Long-Term Outcomes of Liver Transplantation in Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Comparison with Portal Vein Tumor Thrombus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Diagnosis of PVTT and BDTT
2.3. Postoperative Management and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Surgical Characteristics
3.3. Postoperative Characteristics
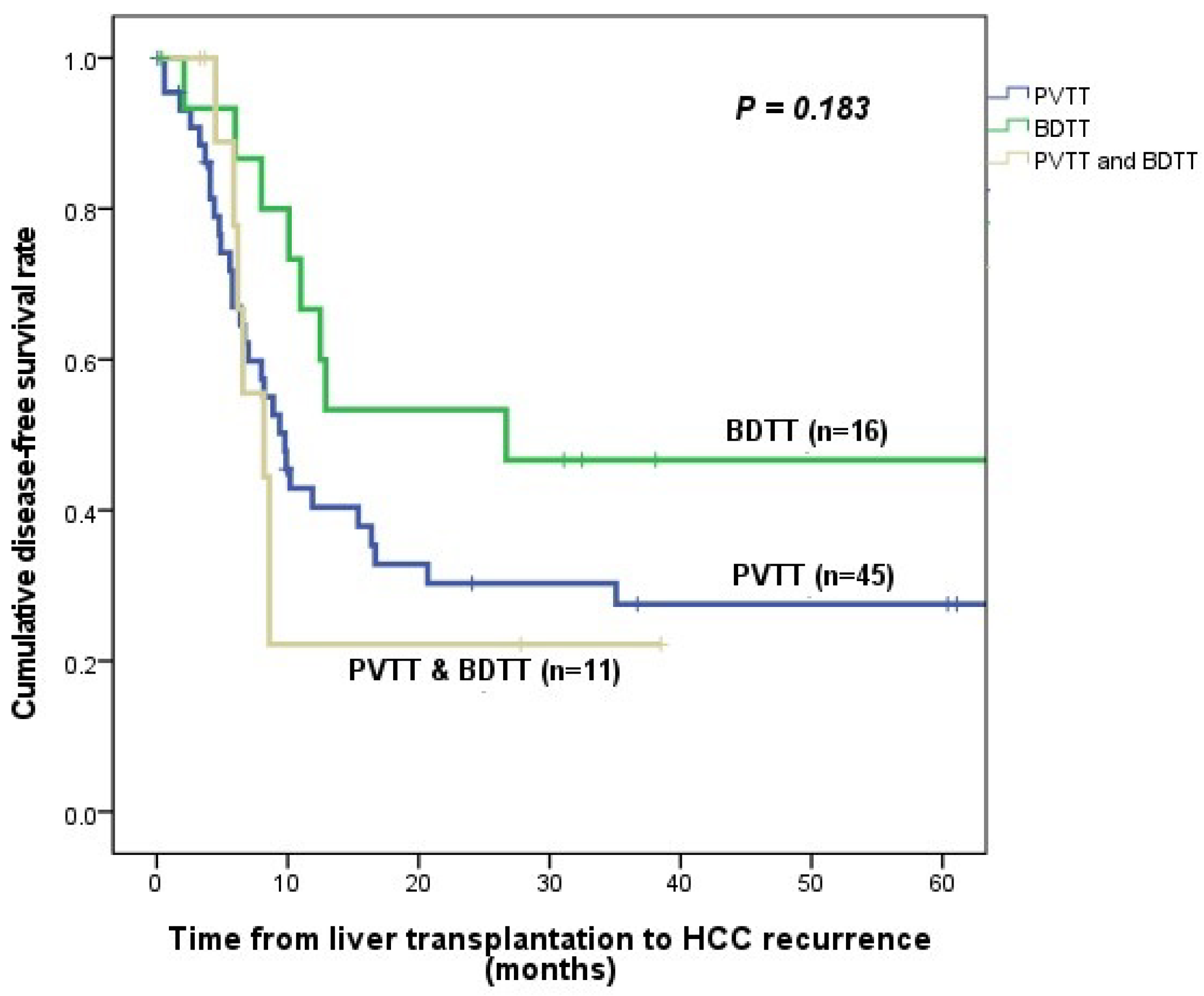
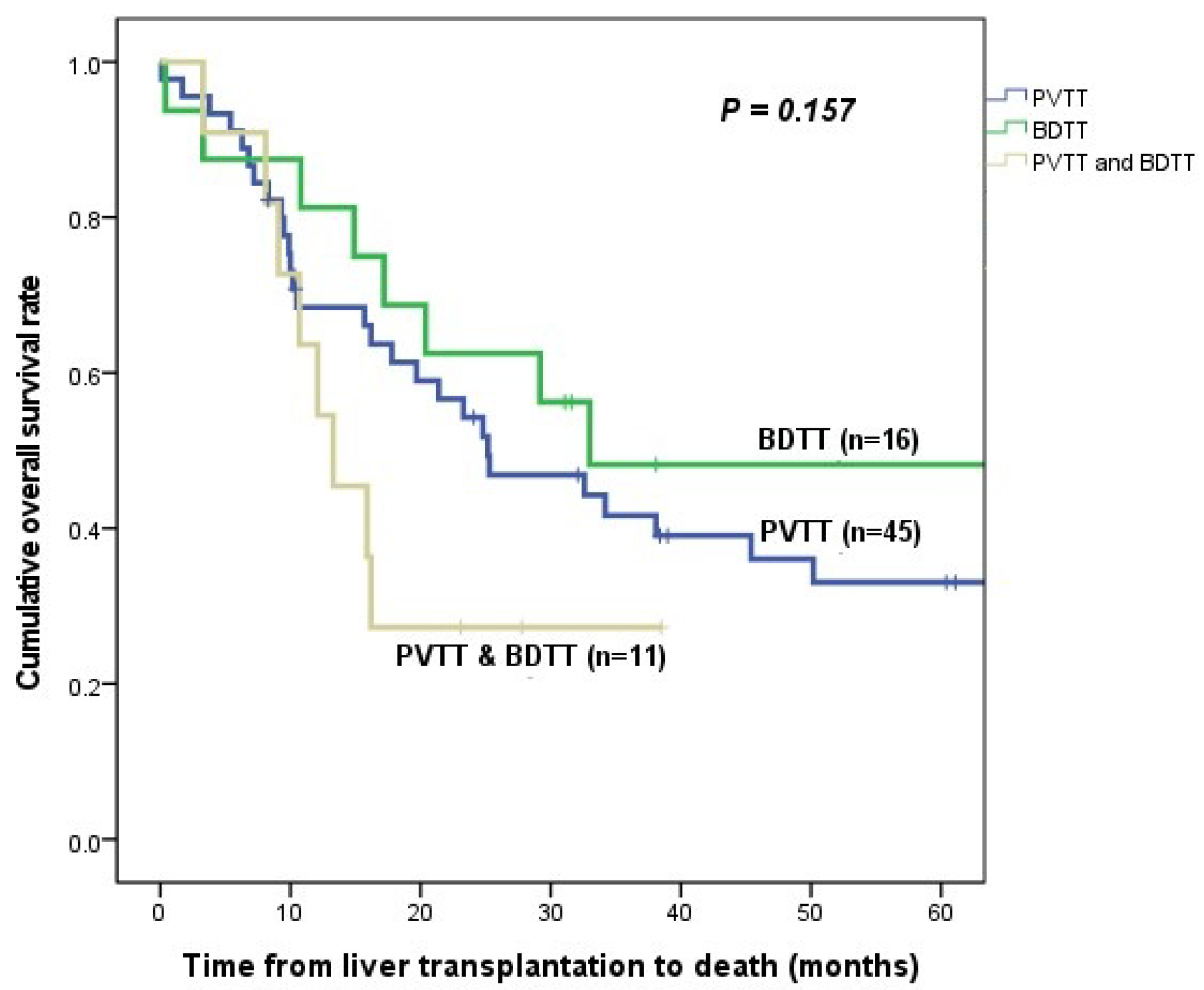
3.4. HCC Recurrence and Patient Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Hsu, C.Y.; Huang, Y.H.; Hsia, C.Y.; Chiou, Y.Y.; Su, C.W.; Lin, H.C.; Huo, T.I. Vascular invasion in hepatocellular carcinoma: Prevalence, determinants and prognostic impact. J. Clin. Gastroenterol. 2014, 48, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Makuuchi, M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J. Gastroenterol. 2006, 12, 7561–7567. [Google Scholar] [CrossRef]
- Shen, J.; Wen, J.; Li, C.; Wen, T.; Yan, L.; Li, B.; Yang, J.; Lu, C. The prognostic value of microvascular invasion in early-intermediate stage hepatocelluar carcinoma: A propensity score matching analysis. BMC Cancer 2018, 18, 278. [Google Scholar] [CrossRef]
- Soin, A.S.; Bhangui, P.; Kataria, T.; Baijal, S.S.; Piplani, T.; Gautam, D.; Choudhary, N.S.; Thiagarajan, S.; Rastogi, A.; Saraf, N.; et al. Experience With LDLT in Patients With Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis Postdownstaging. Transplantation 2020, 104, 2334–2345. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, D.G.; Na, G.H.; Hong, T.H.; Bae, S.H.; You, Y.K.; Choi, J.Y.; Yoon, S.K. The clinical outcomes of patients with portal vein tumor thrombi after living donor liver transplantation. Liver Transpl. 2017, 23, 1023–1031. [Google Scholar] [CrossRef]
- Han, D.H.; Joo, D.J.; Kim, M.S.; Choi, G.H.; Choi, J.S.; Park, Y.N.; Seong, J.; Han, K.H.; Kim, S.I. Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis after Concurrent Chemoradiation Therapy. Yonsei Med. J. 2016, 57, 1276–1281. [Google Scholar] [CrossRef]
- Lee, K.W.; Suh, S.W.; Choi, Y.; Jeong, J.; Yi, N.J.; Kim, H.; Yoon, K.C.; Hong, S.K.; Kim, H.S.; Lee, K.B.; et al. Macrovascular invasion is not an absolute contraindication for living donor liver transplantation. Liver Transpl. 2017, 23, 19–27. [Google Scholar] [CrossRef]
