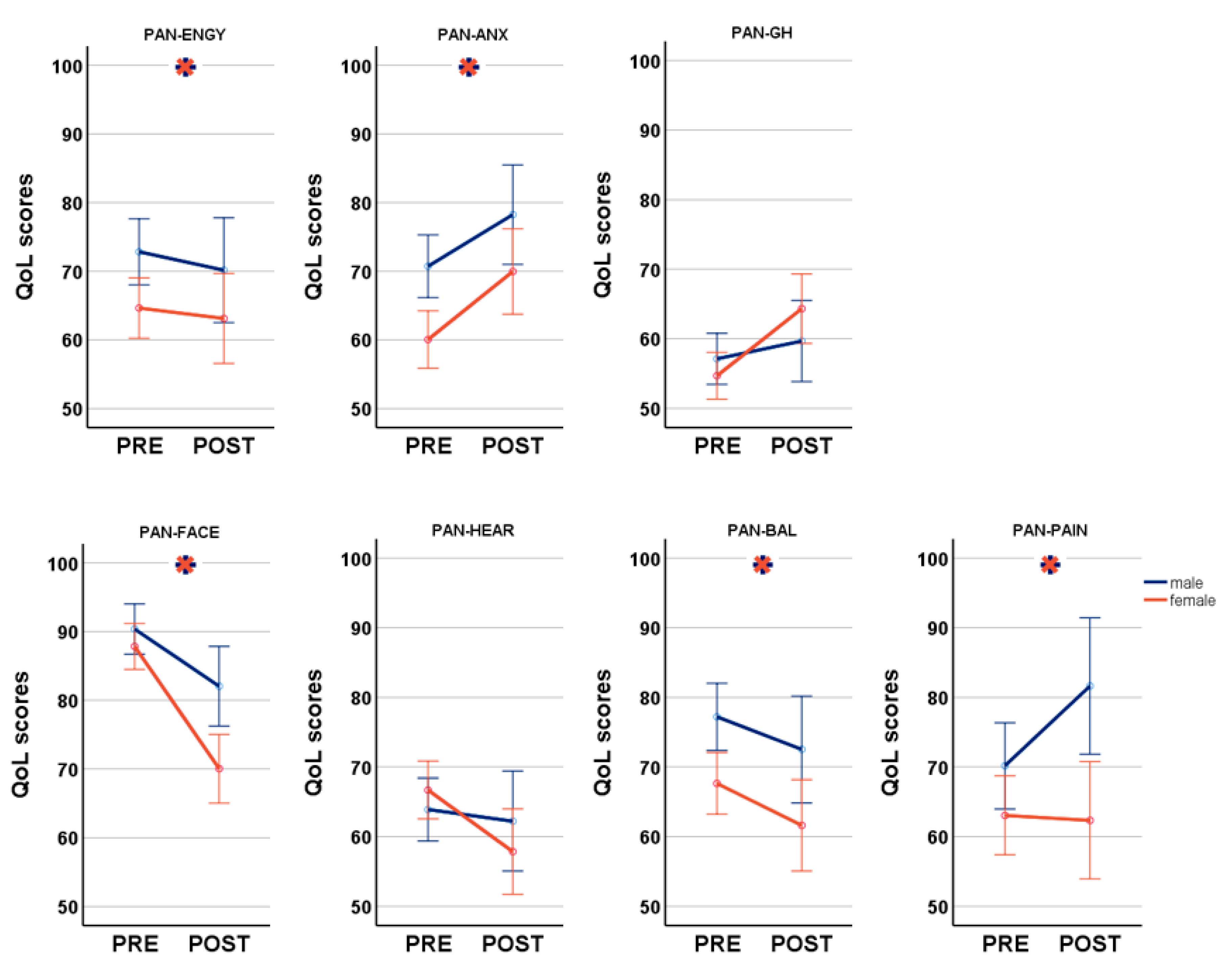
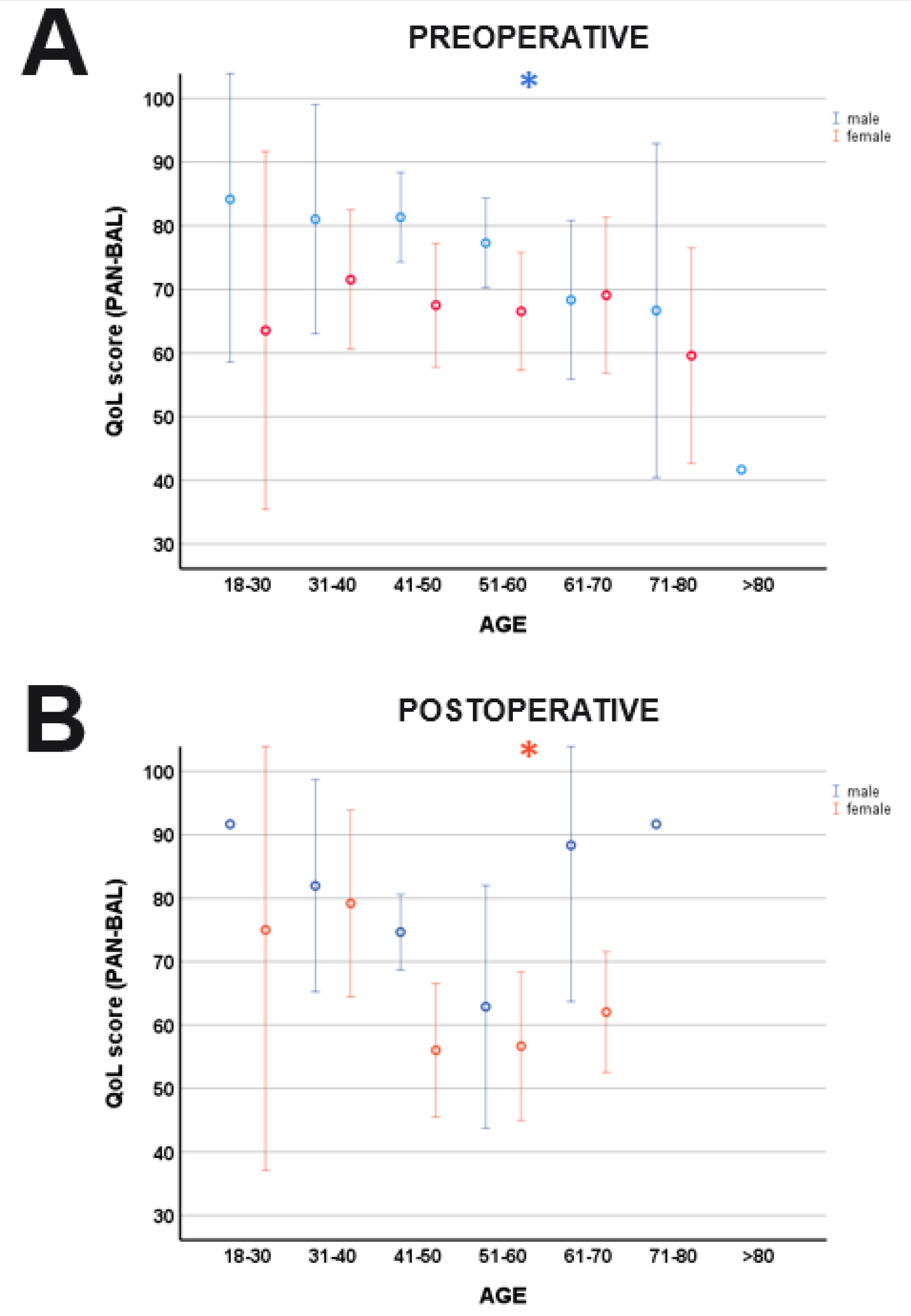
 indicates significant differences between genders. Blue lines: male; orange lines: female.
