Recruitment and Retention Strategies Used in Dietary Randomized Controlled Interventions with Cancer Survivors: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
Aims
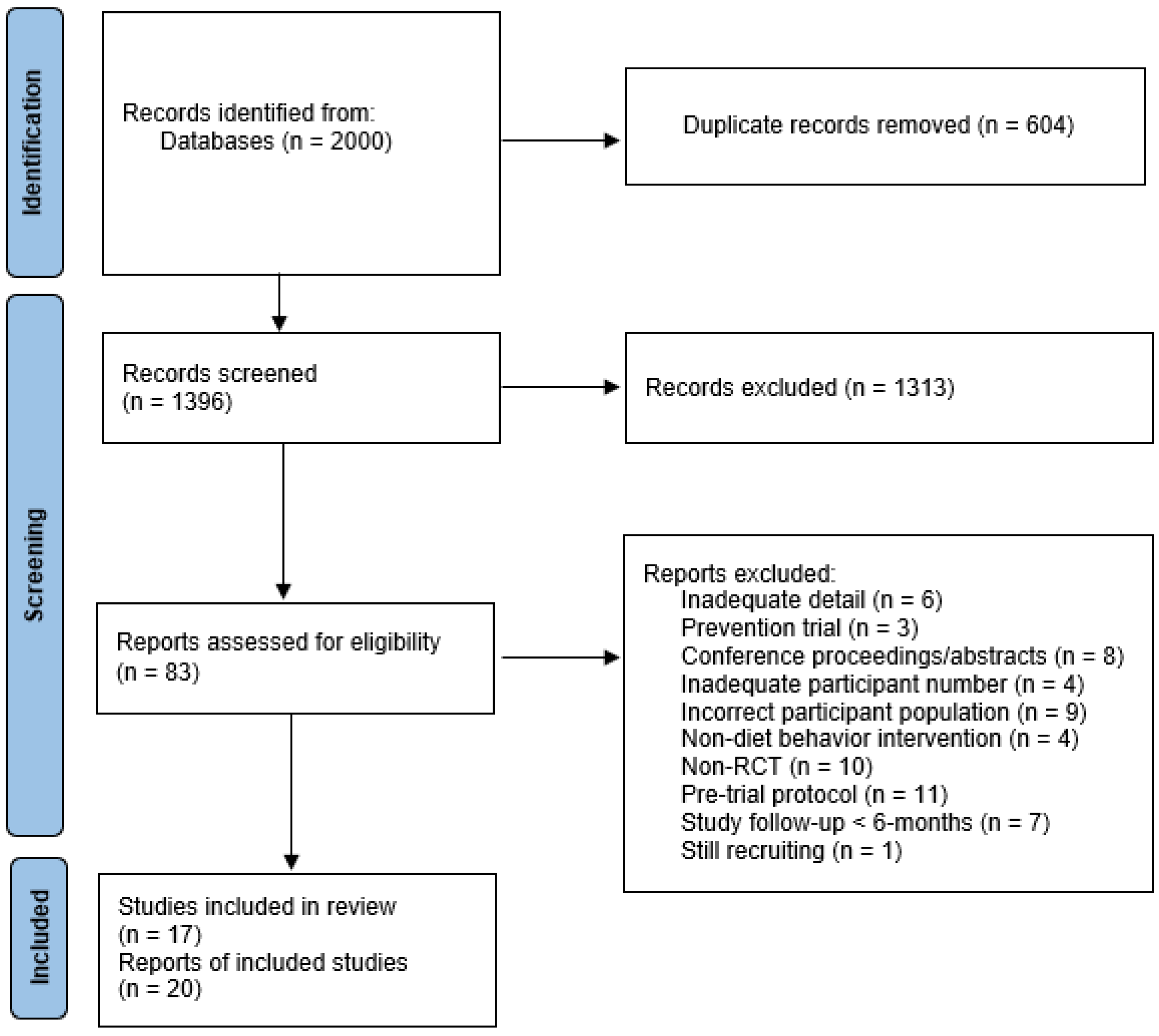
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
3. Results
3.1. Study and Participant Characteristics
3.2. Recruitment Results
3.3. Retention Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and Physical Activity Guidelines for Cancer Survivors. CA Cancer J. Clin. 2012, 62, 242–274. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of Red Meat and Processed Meat and Cancer Incidence: A Systematic Review and Meta-Analysis of Prospective Studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society Nutrition and Physical Activity Guideline for Cancer Survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Green, A.C.; Hayman, L.L.; Cooley, M.E. Multiple Health Behavior Change in Adults with or at Risk for Cancer: A Systematic Review. Am. J. Health Behav. 2015, 39, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.N.; Mosher, C.E.; Blair, C.K.; Snyder, D.C.; Sloane, R.; Demark-Wahnefried, W. Cancer Survivors’ Uptake and Adherence in Diet and Exercise Intervention Trials: An Integrative Data Analysis. Cancer 2015, 121, 77–83. [Google Scholar] [CrossRef]
- Pekmezi, D.W.; Demark-Wahnefried, W. Updated Evidence in Support of Diet and Exercise Interventions in Cancer Survivors. Acta Oncol. 2011, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Terranova, C.O.; Winkler, E.A.H.; Healy, G.N.; Demark-Wahnefried, W.; Eakin, E.G.; Reeves, M.M. Dietary and Physical Activity Changes and Adherence to WCRF/AICR Cancer Prevention Recommendations Following a Remotely Delivered Weight Loss Intervention for Female Breast Cancer Survivors: The Living Well after Breast Cancer Randomized Controlled Trial. J. Acad. Nutr. Diet. 2022, 122, 1644–1664.e7. [Google Scholar] [CrossRef]
- Roberts, A.L.; Fisher, A.; Smith, L.; Heinrich, M.; Potts, H.W.W. Digital Health Behaviour Change Interventions Targeting Physical Activity and Diet in Cancer Survivors: A Systematic Review and Meta-Analysis. J. Cancer Surviv. 2017, 11, 704–719. [Google Scholar] [CrossRef]
- Hofseth, L.J. Getting Rigorous with Scientific Rigor. Carcinogenesis 2018, 39, 21–25. [Google Scholar] [CrossRef]
- Gul, R.B.; Ali, P.A. Clinical Trials: The Challenge of Recruitment and Retention of Participants. J. Clin. Nurs. 2010, 19, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, P.W.; Salzer, M.S. The Conflict between Random Assignment and Treatment Preference: Implications for Internal Validity. Eval. Program. Plan. 2003, 26, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Torgerson, D.J.; Hewitt, C.E. Reporting Attrition in Randomised Controlled Trials. BMJ 2006, 332, 969–971. [Google Scholar] [CrossRef]
- Probstfield, J.L.; Frye, R.L. Strategies for Recruitment and Retention of Participants in Clinical Trials. JAMA 2011, 306, 1798–1799. [Google Scholar] [CrossRef]
- Feathers, A.; Aycinena, A.C.; Lovasi, G.S.; Rundle, A.; Gaffney, A.O.; Richardson, J.; Hershman, D.; Koch, P.; Contento, I.; Greenlee, H. Food Environments Are Relevant to Recruitment and Adherence in Dietary Modification Trials. Nutr. Res. 2015, 35, 480–488. [Google Scholar] [CrossRef]
- Aycinena, A.C.; Valdovinos, C.; Crew, K.D.; Tsai, W.Y.; Mata, J.M.; Sandoval, R.; Hershman, D.; Greenlee, H. Barriers to Recruitment and Adherence in a Randomized Controlled Diet and Exercise Weight Loss Intervention Among Minority Breast Cancer Survivors. J. Immigr. Minor. Health 2017, 19, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.B.; Snyder, D.C.; Demark-Wahnefried, W. Lifestyle Interventions in Cancer Survivors: Designing Programs That Meet the Needs of This Vulnerable and Growing Population. J. Nutr. 2007, 137, 243S–248S. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-Duplication of Database Search Results for Systematic Reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- Bail, J.R.; Frugé, A.D.; Cases, M.G.; De Los Santos, J.F.; Locher, J.L.; Smith, K.P.; Cantor, A.B.; Cohen, H.J.; Demark-Wahnefried, W. A Home-Based Mentored Vegetable Gardening Intervention Demonstrates Feasibility and Improvements in Physical Activity and Performance among Breast Cancer Survivors. Cancer 2018, 124, 3427–3435. [Google Scholar] [CrossRef]