- Shiomi, M.; Kamiya, J.; Nagino, M.; Uesaka, K.; Sano, T.; Hayakawa, N.; Kanai, M.; Yamamoto, H.; Nimura, Y. Hepatocellular carcinoma with biliary tumor thrombi: Aggressive operative approach after appropriate preoperative management. Surgery 2001, 129, 692–698. [Google Scholar] [CrossRef]
- Qin, L.X.; Tang, Z.Y. Hepatocellular carcinoma with obstructive jaundice: Diagnosis, treatment and prognosis. World J. Gastroenterol. 2003, 9, 385–391. [Google Scholar] [CrossRef]
- Meng, K.W.; Dong, M.; Zhang, W.G.; Huang, Q.X. Clinical characteristics and surgical prognosis of hepatocellular carcinoma with bile duct invasion. Gastroenterol. Res. Pract. 2014, 2014, 604971. [Google Scholar] [CrossRef]
- Huang, J.F.; Wang, L.Y.; Lin, Z.Y.; Chen, S.C.; Hsieh, M.Y.; Chuang, W.L.; Yu, M.Y.; Lu, S.N.; Wang, J.H.; Yeung, K.W.; et al. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2002, 17, 190–195. [Google Scholar] [CrossRef]
- Kim, S.; Bolognese, A.; Leverson, G.; Al-Adra, D. Liver Transplantation for Hepatocellular Carcinoma with Associated Bile Duct Tumor Thrombus: A Systematic Pooled Analysis. Am. J. Transpl. 2022, 22, 43–44. [Google Scholar]
- Wang, C.; Yang, Y.; Sun, D.; Jiang, Y. Prognosis of hepatocellular carcinoma patients with bile duct tumor thrombus after hepatic resection or liver transplantation in Asian populations: A meta-analysis. PLoS ONE 2017, 12, e0176827. [Google Scholar] [CrossRef]
- Ha, T.Y.; Hwang, S.; Moon, D.B.; Ahn, C.S.; Kim, K.H.; Song, G.W.; Jung, D.H.; Park, G.C.; Park, H.W.; Park, Y.H.; et al. Long-term survival analysis of liver transplantation for hepatocellular carcinoma with bile duct tumor thrombus. Transpl. Proc. 2014, 46, 774–777. [Google Scholar] [CrossRef]
- Kim, J.M.; Na, B.G.; Lee, K.W.; Oh, D.G.; Ha, Y.E.; Choi, G.S.; Kwon, C.H.D.; Joh, J.W.; Lee, S.K. Oral Valganciclovir as a Preemptive Treatment for Cytomegalovirus (CMV) Infection in CMV-Seropositive Liver Transplant Recipients. Transplantation 2015, 99, 251. [Google Scholar] [CrossRef][Green Version]