indicates significant differences between genders. Blue lines: male; orange lines: female.
 indicates significant differences between genders. Blue lines: male; orange lines: female.
indicates significant differences between genders. Blue lines: male; orange lines: female.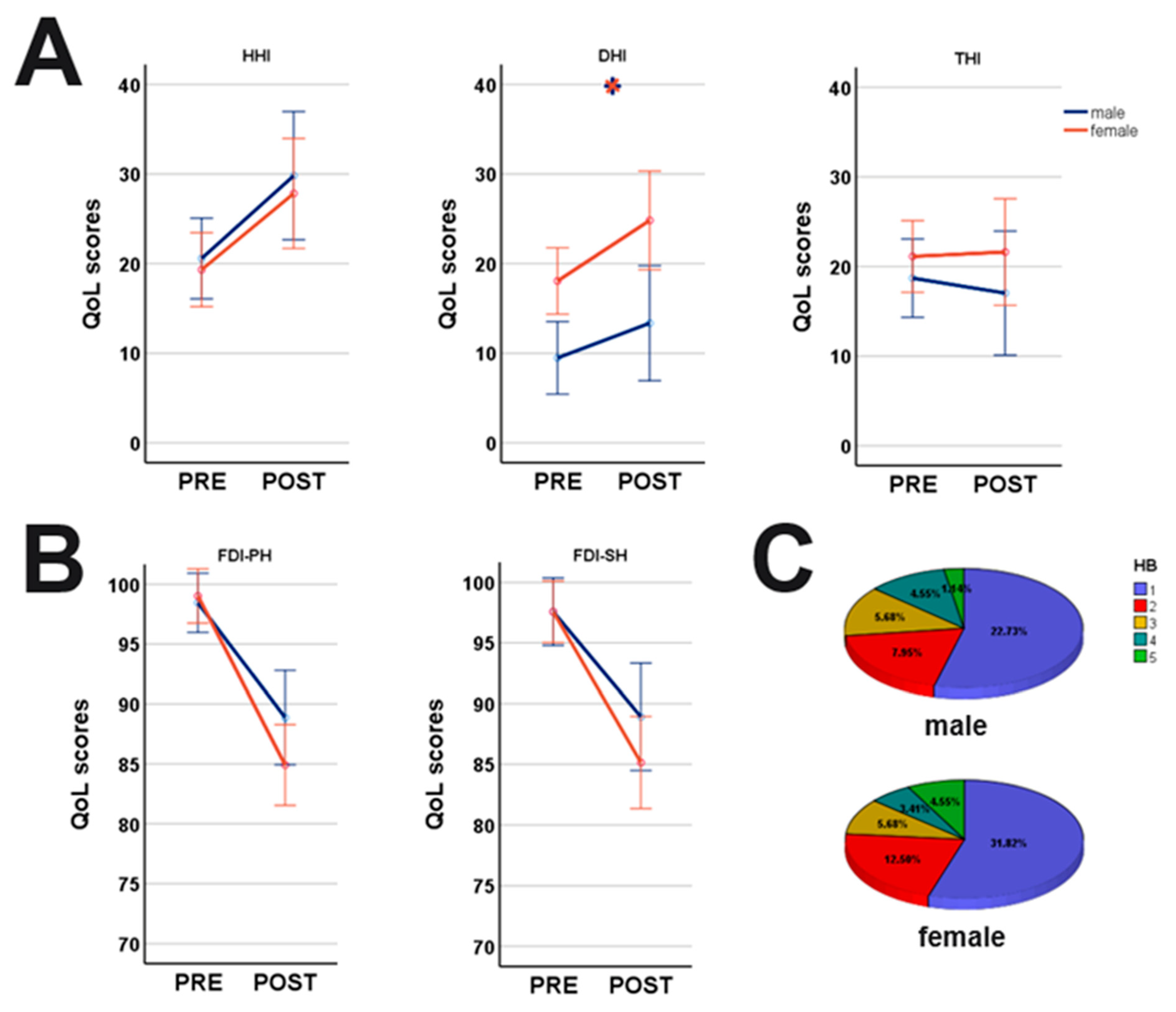
| Total Cohort n = 260 | Preoperative Quest. n = 200 | Postoperative Quest. n = 88 | |||||
|---|---|---|---|---|---|---|---|
| 112 Male/ 148 Female | Male n = 91 | Female n = 109 | Male n = 37 | Female n = 51 | |||
| Age | 52.60 +/− 12.7 | 53.0 +/− 12.4 | 50.59 +/− 11.7 | 50.43 +/− 11.6 | |||
| Koos 1 2 3 4 | 43 (16.5%) 76 (29.2%) 81 (31.2%) 60 (23.1%) | 16 (17.6%) 36 (39.6%) 26 (28.6%) 13 (14.3%) | 26 (23.9%) 31 (28.4%) 33 (30.3%) 19 (17.4%) | H = 0.02 p = 0.892 | 1 (2.7%) 5 (13.5%) 16 (43.2%) 15 (40.5%) | 1 (2.0%) 9 (17.6%) 20 (39.2%) 21 (41.2%) | H = 0.01 p = 0.913 |
| Side Left/ Right | 137 (52.7%) 123 (47.3%) | 56 (61.5%) 35 (38.5%) | 55 (50.5%) 54 (49.5%) | X2 = 2.47 p = 0.116 | 18 (48.6%) 19 (51.4%) | 21 (41.2%) 30 (58.8%) | X2 = 0.49 p = 0.486 |
| Operation Yes No | 165 (63.5%) 95 (36.5%) | 45 (49.5%) 46 (50.5%) | 50 (45.9%) 59 (54.1%) | X2 = 0.04 p = 0.847 | 37 (100%) 0 | 51 (100%) 0 | X2 = 0 p = 1 |
| TSD/TSS | 1.23 +/− 2.3 y | 1.16 +/− 2.2 y | H = 0.25 p = 0.615 | 1.48 +/− 2.1 y | 2.37 +/− 3.1 | H = 0.14 p = 0.137 | |
| HB I II III IV V | 89 (97.8%) 2 (2.2%) 0 0 0 | 104 (95.4%) 5 (4.6%) 0 0 0 | H = 83 p = 0.361 | 20 (54.1%) 7 (18.9%) 5 (13.5%) 4 (10.8%) 1 (2.7%) | 28 (54.9%) 11 (21.6%) 5 (9.8%) 3 (5.9%) 4 (7.8%) | H = 0.01 p = 0.930 | |