- Cases, M.G.; Frugé, A.D.; De Los Santos, J.F.; Locher, J.L.; Cantor, A.B.; Smith, K.P.; Glover, T.A.; Cohen, H.J.; Daniel, M.; Morrow, C.D.; et al. Detailed Methods of Two Home-Based Vegetable Gardening Intervention Trials to Improve Diet, Physical Activity, and Quality of Life in Two Different Populations of Cancer Survivors. Contemp. Clin. Trials 2016, 50, 201–212. [Google Scholar] [CrossRef]
- Bruno, E.; Krogh, V.; Gargano, G.; Grioni, S.; Bellegotti, M.; Venturelli, E.; Panico, S.; Santucci de Magistris, M.; Bonanni, B.; Zagallo, E.; et al. Adherence to Dietary Recommendations after One Year of Intervention in Breast Cancer Women: The DIANA-5 Trial. Nutrients 2021, 13, 2990. [Google Scholar] [CrossRef]
- Bernard-Davila, B.; Aycinena, A.C.; Richardson, J.; Gaffney, A.O.; Koch, P.; Contento, I.; Molmenti, C.S.; Alvarez, M.; Hershman, D.; Greenlee, H. Barriers and Facilitators to Recruitment to a Culturally-Based Dietary Intervention among Urban Hispanic Breast Cancer Survivors. J. Racial Ethn. Health Disparities 2015, 2, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Gaffney, A.O.; Aycinena, A.C.; Koch, P.; Contento, I.; Karmally, W.; Richardson, J.M.; Lim, E.; Tsai, W.-Y.; Crew, K.; et al. ¡Cocinar Para Su Salud!: Randomized Controlled Trial of a Culturally Based Dietary Intervention among Hispanic Breast Cancer Survivors. J. Acad. Nutr. Diet. 2015, 115, 709–723.e3. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.F.; Saxton, J.M.; Rosario, D.J. Lifestyle Changes for Improving Disease-Specific Quality of Life in Sedentary Men on Long-Term Androgen-Deprivation Therapy for Advanced Prostate Cancer: A Randomised Controlled Trial. Eur. Urol. 2014, 65, 865–872. [Google Scholar] [CrossRef]
- Chan, J.M.; Van Blarigan, E.L.; Langlais, C.S.; Zhao, S.; Ramsdill, J.W.; Daniel, K.; Macaire, G.; Wang, E.; Paich, K.; Kessler, E.R.; et al. Feasibility and Acceptability of a Remotely Delivered, Web-Based Behavioral Intervention for Men With Prostate Cancer: Four-Arm Randomized Controlled Pilot Trial. J. Med. Internet Res. 2020, 22, e19238. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Segal, R.J.; Vallis, M.; Ligibel, J.A.; Pond, G.R.; Robidoux, A.; Blackburn, G.L.; Findlay, B.; Gralow, J.R.; Mukherjee, S.; et al. Randomized Trial of a Telephone-Based Weight Loss Intervention in Postmenopausal Women with Breast Cancer Receiving Letrozole: The LISA Trial. J. Clin. Oncol. 2014, 32, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.W.; Lee, A.M.; Macfarlane, D.J.; Fong, D.Y.; Leung, S.; Cerin, E.; Chan, W.Y.; Leung, I.P.; Lam, S.H.; Taylor, A.J.; et al. Study Protocol for “Moving Bright, Eating Smart”—A Phase 2 Clinical Trial on the Acceptability and Feasibility of a Diet and Physical Activity Intervention to Prevent Recurrence in Colorectal Cancer Survivors. BMC Public Health 2013, 13, 487. [Google Scholar] [CrossRef]
- Holtdirk, F.; Mehnert, A.; Weiss, M.; Meyer, B.; Watzl, C. Protocol for the Optimune Trial: A Randomized Controlled Trial Evaluating a Novel Internet Intervention for Breast Cancer Survivors. Trials 2020, 21, 117. [Google Scholar] [CrossRef]
- Kanera, I.M.; Bolman, C.A.W.; Willems, R.A.; Mesters, I.; Lechner, L. Lifestyle-Related Effects of the Web-Based Kanker Nazorg Wijzer (Cancer Aftercare Guide) Intervention for Cancer Survivors: A Randomized Controlled Trial. J. Cancer Surviv. 2016, 10, 883–897. [Google Scholar] [CrossRef]