- Shah, S.A.; Tan, J.C.; McGilvray, I.D.; Cattral, M.S.; Levy, G.A.; Greig, P.D.; Grant, D.R. Does microvascular invasion affect outcomes after liver transplantation for HCC? A histopathological analysis of 155 consecutive explants. J. Gastrointest. Surg. 2007, 11, 464–471. [Google Scholar] [CrossRef]
- Pommergaard, H.C.; Rostved, A.A.; Adam, R.; Thygesen, L.C.; Salizzoni, M.; Gomez Bravo, M.A.; Cherqui, D.; Filipponi, F.; Boudjema, K.; Mazzaferro, V.; et al. Vascular invasion and survival after liver transplantation for hepatocellular carcinoma: A study from the European Liver Transplant Registry. HPB 2018, 20, 768–775. [Google Scholar] [CrossRef]
- Yu, J.; Zhuang, L.; Liu, P.; Liu, Z.; Ling, S.; Deng, Y.; Li, J.; Yang, B.; Chen, Z.; Wang, Z.; et al. Long-term outcomes of deceased donor liver transplantation in hepatocellular carcinoma patients with portal vein tumor thrombus: A multicenter study. Eur. J. Surg. Oncol. 2022, 48, 121–132. [Google Scholar] [CrossRef]
- Nam, J.Y.; Lee, J.H.; Bae, J.; Chang, Y.; Cho, Y.; Sinn, D.H.; Kim, B.H.; Kim, S.H.; Yi, N.J.; Lee, K.W.; et al. Novel Model to Predict HCC Recurrence after Liver Transplantation Obtained Using Deep Learning: A Multicenter Study. Cancers 2020, 12, 2791. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.J.; Lee, K.W.; Kim, S.H.; et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.M.; Yi, N.J.; Choi, G.S.; Lee, K.W.; Suh, K.S.; Joh, J.W. Validation for models for tumor recurrence after liver transplantation in hepatectomy patients. Ann. Surg. Treat. Res. 2022, 102, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cho, Y.; Lee, J.H.; Lee, Y.B.; Cho, E.J.; Yu, S.J.; Sinn, D.H.; Kim, B.H.; Kim, S.H.; Yi, N.J.; et al. Comparison of Models for Tumor Recurrence after Liver Transplantation for the Patients with Hepatocellular Carcinoma: A Multicenter Long-Term Follow-Up Study. Cancers 2019, 11, 1295. [Google Scholar] [CrossRef]
- Moon, D.B.; Hwang, S.; Wang, H.J.; Yun, S.S.; Kim, K.S.; Lee, Y.J.; Kim, K.H.; Park, Y.K.; Xu, W.; Kim, B.W.; et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: A Korean multicenter study. World J. Surg. 2013, 37, 443–451. [Google Scholar] [CrossRef]
- Yeh, C.N.; Jan, Y.Y.; Lee, W.C.; Chen, M.F. Hepatic resection for hepatocellular carcinoma with obstructive jaundice due to biliary tumor thrombi. World J. Surg. 2004, 28, 471–475. [Google Scholar] [CrossRef]