| EOR GTR STR PR | 64 (72.7%) 19 (21.6%) 5 (5.7%) | - - - | - - - | 25 (67.6%) 8 (21.6%) 4 (10.8%) | 39 (76.5%) 11 (21.6%) 1 (2.0%) | X2 = 3.19 p = 0.203 | |
| SF-36 physical function role physical bodily pain general health vitality social function role emotional mental health | 89.78 ± 17.7 75.27 ± 36.6 76.45 ± 27.8 63.87 ± 17.5 58.52 ± 19.9 77.20 ± 25.2 73.35 ± 40.1 69.71 ± 19.3 | 81.06 +/− 24.2 64.45 +/− 42.3 66.58 +/− 30.8 59.73 +/− 17.6 52.16 +/− 20.5 70.64 +/− 25.8 65.14 +/− 41.7 64.62 +/− 17.9 | p = 0.001 * p = 0.067 p = 0.023 * p = 0.097 p = 0.032 * p = 0.053 p = 0.089 p = 0.043 * | 86.89 +/− 16.6 72.16 +/− 33.6 80.46 +/− 27.0 66.08 +/− 19.6 59.65 +/− 16.7 78.11 +/− 21.9 84.01 +/− 29.9 75.89 +/− 18.7 | 78.76 +/− 21.7 60.00 +/− 41.2 71.57 +/− 30.2 65.24 +/− 19.2 55.53 +/− 24.0 73.97 +/− 26.7 70.59 +/− 43.5 69.65 +/− 20.5 | p = 0.079 p = 0.256 p = 0.132 p = 0.879 p = 0.425 p = 0.579 p = 0.251 p = 0.129 | |
| PANQOL anxiety facial general health balance hearing energy pain Total | 70.54 ± 22.9 90.57 ± 14.2 56.32 ± 19.0 76.69 ± 22.0 63.46 ± 22.5 71.98 ± 21.5 70.05 ± 28.9 71.37 ± 15.6 | 59.98 +/− 22.1 87.92 +/− 14.3 54.0 +/− 15.3 67.05 +/− 25.7 66.17 +/− 21.0 63.80 +/− 24.2 63.07 +/− 30.2 66.10 +/− 14.6 | p = 0.001 * p = 0.129 p = 0.267 p = 0.008 * p = 0.332 p = 0.015 * p = 0.086 p = 0.006 * | 78.55 +/− 22.8 81.67 +/− 20.6 61.49 +/− 19.6 73.76 +/− 22.2 63.34 +/− 18.0 72.07 +/− 20.5 81.76 +/− 24.0 73.21 +/− 14.3 | 70.21 +/− 19.4 69.73 +/− 25.9 66.10 +/− 19.2 62.91 +/− 21.7 58.99 +/− 24.9 64.99 +/− 25.6 62.42 +/− 33.4 64.29 +/− 17.8 | p = 0.027 * p = 0.027 * p = 0.420 p = 0.008 * p = 0.468 p = 0.242 p = 0.006 * p = 0.016 * | |