- Mohamad, H.; Ntessalen, M.; Craig, L.C.A.; Clark, J.; Fielding, S.; N’Dow, J.; Heys, S.D.; McNeill, G. A Self-Help Diet and Physical Activity Intervention with Dietetic Support for Weight Management in Men Treated for Prostate Cancer: Pilot Study of the Prostate Cancer Weight Management (PRO-MAN) Randomised Controlled Trial. Br. J. Nutr. 2019, 122, 592–600. [Google Scholar] [CrossRef]
- O’Neill, R.F.; Haseen, F.; Murray, L.J.; O’Sullivan, J.M.; Cantwell, M.M. A Randomised Controlled Trial to Evaluate the Efficacy of a 6-Month Dietary and Physical Activity Intervention for Patients Receiving Androgen Deprivation Therapy for Prostate Cancer. J. Cancer Surviv. 2015, 9, 431–440. [Google Scholar] [CrossRef]
- Park, C.L.; Cho, D.; Salner, A.L.; Dornelas, E. A Randomized Controlled Trial of Two Mail-Based Lifestyle Interventions for Breast Cancer Survivors. Support. Care Cancer 2016, 24, 3037–3046. [Google Scholar] [CrossRef]
- Ramirez, A.G.; Parma, D.L.; Muñoz, E.; Mendoza, K.D.; Harb, C.; Holden, A.E.C.; Wargovich, M. An Anti-Inflammatory Dietary Intervention to Reduce Breast Cancer Recurrence Risk: Study Design and Baseline Data. Contemp. Clin. Trials 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Rock, C.L.; Byers, T.E.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J.; Naughton, M.; et al. Reducing Breast Cancer Recurrence with Weight Loss, a Vanguard Trial: The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial. Contemp. Clin. Trials 2013, 34, 282–295. [Google Scholar] [CrossRef]
- Sedjo, R.L.; Flatt, S.W.; Byers, T.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J.; et al. Impact of a Behavioral Weight Loss Intervention on Comorbidities in Overweight and Obese Breast Cancer Survivors. Support. Care Cancer 2016, 24, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Kenfield, S.A.; Chan, J.M.; Van Loon, K.; Paciorek, A.; Zhang, L.; Chan, H.; Savoie, M.B.; Bocobo, A.G.; Liu, V.N.; et al. Feasibility and Acceptability of a Web-Based Dietary Intervention with Text Messages for Colorectal Cancer: A Randomized Pilot Trial. Cancer Epidemiol. Biomark. Prev. 2020, 29, 752–760. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Reeves, M.; Winkler, E.; Mccarthy, N.; Lawler, S.; Terranova, C.; Hayes, S.; Janda, M.; Demark-Wahnefried, W.; Eakin, E. The Living Well after Breast CancerTM Pilot Trial: A Weight Loss Intervention for Women Following Treatment for Breast Cancer. Asia Pac. J. Clin. Oncol. 2017, 13, 125–136. [Google Scholar] [CrossRef]
- Spark, L.C.; Reeves, M.M.; Fjeldsoe, B.S.; Eakin, E.G. Physical Activity and/or Dietary Interventions in Breast Cancer Survivors: A Systematic Review of the Maintenance of Outcomes. J. Cancer Surviv. 2013, 7, 74–82. [Google Scholar] [CrossRef]
- Lawrence, R.A.; McLoone, J.K.; Wakefield, C.E.; Cohn, R.J. Primary Care Physicians’ Perspectives of Their Role in Cancer Care: A Systematic Review. J. Gen. Intern Med. 2016, 31, 1222–1236. [Google Scholar] [CrossRef]
- Regnante, J.M.; Richie, N.A.; Fashoyin-Aje, L.; Vichnin, M.; Ford, M.; Roy, U.B.; Turner, K.; Hall, L.L.; Gonzalez, E.; Esnaola, N.; et al. US Cancer Centers of Excellence Strategies for Increased Inclusion of Racial and Ethnic Minorities in Clinical Trials. JOP 2019, 15, e289–e299. [Google Scholar] [CrossRef] [PubMed]
- Chen Jr, M.S.; Lara, P.N.; Dang, J.H.T.; Paterniti, D.A.; Kelly, K. Twenty Years Post-NIH Revitalization Act: Enhancing Minority Participation in Clinical Trials (EMPaCT): Laying the Groundwork for Improving Minority Clinical Trial Accrual. Cancer 2014, 120, 1091–1096. [Google Scholar] [CrossRef]
- American Cancer Society Cancer Action Network. Barriers to Patient Enrollment in Therapeutic Clinical Trials. 2018. Available online: https://www.fightcancer.org/sites/default/files/National%20Documents/Clinical-Trials-Landscape-Report.pdf (accessed on 30 August 2023).