- Ikenaga, N.; Chijiiwa, K.; Otani, K.; Ohuchida, J.; Uchiyama, S.; Kondo, K. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J. Gastrointest. Surg. 2009, 13, 492–497. [Google Scholar] [CrossRef]
- Navadgi, S.; Chang, C.C.; Bartlett, A.; McCall, J.; Pandanaboyana, S. Systematic review and meta-analysis of outcomes after liver resection in patients with hepatocellular carcinoma (HCC) with and without bile duct thrombus. HPB 2016, 18, 312–316. [Google Scholar] [CrossRef]
- Satoh, S.; Ikai, I.; Honda, G.; Okabe, H.; Takeyama, O.; Yamamoto, Y.; Yamamoto, N.; Iimuro, Y.; Shimahara, Y.; Yamaoka, Y. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery 2000, 128, 779–783. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.B.; Wen, J.M.; Zhang, R.; Zhu, M.S.; Shi, X.D.; Liu, C. Hepatocellular carcinoma with bile duct tumor thrombus: A clinicopathological analysis of factors predictive of recurrence and outcome after surgery. Medicine 2015, 94, e364. [Google Scholar] [CrossRef]
| PVTT (n = 45) | BDTT (n = 16) | Coexisted (n = 11) | p-Value | |
|---|---|---|---|---|
| Sex, male | 44 (97.8%) | 16 (100%) | 9 (81.8%) | 0.057 |
| Age, years | 53 (37–72) | 53 (23–66) | 52 (40–72) | 0.996 |
| BMI | 24.8 (17.1–32.9) | 23.9 (18.5–27.0) | 23.6 (19.1–37.1) | 0.164 |
| Hypertension | 7 (15.6%) | 3 (18.8%) | 1 (9.1%) | 0.725 |
| Diabetes mellitus | 14 (31.1%) | 6 (37.5%) | 4 (36.4%) | 0.657 |
| Hepatic encephalopathy | 2 (3.4%) | 3 (18.8%) | 0 (0%) | 0.823 |
| Varix bleeding | 3 (5.1%) | 0 (0%) | 0 (0%) | 0.213 |
| Ascites | 0.331 | |||
| None Diuretics—controlled Diuretics—uncontrolled | 26 (57.6%) 14 (28.8%) 5 (13.6%) | 7 (43.8%) 4 (25.0%) 5 (31.3%) | 6 (54.5%) 3 (27.3%) 2 (18.2%) | |
| ABO incompatibility | 9 (12.5%) | 0 (0.0%) | 2 (18.2%) | 0.430 |
| HBV | 41 (91.9%) | 11 (68.8%) | 11 (100%) | 0.905 |
| HCV | 5 (8.1%) | 1 (6.3%) | 0 (0%) | 0.218 |
| Alcoholic | 1 (2.2%) | 2 (12.5%) | 1 (9.1%) | 0.195 |
| NASH | 0 (0%) | 2 (12.5%) | 0 (0%) | 0.367 |
| Child–Pugh class | 0.138 | |||
| A B C | 21 (46.7%) 17 (37.8%) 7 (15.6%) | 6 (37.5%) 5 (31.3%) 5 (31.3%) | 4 (36.4%) 2 (18.2%) 5 (45.5%) | |
| MELD score | 10 (7–40) | 10 (0–40) | 13 (8–40) | 0.220 |
