Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathophysiology of Lung Cancer
3. Cell Signaling Pathways in Lung Cancer
3.1. RAS/RAF/MEK/ERK Pathway
3.2. PI3K/Akt/mTOR Pathway
3.3. JAK-STAT Pathway
3.4. NRF2-KEAP1-ARE Pathway
3.5. PD-1/PD-L1 Pathway
4. Potential Therapeutic Targets for Lung Cancer
5. Current Therapeutic Strategies for Mitigating Lung Cancer and Associated Adversities
6. Literature Search and Selection Process
7. Anticancer Potential of Bioactive Phytocompounds in Lung Cancer
7.1. Preclinical Studies
7.1.1. Alkaloids
Acutiaporberine
β-Carboline
Berberine
Evodiamine
Hirsutine
Homoharringtonine
Indole-3-Carbinol
Melosine B
Piperine
Solamargine
Vallesiachotamine and Iso-Vallesiachotamine
7.1.2. Phenolics
Acacetin
Apocynin
Baicalein
Batatasin
Caffeic Acid
Cardamonin
Casticin
Chrysin
Curcumin
p-Coumaric Acid
Epigallocatechin Gallate
Ferulic Acid
Fisetin
Gallic Acid
Genistein
Gigantol
Hesperidin
Honokiol
Isorhamnetin
Kaempferol
Kurarinone
Luteolin
Moscatilin
Naringenin
Nobiletin
Osthol
Phloretin
Polydatin
Polymethoxyflavones
Pterostilbene
Quercetin
Resveratrol
Salicylic Acid
Tangeretin Derivative
Tatariside
7.1.3. Sulfur-Containing Compounds
Allicin
Sulforaphane
7.1.4. Terpenoids
Abietane Diterpene
β-Sitosterol
Betulinic Acid
Cucurbitacin B
Dihydroartemisinin
Oridonin
Scabertopin
Soyasapogenol
Thymoquinone
Ursolic Acid
Withaferin A
7.1.5. Miscellaneous Compounds
Cannabidiol
Cypripedin
Daucosterol
Emodin
Glossogin
Hypericin
Ouabain
Physalin A
Rhein
Withanone
7.2. Clinical Studies
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Wspolczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.R.; Gazdar, A.F.; Clarke, B.E. The pivotal role of pathology in the management of lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S463–S478. [Google Scholar] [CrossRef]
- Nojiri, T.; Hamasaki, T.; Inoue, M.; Shintani, Y.; Takeuchi, Y.; Maeda, H.; Okumura, M. Long-Term Impact of Postoperative Complications on Cancer Recurrence Following Lung Cancer Surgery. Ann. Surg. Oncol. 2017, 24, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.I.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Ghadiri, M.; Leong, C.R.; Young, P.M.; Traini, D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv. Drug Deliv. Rev. 2018, 133, 107–130. [Google Scholar] [CrossRef]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef]
- Ban, H.; Kim, K.S.; Oh, I.J.; Yoon, S.H.; Lee, B.; Yu, J.; Kim, S.; Lee, H.S.; Shin, H.J.; Park, C.K.; et al. Efficacy and safety of docetaxel plus oxaliplatin as a first-line chemotherapy in patients with advanced or metastatic non-small cell lung cancer. Thorac. Cancer 2014, 5, 525–529. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Choudhary, N.; Prabhakar, P.K.; Khatik, G.L.; Chamakuri, S.R.; Tewari, D.; Suttee, A. Evaluation of acute toxicity, in-vitro, in-vivo antidiabetic potential of the flavonoid fraction of the plant Chenopodium album L. Pharmacogn. J. 2021, 13, 765–779. [Google Scholar] [CrossRef]
- Choudhary, N.; Khatik, G.L.; Sharma, R.; Khurana, N.; Lobo, R.; Bhatt, S.; Tewari, D.; Suttee, A. Ameliorative potential of Operculina turpethum against streptozotocin-induced diabetes in rats: Biochemical and histopathological studies. 3 Biotech 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Hayakawa, Y.; Pongrakhananon, V. Molecular mechanisms of natural compounds in cell death induction and sensitization to chemotherapeutic drugs in lung cancer. Phyther. Res. 2019, 33, 2531–2547. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, T.M.; Di, L. Natural products with activity against lung cancer: A review focusing on the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 10827. [Google Scholar] [CrossRef]
- Ahmed, B.; Joseph, A.; Das, S.; Roy, S.; Rahaman, S.B.; Akbar, S.; Halder, D.; Ramachandran, A.K. Structure-Activity Relationship Insight of Naturally Occurring Bioactive Molecules and Their Derivatives against Non-Small Cell Lung Cancer: A Comprehensive Review. Curr. Med. Chem. 2022, 29, 6030–6062. [Google Scholar] [CrossRef]
- Heng, W.S.; Kruyt, F.A.E.; Cheah, S.-C. Understanding lung carcinogenesis from a morphostatic perspective: Prevention and therapeutic potential of phytochemicals for targeting cancer stem cells. Int. J. Mol. Sci. 2021, 22, 5697. [Google Scholar] [CrossRef]