- Byrd, D.A.; Agurs-Collins, T.; Berrigan, D.; Lee, R.; Thompson, F.E. Racial and Ethnic Differences in Dietary Intake, Physical Activity, and Body Mass Index (BMI) Among Cancer Survivors: 2005 and 2010 National Health Interview Surveys (NHIS). J. Racial Ethn. Health Disparities 2017, 4, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Paxton, R.J.; Holmes, H.; Thanh Nguyen, H.; Elting, L.S. Racial and Ethnic Differences in Health Behaviors among Cancer Survivors. Am. J. Prev. Med. 2015, 48, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.M.; Kue, J.; Brophy, L.; Peabody, A.L.; Foraker, R.E.; Yen, P.-Y.; Tucker, S. Mobile Health Applications, Cancer Survivors, and Lifestyle Modification: An Integrative Review. CIN Comput. Inform. Nurs. 2021, 39, 755. [Google Scholar] [CrossRef]
- Wilkins, C.H.; Edwards, T.L.; Stroud, M.; Kennedy, N.; Jerome, R.N.; Lawrence, C.E.; Kusnoor, S.V.; Nelson, S.; Byrne, L.M.; Boone, L.R.; et al. The Recruitment Innovation Center: Developing Novel, Person-Centered Strategies for Clinical Trial Recruitment and Retention. J. Clin. Transl. Sci. 2021, 5, e194. [Google Scholar] [CrossRef]
- Cunningham-Erves, J.; Joosten, Y.; Kusnoor, S.V.; Mayers, S.A.; Ichimura, J.; Dunkel, L.; Israel, T.L.; Ray, D.; Stroud, M.; Harris, P.A.; et al. A Community-Informed Recruitment Plan Template to Increase Recruitment of Racial and Ethnic Groups Historically Excluded and Underrepresented in Clinical Research. Contemp. Clin. Trials 2023, 125, 107064. [Google Scholar] [CrossRef]
- Chhatre, S.; Jefferson, A.; Cook, R.; Meeker, C.R.; Kim, J.H.; Hartz, K.M.; Wong, Y.-N.; Caruso, A.; Newman, D.K.; Morales, K.H.; et al. Patient-Centered Recruitment and Retention for a Randomized Controlled Study. Trials 2018, 19, 205. [Google Scholar] [CrossRef]
- Kelsey, M.D.; Patrick-Lake, B.; Abdulai, R.; Broedl, U.C.; Brown, A.; Cohn, E.; Curtis, L.H.; Komelasky, C.; Mbagwu, M.; Mensah, G.A.; et al. Inclusion and Diversity in Clinical Trials: Actionable Steps to Drive Lasting Change. Contemp. Clin. Trials 2022, 116, 106740. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.T.; Watkins, L.; Piña, I.L.; Elmer, M.; Akinboboye, O.; Gorham, M.; Jamerson, B.; McCullough, C.; Pierre, C.; Polis, A.B.; et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr. Probl. Cardiol. 2019, 44, 148–172. [Google Scholar] [CrossRef] [PubMed]
- Michaels, M.; Weiss, E.S.; Guidry, J.A.; Blakeney, N.; Swords, L.; Gibbs, B.; Yeun, S.; Rytkonen, B.; Goodman, R.; Jarama, S.L.; et al. The Promise of Community-Based Advocacy and Education Efforts for Increasing Cancer Clinical Trials Accrual. J. Cancer Educ. 2012, 27, 67–74. [Google Scholar] [CrossRef]
- Hopewell, S.; Loudon, K.; Clarke, M.J.; Oxman, A.D.; Dickersin, K. Publication Bias in Clinical Trials Due to Statistical Significance or Direction of Trial Results. Cochrane Database Syst. Rev. 2009, 1, MR000006. [Google Scholar] [CrossRef]
- Beller, J.; Geyer, S.; Epping, J. Health and Study Dropout: Health Aspects Differentially Predict Attrition. BMC Med. Res. Methodol. 2022, 22, 31. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; Friedlander, M.; Calvert, M.; Stockler, M.; Kyte, D.; Kok, P.-S.; King, M.T. A Systematic Evaluation of Compliance and Reporting of Patient-Reported Outcome Endpoints in Ovarian Cancer Randomised Controlled Trials: Implications for Generalisability and Clinical Practice. J. Patient-Rep. Outcomes 2017, 1, 5. [Google Scholar] [CrossRef]
| Study Name, First Author, Year, Country | Study Design, a Priori Sample Size Inclusion Criteria | Mean Age (SD), Enrollment by Sex, Race, Education | Intervention Behavioral Targets, Duration | Mode of Intervention Delivery | Recruitment Source and Time Frame for Accrual | Recruitment Methods, Number Contacted, and Number Randomized | Did Study Meet Recruitment (Accrual) Goal? | Retention Methods (Rates by Time-Point and Group, if Available) | Did Study Meet Retention (Attrition) Goal? |
|---|---|---|---|---|---|---|---|---|---|
| Harvest for Health, Birmingham Breast Cancer Survivors (BBCS), Bail, 2018; and Cases, 2016 (United States) [23,24] | Two-arm wait-list control, crossover RCT Target accrual: n = 100 (1) BC (2) post-primary treatment (3) eating <5 servings of vegetables and fruits/day (4) exercising <150 min/week (5) ≥1 physical function limitation | 60.2 (11.1) years 100% female 73.2% (n = 60) White 83.0% (n = 69) ≥ some college | Diet and PA 24 months | Home-based and in-person, Master gardener led gardening intervention, gardening journals/notebooks | University of Alabama cancer registry, self-referrals August 2013–May 2014 (10 months) | Cancer registry, n = 1279 mailed study invitations, n = 1128 survivors contacted, n = 194 screened, n = 82 deemed eligible 32.6% response rate n = 82 randomized (n = 44 intervention, n = 38 delayed intervention) | No, 82% of accrual target Reason: lack of time to recruit equal number of participants from surrounding 5 counties, many rural county participants were already gardening and ineligible | Methods: Participant compensation, USD 500 worth of gardening tools Total: 97.6% (n = 80) Intervention: 95.4% (n = 42) Control: 94.7% (n = 36) | Yes, goal was 80% retention |