| HRS | 0.444 | |||
| None Without CRRT CRRT or HD | 42 (93.3%) 2 (4.4%) 1 (1.4%) | 15 (93.8%) 0 (0%) 1 (6.3%) | 9 (81.8%) 2 (18.2%) 0 (0%) | |
| SBP | 4 (5.6%) | 1 (6.3%) | 0 (0%) | 0.311 |
| Intrahepatic metastasis | 33 (75%) | 10 (62.5%) | 4 (36.4%) | 0.017 |
| Neoadjuvant treatments | 32 (71.1%) | 12 (75.0%) | 8 (72.7%) | 0.845 |
| TACE | 28 (62.2%) | 12 (75.0%) | 8 (72.7%) | 0.216 |
| Liver resection | 7 (15.6%) | 3 (18.8%) | 1 (9.1%) | 0.725 |
| Radiation therapy | 10 (23.7%) | 5 (31.3%) | 4 (36.4%) | 0.268 |
| RFA | 9 (20%) | 5 (31.3%) | 3 (27.3%) | 0.362 |
| Intra-arterial chemotherapy | 0 (0%) | 0 (0%) | 1 (9.1%) | 0.048 |
| Treatment response | 0.449 | |||
| CR PR SD PD | 35 (77.8%) 3 (6.7%) 2 (4.4%) 5 (11.1%) | 11 (68.8%) 2 (12.5%) 3 (18.8%) 0 (0%) | 7 (63.6%) 3 (27.3%) 0 (0%) 1 (9.1%) | |
| Donor type, LDLT | 40 (88.9%) | 15 (93.8%) | 7 (63.6%) | 0.091 |
| Donor sex, male | 17 (37.8%) | 10 (62.5%) | 3 (27.3%) | 0.958 |
| Donor age, years | 32 (17–61) | 27 (19–72) | 44 (16–82) | 0.195 |
| Donor BMI | 23.4 (18.7–32.2) | 23.2 (18.4–27.1) | 21.6 (18.8–32.5) | 0.580 |
| Donor hospitalization | 10 (6–20) | 11 (6–28) | 9 (7–15) | 0.208 |
| PVTT (n = 45) | BDTT (n = 16) | Coexisted (n = 11) | p-Value | |
|---|---|---|---|---|
| Preoperative AFP, ng/mL | 45.3 (2.2–17,342.0) | 18.3 (4.7–7933) | 40.3 (3.9–37,478.0) | 0.462 |
| Preoperative PIVKA-II, ng/mL | 473 (15–16,650) | 52 (26–500) | 132 (16–520) | 0.079 |
| MoRAL score at diagnosis time | 336.2 (45.6–1504.1) | 83.7 (64.9–424.1) | 133.2 (53.8–447.1) | 0.079 |
| MoRAL score at LT | 273.2 (48.9–3279.1) | 161.5 (69.2–1478.5) | 107.9 (54.8–458.3) | 0.201 |
| MoRAL score, maximum | 291.3 (63.9–3017.9) | 137.3 (73.3–1684.1) | 140.7 (75.1–765.9) | 0.111 |
| Tumor number, n | 0.189 | |||
| Solitary | 9 | 9 | 7 | |
| Multiple | 33 (2–50) | 7 (2–10) | 4 (2–13) | |
| Tumor differentiation | 0.551 | |||
| Moderate | 34 (75.6%) | 13 (81.3%) | 9 (81.8%) | |
| Poor | 11 (24.4%) | 3 (18.8%) | 2 (18.2%) | |
| Maximum tumor size, cm | 4.3 (1.0–17.0) | 3.8 (1.0–16.0) | 4.8 (2–14) | 0.838 |
| Total tumor size, cm | 10.0 (2.0–29.0) | 10.0 (3.0–31.0) | 9.6 (3.0–30.0) | 0.962 |
| Pathologic viability | 11 (24.4%) | 5 (31.3%) | 4 (36.4%) | 0.391 |