- Singh, J.; Luqman, S.; Meena, A. Emerging role of phytochemicals in targeting predictive, prognostic, and diagnostic biomarkers of lung cancer. Food Chem. Toxicol. 2020, 144, 111592. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.G.; Sarker, S.D.; Saleem, I.Y.; Hutcheon, G.A. Delivery of natural phenolic compounds for the potential treatment of lung cancer. DARU J. Pharm. Sci. 2019, 27, 433–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [Green Version]
- Byers, L.A.; Rudin, C.M. Small cell lung cancer: Where do we go from here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Santarpia, M.; Daffinà, M.G.; Karachaliou, N.; González-Cao, M.; Lazzari, C.; Altavilla, G.; Rosell, R. Targeted drugs in small-cell lung cancer. Transl. Lung Cancer Res. 2016, 5, 51–70. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Gazdar, A.F. Lung cancer preneoplasia. Annu. Rev. Pathol. 2006, 1, 331–348. [Google Scholar] [CrossRef]
- Sánchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in Lung Cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Kadara, H.; Scheet, P.; Wistuba, I.I.; Spira, A.E. Early Events in the Molecular Pathogenesis of Lung Cancer. Cancer Prev. Res. 2016, 9, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhardt, E.B.; Jalal, S.I. Small Cell Lung Cancer. Cancer Treat. Res. 2016, 170, 301–322. [Google Scholar] [CrossRef]
- Kadara, H.; Wistuba, I.I. Field cancerization in non-small cell lung cancer: Implications in disease pathogenesis. Proc. Am. Thorac. Soc. 2012, 9, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Steiling, K.; Ryan, J.; Brody, J.S.; Spira, A. The field of tissue injury in the lung and airway. Cancer Prev. Res. 2008, 1, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, O.; Stout, A.P.; Hammond, E.C.; Garfinkel, L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N. Engl. J. Med. 1961, 265, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Pirlog, R.; Cismaru, A.; Nutu, A.; Berindan-Neagoe, I. Field Cancerization in NSCLC: A New Perspective on MicroRNAs in Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 746. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Zalzale, H.; Rahal, Z.; Khalifeh, Y.; Sinjab, A.; Kadara, H. Insights Into Lung Cancer Immune-Based Biology, Prevention, and Treatment. Front. Immunol. 2020, 11, 159. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.A.; Hughes, B.G.M. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl. Lung Cancer Res. 2015, 4, 36–54. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef] [Green Version]
- Wistuba, I.I.; Gazdar, A.F.; Minna, J.D. Molecular genetics of small cell lung carcinoma. Semin. Oncol. 2001, 28, 3–13. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, Y.; Arkenau, H.-T.; Lao, T.; Chu, L.; Xu, Q. Signal pathways and precision therapy of small-cell lung cancer. Signal Transduct. Target. Ther. 2022, 7, 187. [Google Scholar] [CrossRef]
- Abolfathi, H.; Arabi, M.; Sheikhpour, M. A literature review of microRNA and gene signaling pathways involved in the apoptosis pathway of lung cancer. Respir. Res. 2023, 24, 55. [Google Scholar] [CrossRef]
- Iksen; Pothongsrisit, S.; Pongrakhananon, V. Targeting the PI3K/AKT/mTOR Signaling Pathway in Lung Cancer: An Update Regarding Potential Drugs and Natural Products. Molecules 2021, 26, 4100. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Hasan, G.M.; Eldin, S.M.; Adnan, M.; Riaz, M.B.; Islam, A.; Khan, I.; Hassan, M.I. Investigating regulated signaling pathways in therapeutic targeting of non-small cell lung carcinoma. Biomed. Pharmacother. 2023, 161, 114452. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.F.A.; Barros, P.; Moyer, M.P.; Matos, P.; Jordan, P. Expression of tumor-related Rac1b antagonizes B-Raf-induced senescence in colorectal cells. Cancer Lett. 2015, 369, 368–375. [Google Scholar] [CrossRef]
- Ritt, D.A.; Abreu-Blanco, M.T.; Bindu, L.; Durrant, D.E.; Zhou, M.; Specht, S.I.; Stephen, A.G.; Holderfield, M.; Morrison, D.K. Inhibition of Ras/Raf/MEK/ERK Pathway Signaling by a Stress-Induced Phospho-Regulatory Circuit. Mol. Cell 2016, 64, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Avery, T.Y.; Köhler, N.; Zeiser, R.; Brummer, T.; Ruess, D.A. Onco-immunomodulatory properties of pharmacological interference with RAS-RAF-MEK-ERK pathway hyperactivation. Front. Oncol. 2022, 12, 931774. [Google Scholar] [CrossRef]