| Diet and Androgen-5 (DIANA-5) Trial, Bruno, 2021 (Italy) [25] | Two-arm RCT A priori target accrual not defined (1) invasive BC (2) post-primary treatment within 5 years (3) 35–70 years | 52.0 (8.5) years 100% female Not reported Not reported | Diet 5 years | In person classes (4) and 10 food-centric meetings | Tumor registries, oncology units, breast cancer screening units, breast cancer support groups, media January 2008–December 2010 (3 years) | No details n = 1542 randomized (n = 769 intervention, n = 773 control) | Not reported | Methods: Not reported 1 year: Total: 87.1% (n = 1344) Intervention: 89.6% (n = 689) Control: 84.7% (n = 655) | No retention goal identified |
| ¡Cocinar para su salud!, Bernard-Davilla, 2015; and Greenlee, 2015 (United States) [26,27] | Two-arm RCT Target accrual: n = 70 (1) BC, stage 0–III (2) >3-months post-treatment (3) Hispanic descent | 56.6 (9.7) years 100% female 40.0% (n = 28) White 38.6% (n = 27) ≥ some college | Culturally based diet intervention 12 weeks, 9 sessions Extended follow-up at 6 months | In-person, community based: culturally based group cooking classes, grocery shopping field trip, nutrition education | Columbia University Medical Center (CUMC) Breast Oncology Clinic January 2011–March 2012 (1 year and 3 months) | Clinical recruitment, n = 405 potentially eligible (n = 142 refused to participate, n = 37 unable to contact), n = 111 screened, n = 102 eligible, n = 70 enrolled n = 70 randomized (n = 34 intervention, n = 36 control) | Yes, 100% of accrual target | Methods: Culturally adapted and tailored materials provided in Spanish 3 months: Total: 96% (n = 67) Intervention: 91.2% (n = 31) Control: 100% (n = 36) 6 months: Total: 87% (n = 61) Intervention: 88.2% (n = 30) Control: 86.1% (n = 31) | Yes, expected 15% attrition at 6 months |
| Effect of lifestyle intervention in men with advanced PC on ADT, Bourke, 2014 (England) [28] | Two-arm single blind RCT Target accrual: n = 100 (1) PC on ADT for locally advanced and metastatic PC (2) taking ADT for ≥6 months | 71 years, range: 53–87 years 100% male Not reported Not reported | Diet and PA 12 weeks Extended follow-up at 6 months | In-person: supervised exercise program and small-group healthy eating seminars, home-based: nutrition advice pack | Outpatient clinics 2008–2012 (4 years) | No details on number screened, n = 135 deemed eligible n = 100 randomized (n = 50/arm) | Yes, 100% of accrual target | Methods: Extended supervision and contact during follow-up 12 weeks: Total: 85% (n = 85) Intervention: 86% (n = 43) Control: 84% (n = 42) 6 months: Total: 68% (n = 68) Intervention: 70% (n = 35) Control: 66% (n = 33) | No, only expected 25% attrition at 6-months |
| TrueNTH Community of Wellness, Chan, 2020 (United States) [29] | Four-arm pilot RCT Target accrual: n = 200 (1) PC (2) physician clearance | Median 70 years old, IQR: 65–75 years 100% male 92.6% (n = 187) White 92.6% (n = 187) ≥ some college | Diet and PA 3 months Extended follow-up at 6 months | Level 1: information on website, Level 2: information on website and exercise videos, Level 3: information on website, exercise videos, Fitbit, text messaging, Level 4: information on website, exercise videos, Fitbit, text messaging, two 30-min telephone calls with a dietician and/or exercise trainer | Hospital cancer registry databases, the Cancer of the Prostate Strategic Urologic Research Endeavor registry, clinics 2017–2019 (13 months) | Potentially eligible participants identified from the cancer registry mailed study letter, n = 6406 mailed study information, n = 292 expressed interest, n = 240 screened, n = 220 deemed eligible n = 202 randomized Level 1 n = 49 Level 2 n = 51 Level 3 n = 50 Level 4 n = 52 | Yes, 101% of total accrual target (did not meet goal of n = 50 per arm) | Methods: not reported 3 months: Total: 82.7% (n = 167) Level 1: 79.6% (n = 39) Level 2: 84.3% (n = 43) Level 3: 86% (n = 43) Level 4: 80.8% (n = 42) 6 months: Total: 77.2% (n = 156) Level 1: 77.6% (n = 38) Level 2: 78.4% (n = 40) Level 3: 76% (n = 38) Level 4: 76.9% (n = 40) | Yes, expected 20% attrition at 3 months and 36% attrition at 6-months |