| MVI | 44 (97.8%) | 14 (87.5%) | 44 (100%) | 0.743 |
| GRWR | 0.98 (0.62–2.21) | 1.08 (0.81–1.69) | 0.95 (0.76–2.29) | 0.280 |
| Donor operation time, min | 327 (210–545) | 347 (203–469) | 336 (211–373) | 0.805 |
| Recipient operation time, min | 526 (247–758) | 511 (358–715) | 366 (264–752) | 0.180 |
| Cold ischemic time, min | 87 (37–448) | 85 (66–141) | 91 (58–415) | 0.789 |
| Warm ischemic time, min | 36 (13–88) | 36 (18–90) | 40 (17–60) | 0.779 |
| Macrosteatosis, % | 5 (1–25) | 5 (1–60) | 5 (1–25) | 0.238 |
| Microsteatosis, % | 5 (1–20) | 5 (1–25) | 10 (1–75) | 0.355 |
| PVTT (n = 45) | BDTT (n = 16) | Coexisted (n = 11) | p-Value | |
|---|---|---|---|---|
| mTOR inhibitor | 0.879 | |||
| None Combined tacrolimus Only mTOR inhibitor use | 14 (31.1%) 29 (64.4%) 2 (4.4%) | 7 (43.8%) 6 (37.5%) 3 (18.8%) | 4 (36.4%) 6 (54.5%) 1 (9.1%) | |
| Acute cellular rejection | 10 (22.2%) | 0 (0%) | 2 (18.2%) | 0.325 |
| Frequency of acute rejection | 0.158 | |||
| 1 time 2 times 3 times | 7 2 1 | 0 0 0 | 2 0 0 | |
| HCC recurrence | 31 (68.9%) | 8 (50.0%) | 7 (63.6%) | 0.456 |
| Mortality | 30 (66.7%) | 8 (50.0%) | 8 (72.7%) | 0.928 |
| HCC recurrence organ | 0.062 | |||
| Liver Lung Bone Lymph node Others | 10 (22.2%) 18 (40%) 3 (6.7%) 0 (0%) 10 (22.2%) | 6 (75.0%) 1 (12.5%) 0 (0%) 1 (12.5%) 0 (0%) | 4 (57.1%) 1 (14.3%) 0 (0%) 0 (0%) 2 (28.6%) | |
| ICU stay before LT, days | 0 (0–8) | 0 (0–2) | 0 (0–3) | 0.530 |
| ICU stay after LT, days | 6 (3–11) | 6 (3–20) | 5 (3–29) | 0.676 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex, female | 2.490 | 0.750–8.270 | 0.136 | |||
| HBV | 1.125 | 0.476–2.658 | 0.788 | |||
| MELD score | 1.046 | 1.013–1.080 | 0.006 | 1.041 | 1.007–1.076 | 0.018 |
| Maximum tumor size | 1.082 | 1.002–1.169 | 0.045 | |||
| Total tumor size | 1.038 | 0.997–1.081 | 0.067 | |||
| Pathologic viability | 0.547 | 0.271–1.103 | 0.092 | |||
| PVTT | 1 | 1 | 0.197 | |||
| BDTT | 0.704 | 0.380–1.305 | 0.265 | |||
| BDTT only | 0.519 | 0.238–1.130 | 0.099 | |||
| PVTT and BDTT | 1.187 | 0.519–2.715 | 0.685 | |||
| Intrahepatic metastasis | 1.414 | 0.754–2.654 | 0.280 | |||
| Locoregional treatments | 1.170 | 0.615–2.225 | 0.632 | |||
| Treatment response | ||||||