| DHI | 9.65 ± 16.3 | 18.26 +/− 20.8 | p = 0.001 * | 12.92 +/− 16.7 | 24.39 +/− 22.7 | p = 0.011 * | |
| THI | 19.41 ± 21.1 | 21.71 +/− 21.5 | p = 0.431 | 15.51 +/− 18.1 | 20.20 +/− 22.3 | p = 0.432 | |
| HHI | 20.59 ± 20.9 | 19.43 +/− 22.2 | p = 0.473 | 29.68 +/−19.8 | 27.65 +/−23.1 | p = 0.475 | |
| FDI physical function social function | 98.74 ± 10.5 97.89 ± 12.1 | 99.27 +/− 3.3 97.87 +/− 8.1 | p = 0.319 p = 0.402 | 88.24 +/−16.5 88.22 +/−16.3 | 84.31 +/− 19.6 84.47 +/− 20.4 | p = 0.382 p = 0.491 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machetanz, K.; Wang, S.S.; Oberle, L.; Tatagiba, M.; Naros, G. Sex Differences in Vestibular Schwannoma. Cancers 2023, 15, 4365. https://doi.org/10.3390/cancers15174365
Machetanz K, Wang SS, Oberle L, Tatagiba M, Naros G. Sex Differences in Vestibular Schwannoma. Cancers. 2023; 15(17):4365. https://doi.org/10.3390/cancers15174365
Chicago/Turabian StyleMachetanz, Kathrin, Sophie S. Wang, Linda Oberle, Marcos Tatagiba, and Georgios Naros. 2023. "Sex Differences in Vestibular Schwannoma" Cancers 15, no. 17: 4365. https://doi.org/10.3390/cancers15174365
APA StyleMachetanz, K., Wang, S. S., Oberle, L., Tatagiba, M., & Naros, G. (2023). Sex Differences in Vestibular Schwannoma. Cancers, 15(17), 4365. https://doi.org/10.3390/cancers15174365