| The LISA Trial, Goodwin, 2014 (North America) [30] | Multicenter, 2-arm RCT Target accrual: n = 2150 (1) postmenopausal women (2) diagnosed with T1-3N0-3M0 BC in the previous 36 months (3) currently using letrozole® | 60.4 years (7.8) for mail-based vs. 61.6 years (6.7) for intervention 100% female 95.6% (n = 323) White Not reported | Diet and PA 24 months | Home-based, mailed information on healthy living, workbook, and individualized telephone-based lifestyle coaching intervention in English or French | 16 Canadian and American participating medical centers May 2007–January 2010 (2 years 8 months) | Clinical recruitment, n = 682 identified as potentially eligible, n = 546 consented and screened, n = 338 deemed eligible n = 338 randomized (n = 167 mail-based, n = 171 intervention) | No, 15.7% of accrual target Reason: enrollment was terminated due to loss of funding | Methods: Mailed and telephone reminders 6 months: Total: 93.4% (n = 316) Mail-based: 92.8% (n = 155) Intervention: 94.2% (n = 161) 12 months: Total: 85.5% (n = 289) Mail-based: 88% (n = 147) Intervention: 83% (n = 142) 18 months: Total: 82.5% (n = 279) Mail-based: 86.2% (n = 144) Intervention: 78.9% (n = 135) 24 months: Total: 78.1% (n = 264) Mail-based: 78.4% (n = 131) Intervention: 77.8% (n = 133) | No retention goal identified |
| Moving Bright, Eating Smart, Ho, 2013 (Hong Kong) [31] | Multicenter, 2 × 2 factorial RCT Target accrual: n = 224 (n = 56 per cell) (1) CRC (2) within one-year post-primary treatment | 65.2 years (10.1), range: 25 to 86 years 36.8% female (n = 82), 63.2% male (n = 141) Not reported 87.4% (n = 195) ≥ some college | Diet and PA 12 months Extended follow-up at 24 months | Motivational interviews with registered dietitian, telephone calls, mailed newsletters and pamphlets, quarterly group meetings | CRC case management program, surgical and clinical oncology departments May 2013–April 2014 (1 year) | EHR review, n = 1613 medical records reviewed, n = 341 assessed for eligibility, n = 229 deemed eligible n = 223 randomized Group A—Diet and PA (n = 55) Group B—Diet only (n = 56) Group C—PA only (n = 56) Group D—control (n = 56) | No, 99.6% of accrual target | Methods: Flexible scheduling, travel allowance 6 months: Total: 95% (n = 212) Group A 96.4% (n = 53) Group B 92.9% (n = 52) Group C 96.4% (n = 54) Group D 94.6% (n = 53) 12 months: Total: 91.9% (n = 205) Group A 94.5% (n = 52) Group B 85.7% (n = 48) Group C 92.9% (n = 52) Group D 94.6% (n = 53) 18 months: Total: 88.8% (n = 198) Group A 89.1% (n = 49) Group B 83.9% (n = 47) Group C 92.9% (n = 52) Group D 89.3% (n = 50) 24 months: Total: 86.1% (n = 192) Group A 85.5% (n = 47) Group B 82.1% (n = 46) Group C 92.9% (n = 52) Group D 85.7% (n = 48) | No, expected 10% total attrition. Met goal at year 1 but not year 2 |
| Optimune trial, Holtdirk, 2020 (Germany) [32] | Two-arm RCT Target accrual: n = 360 (1) BC (2) diagnosed within 5 years (3) completed primary treatment >1 month before enrollment | 49.9 years (8.2), range: 30–70 years 100% female Not reported 62.3% (n = 226) ≥ some college | Diet, PA, psychosocial support, sleep 6 months | Internet-based | Online (Google ads, internet forums, Facebook), treatment centers, patient associations, support groups, health insurance companies October 2018–April 2020 (1.5 years) | Potential participants indicated their interest using a survey on the study webpage after hearing about the study from one of the recruitment sources, n = 749 showed interest in the study, n = 609 provided consent and were screened, n = 363 deemed eligible n = 363 randomized (n = 181 intervention, n = 182 control) | Yes, 100.8% of accrual target | Methods: Personalized recommendations, responsive web-design approach 3 months: Total: 84.3% (n = 306) Intervention: 77.9% (n = 141) Control: 90.7% (n = 165) 6 months: Total: 72.4% (n = 263) Intervention: 69.6% (n = 126) Control: 75.3% (n = 137) | No retention goal identified |
| Kanker Nazorg Wijzer (Cancer Aftercare Guide) Kanera, 2016 (Netherlands) [33] | Two-arm RCT Target accrual: n = 376 (1) cancer diagnosis (2) completed primary treatment between 4 and 56 weeks previous | Intervention: 55.6 (11.5) years; Control: 56.2 (11.3) years 80% female (n = 369), 20% male (n = 93) Not reported 27.6% (n = 143) ≥ some college | Diet, PA, smoking cessation, psychosocial support 6 months | Home-based, online modules | Hospitals, outpatient clinics November 2013 to June 2014 (8 months) | EHR review, n = 1303 assessed for eligibility, n = 492 completed baseline, n = 30 did not complete informed consent, n = 475 deemed eligible, n = 240 eligible in control, n = 252 intervention n = 518 randomized (n = 253 control, n = 265 intervention) | Yes, 137.8% of accrual target | Methods: Email reminders 6 months: Total: 79% (n = 409) Intervention: 70.9% (n = 188) Control: 87.4% (n = 221) | Yes, expected 20–23% attrition |
| PRO-MAN trial, Mohamad, 2019 (Scotland) [34] | Two-arm RCT A priori target accrual not defined (1) PC (2) diagnosis within the previous 36 months | 65.5 (5.6) years 100% male Not reported Not reported | Diet and PA 12 weeks Extended follow-up at 6 and 12 months | Group meeting, pedometer, telephone-based dietary advice, online resources | Urological Cancer Database October 2013–December 2013 (3 months) | EHR review, n = 286 screened, n = 92 assessed for eligibility, n = 63 deemed eligible n = 62 randomized (n = 31 intervention, n = 31 control) | Not reported | Methods: Phone calls 12 weeks: Total: 87.1% (n = 54) Intervention: 83.9% (n = 26) Control: 90.3% (n = 28) 6 months: Total: 82.3% (n = 51) Intervention: 77.4% (n = 24) Control: 87.1% (n = 27) 12 months: Total: 43.5% (n = 27) Intervention: 35.5% (n = 11) Control: 51.6% (n = 16) | No goal identified |