| CR PR SD PD | 1 0.506 0.170 1.972 | 1 0.180–1.422 0.023–1.242 0.766–5.076 | 0.067 0.196 0.081 0.159 | |||
| MoRAL score before LT | 1.001 | 1.001–1.001 | <0.001 | 1.001 | 1.000–1.001 | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 1.018 | 0.982–1.056 | 0.338 | |||
| Sex, female | 2.568 | 0.773–8.528 | 0.124 | |||
| HBV | 1.143 | 0.451–2.896 | 0.777 | |||
| MELD score | 1.055 | 1.023–1.088 | 0.001 | 1.105 | 1.024–1.192 | 0.010 |
| Child–Pugh class | ||||||
| A B C | 1 2.935 4.441 | 1 1.419–6.074 2.063–9.563 | <0.001 0.004 <0.001 | |||
| HRS, HD, or CRRT | 9.076 | 2.032–40.548 | 0.004 | |||
| Maximum tumor size | 1.074 | 0.992–1.164 | 0.079 | |||
| PVTT | 1 | 1 | 0.168 | |||
| BDTT | 0.922 | 0.502–1.694 | 0.794 | |||
| BDTT only | 0.640 | 0.293–1.398 | 0.263 | |||
| PVTT and BDTT | 1.674 | 0.754–3.720 | 0.206 | |||
| Treatment response | ||||||
| CR PR SD PD | 1 0.473 0.721 2.616 | 1 0.145–1.544 0.221–2.350 1.087–6.298 | 0.064 0.215 0.587 0.032 | |||
| MoRAL score before LT | 1.001 | 1.000–1.001 | 0.003 | 1.001 | 1.000–1.001 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Kim, J.; Rhu, J.; Choi, G.-S.; Joh, J.-W. Long-Term Outcomes of Liver Transplantation in Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Comparison with Portal Vein Tumor Thrombus. Cancers 2023, 15, 4225. https://doi.org/10.3390/cancers15174225
Lee JS, Kim J, Rhu J, Choi G-S, Joh J-W. Long-Term Outcomes of Liver Transplantation in Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Comparison with Portal Vein Tumor Thrombus. Cancers. 2023; 15(17):4225. https://doi.org/10.3390/cancers15174225
Chicago/Turabian StyleLee, Ji Soo, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, and Jae-Won Joh. 2023. "Long-Term Outcomes of Liver Transplantation in Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Comparison with Portal Vein Tumor Thrombus" Cancers 15, no. 17: 4225. https://doi.org/10.3390/cancers15174225
APA StyleLee, J. S., Kim, J., Rhu, J., Choi, G.-S., & Joh, J.-W. (2023). Long-Term Outcomes of Liver Transplantation in Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Comparison with Portal Vein Tumor Thrombus. Cancers, 15(17), 4225. https://doi.org/10.3390/cancers15174225