| Efficacy of a diet and physical activity intervention for patients on ADT, O’Neill, 2015 (North Ireland) [35] | Two-arm RCT Target accrual: n = 94 (1) PC (2) planning to or are already being treated with LHRHa for 6 months | Intervention: 69.7 (6.8) years; Control: 69.9 (7.0) years 100% male Not reported 36.2% (n = 34) ≥ some college | Diet and PA 6 months | Home-based meetings with a nutritionist, guidebook, pedometer, involved partner or caregiver if possible | Cancer center at Belfast City Hospital August 2009–March 2011 (1.5 years) | Clinical recruitment, n = 158 patients deemed eligible, n = 94 enrolled, 59.5% recruitment rate n = 94 randomized (n = 47 intervention, n = 47 control) | Yes, 100% of accrual target | Methods: Provision of intervention materials to control group at the end of the trial, telephone calls to monitor participant progress 3 months: Total: 96.8% (n = 91) Intervention: 95.7% (n = 45) Control: 97.9% (n = 46) 6 months: Total: 95.7% (n = 90) Intervention: 95.7% (n = 45) Control: 95.7% (n = 45) | Yes, expected 30% attrition |
| Mail-based lifestyle interventions for BC survivors, Park, 2016 (United States) [36] | Three-arm RCT Target accrual: n = 225 (1) BC, stage I–II (2) post-primary treatment in the previous 3 months (3) eligibility expanded to stage 0–II with diagnosis in the previous 1.5 years due to recruitment difficulties | 56.4 years (TTMI: 55.7 (10.9) years, SLM: 57.7 (10.7) years, Control: 55.7 (10.9) years) 100% female 94.2% (n = 163) White 87.2% (n = 151) ≥ some college | Diet and PA 4 months Extended follow-up at 7 months | Home-based: mailed materials with 5 or more f/v per day, <30% calories from lipids, increase to moderate PA 150 min/week. | Cancer centers, flyers, hospitals, ClinicalTrials.gov, mailings September 2011–October 2013 (2 years) | 85% recruited through cancer center registry, 7.5% through ClinicalTrials.gov and a regional hospital, 6.4% through mailings, and 1.2% through flyers, n = 2279 identified as potentially eligible, n = 177 screened, n = 173 deemed eligible n = 173 randomized TTMI n = 57 SLM n = 58 Control n = 58 | No, 76.9% of accrual target Reason: funding period ended | Methods: USD 60 compensation for completion of 4- and 7-month follow-up, provision of intervention materials to control group at the end of the trial 4 months: Total: 76.9% (n = 133) TTMI 70.2% (n = 40) SLM 74.1% (n = 43) Control 86.2% (n = 50) 7 months: Total: 75.7% (n = 131) TTMI 75.4% (n = 43) SLM 70.7% (n = 41) Control 81% (n = 47) | No retention goal identified |
| Rx for Better Breast Health Trial, Ramirez, 2017 (United States) [37] | Two-arm RCT Target accrual: n = 150 (1) BC (2) ≥2 months post-treatment | 56.6 years (intervention: 55.3 (9.8) years, control: 57.9 (8.8) years) 100% female 43.1% (n = 66) White 85.6% (n = 131) ≥ some college | Diet 6 months Extended follow-up at 12 months | In-person nutrition workshops, telephone calls with patient navigators, newsletters, motivational interviewing | Not reported Not reported | No details on recruitment methods, n = 301 screened for eligibility, n = 180 deemed eligible n = 153 randomized (n = 76 intervention, n = 77 control) | Yes, 102% of accrual target | Methods: Phone call to follow-up after a missed session and electronic copies of all workshop materials, tailored newsletter Total: 81.7% (n = 125) Intervention: 78.9% (n = 60) Control: 84.4% (n = 65) | No retention goal identified |
| Living Well after Breast Cancer, Reeves, 2017 (Australia) [42] | Two-arm pilot feasibility RCT Target accrual: n = 90 (1) BC, stage I-III (2) diagnosed in the previous 9–18 months (3) BMI 25–40 kg/m2 | 55.3 (8.7) years 100% female 96.7% (n = 87) White 67.8% (n = 61) ≥ some college | Diet and PA 6 months | Telephone-based calls with coaches, pedometer, self-monitoring diary, workbook, digital scale, calorie counter book | State-based cancer registries, with oncologist permission to contact October 2010 to February 2012 (2.3 years) | Cancer registry, n = 927 potentially eligible participants identified, n = 743 participants given oncologist consent and sent study letters, n = 248 participants consented to contact, n = 213 assessed for eligibility n = 90 randomized (n = 45 intervention, n = 45 control) | Yes, 100% of accrual target | Methods: Individualized coaching Total: 82.2% (n = 74) Intervention: 88.9% (n = 40) Control: 75.6% (n = 34) | No, expected 10% attrition |
| Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial, Rock, 2013; and Sedjo, 2016 (United States) [38,39] | Two-arm RCT Target accrual: n = 690 (1) BC, stage I–III (2) diagnosed in the previous 5 years | Intervention: 56.1 (9.4) years, control: 56.5 (9.5) years 100% female 78.3% (n = 546) White 85.1% (n = 593) ≥ some college | Diet and PA 24 months | In-person, group sessions, individualized telephone/email contact to support study goals, print newsletters | Local or regional cancer registries, clinics, television, radio, local print media, community support groups, local events or organizations Fall of 2010–May 2012 (1.5 years) | Potentially eligible participants identified from the cancer registry were mailed study invitations or participants called study staff from distributed flyers, n = 11,311 tumor registry or oncology referral letters sent, n = 2740 flyers distributed, n = 7501 telephone contacts or records reviewed, n = 5027 breast cancer cases screened, n = 714 baseline visits completed n = 697 randomized (n = 348 intervention, n = 349 control) | Yes, 101% of accrual target | Methods: Monthly standardized contacts, invitation to bi-monthly seminars, mailing personalized cards, distribution of donated items (i.e., massage vouchers, coupons, etc.), monthly newsletters Total: 84.2% (n = 587) Intervention: 86.2% (n = 300) Control: 82.2% (n = 287) | No, expected 10% attrition |
| Living Well After Breast Cancer, Terranova, 2022 (Australia) [10] | Two-arm RCT Target accrual: n = 156 (1) BC, stage II–III (2) diagnosis within 2 years (3) post-primary treatment (4) BMI 25–45 kg/m2 | 55.4 (9.2) years 100% female 98.1% (n = 159) White 59.8% (n = 95) ≥ some college | Diet and PA 18 months | Telephone-based calls with coaches, pedometer, self-monitoring diary, workbook, digital scale, calorie counter book | Hospitals in Brisbane and state cancer registry October 2012–December 2014 (2 years) | Cancer registry, n = 1040 identified, 394 contacted, 170 ineligible and 65 declined, 159 consented and were randomized n = 159 randomized (n = 79 intervention, n = 80 usual care) | Yes, 101.9% of accrual target | Methods: Rapport building, flexible scheduling, travel and parking reimbursement, brief feedback on assessments and newsletter, birthday cards, emergency contact collection 6 months: Total: 89.9% (n = 143) Intervention: 92.4% (n = 73) Control: 87.5% (n = 70) 12 months: Total: 81.8% (n = 130) Intervention: 88.6% (n = 70) Control: 75% (n = 60) 18 months: Total: 80.5% (n = 128) Intervention: 86.1% (n = 68) Control: 75% (n = 60) | Yes, expected 20% attrition at 12 months |
| Survivor Choices for Eating and Drinking (SUCCEED) Trial, Van Blarigan, 2020 (United States) [40] | Two-arm, wait-list control RCT Target accrual: n = 50 (1) CRC (2) not being treated with chemotherapy (3) considered disease-free or have stable disease | 55 years, IQR: 55–62 years 64% female (n = 33), 34% male (n = 17) 70.0% (n = 35) White 96.0% (n = 48) college degree | Diet 12 weeks Extended follow-up at 24 weeks | Web-based intervention, text messaging | University of California San Francisco (UCSF) gastrointestinal oncology clinic, clinics, support groups, national conference April 2017–May 2018 (1 year) | Interested individuals were asked to complete a screening survey online and have a provider complete a form to verify clinical information, n = 94 assessed for eligibility, n = 19 ineligible, n = 11 refused, n = 14 did not complete enrollment procedures n = 50 randomized (n = 25 intervention, n = 25 control) | Yes, 100% of accrual target | Methods: Provision of intervention materials to control group at the end of the trial, text prompts for participation 12 weeks: Total: 90% (n = 45) Intervention: 88% (n = 22) Control: 92% (n = 23) 24-weeks: Total: 84% (n = 42) Intervention: 88% (n = 22) Control: 80% (n = 20) | Yes, expected 20% attrition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werts, S.J.; Lavelle, S.A.; Crane, T.E.; Thomson, C.A. Recruitment and Retention Strategies Used in Dietary Randomized Controlled Interventions with Cancer Survivors: A Systematic Review. Cancers 2023, 15, 4366. https://doi.org/10.3390/cancers15174366
Werts SJ, Lavelle SA, Crane TE, Thomson CA. Recruitment and Retention Strategies Used in Dietary Randomized Controlled Interventions with Cancer Survivors: A Systematic Review. Cancers. 2023; 15(17):4366. https://doi.org/10.3390/cancers15174366
Chicago/Turabian StyleWerts, Samantha J., Sarah A. Lavelle, Tracy E. Crane, and Cynthia A. Thomson. 2023. "Recruitment and Retention Strategies Used in Dietary Randomized Controlled Interventions with Cancer Survivors: A Systematic Review" Cancers 15, no. 17: 4366. https://doi.org/10.3390/cancers15174366
APA StyleWerts, S. J., Lavelle, S. A., Crane, T. E., & Thomson, C. A. (2023). Recruitment and Retention Strategies Used in Dietary Randomized Controlled Interventions with Cancer Survivors: A Systematic Review. Cancers, 15(17), 4366. https://doi.org/10.3390/cancers15174366