- Cristea, S.; Sage, J. Is the Canonical RAF/MEK/ERK Signaling Pathway a Therapeutic Target in SCLC? J. Thorac. Oncol. 2016, 11, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Xu, X.; Fan, Y. KRAS-Mutant Non-Small Cell Lung Cancer: An Emerging Promisingly Treatable Subgroup. Front. Oncol. 2021, 11, 672612. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [Green Version]
- Chilamakuri, R.; Agarwal, S. Direct Targeting of the Raf-MEK-ERK Signaling Cascade Inhibits Neuroblastoma Growth. Curr. Oncol. 2022, 29, 6508–6522. [Google Scholar] [CrossRef]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Papadimitrakopoulou, V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non–small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurutani, J.; Fukuoka, J.; Tsurutani, H.; Shih, J.H.; Hewitt, S.M.; Travis, W.D.; Jen, J.; Dennis, P.A. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J. Clin. Oncol. 2006, 24, 306–314. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Ligorio, C.; Jänne, P.A.; Toschi, L.; Rossi, E.; Trisolini, R.; Paioli, D.; Holmes, A.J.; Magrini, E.; Finocchiaro, G.; et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: The ONCOBELL trial. J. Clin. Oncol. 2007, 25, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J.; Zheng, S.; Aldape, K.; Hinds, P.W.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. PTEN expression in non-small-cell lung cancer: Evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 2005, 36, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Lee, H.-Y.; Lee, J.I.; Wang, L.; Issa, J.-P.; Kemp, B.L.; Liu, D.D.; Kurie, J.M.; Mao, L.; Khuri, F.R. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin. Cancer Res. 2002, 8, 1178–1184. [Google Scholar]
- Okudela, K.; Suzuki, M.; Kageyama, S.; Bunai, T.; Nagura, K.; Igarashi, H.; Takamochi, K.; Suzuki, K.; Yamada, T.; Niwa, H.; et al. PIK3CA mutation and amplification in human lung cancer. Pathol. Int. 2007, 57, 664–671. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shigematsu, H.; Nomura, M.; Lockwood, W.W.; Sato, M.; Okumura, N.; Soh, J.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008, 68, 6913–6921. [Google Scholar] [CrossRef] [Green Version]
- Kawano, O.; Sasaki, H.; Okuda, K.; Yukiue, H.; Yokoyama, T.; Yano, M.; Fujii, Y. PIK3CA gene amplification in Japanese non-small cell lung cancer. Lung Cancer 2007, 58, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Angulo, B.; Suarez-Gauthier, A.; Lopez-Rios, F.; Medina, P.P.; Conde, E.; Tang, M.; Soler, G.; Lopez-Encuentra, A.; Cigudosa, J.C.; Sanchez-Cespedes, M. Expression signatures in lung cancer reveal a profile for EGFR-mutant tumours and identify selective PIK3CA overexpression by gene amplification. J. Pathol. 2008, 214, 347–356. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Chua, P.J.; Bay, B.H.; Baeg, G.H. The JAK/STAT signaling cascade in gastric carcinoma (Review). Int. J. Oncol. 2015, 47, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wu, S.; Chen, H.; Huang, Y.; Qiu, G.; Liu, L.; Li, Y. Crizotinib induces apoptosis of lung cancer cells through JAK-STAT pathway. Oncol. Lett. 2018, 16, 5992–5996. [Google Scholar] [CrossRef] [Green Version]
- Espert, L.; Dusanter-Fourt, I.; Chelbi-Alix, M.K. Negative regulation of the JAK/STAT: Pathway implication in tumorigenesis. Bull. Cancer 2005, 92, 845–857. [Google Scholar]
- Camiña, N.; Penning, T.M. Genetic and epigenetic regulation of the NRF2-KEAP1 pathway in human lung cancer. Br. J. Cancer 2022, 126, 1244–1252. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, Z.; Aldrich, K.E.; Lockwood, L.; Odom, A.L.; Liby, K.T. A novel NRF2 pathway inhibitor sensitizes keap1-mutant lung cancer cells to chemotherapy. Mol. Cancer Ther. 2021, 20, 1692–1701. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Q.; Yu, S.; Chu, Q.; Chen, Y.; Wu, K.; Wang, L. NRF2-Driven KEAP1 transcription in human lung cancer. Mol. Cancer Res. 2020, 18, 1465–1476. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. The NRF2, thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Rocha, C.R.R.; Kinker, G.S.; Pelegrini, A.L.; Menck, C.F.M. The balance between NRF2/GSH antioxidant mediated pathway and DNA repair modulates cisplatin resistance in lung cancer cells. Sci. Rep. 2019, 9, 17639. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.; Kim, D.; Min, D.W.; Kwon, S.H.; Lee, J.Y. NRF2 regulates cell motility through RhoA–ROCK1 signalling in non-small-cell lung cancer cells. Sci. Rep. 2021, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Lignitto, L.; LeBoeuf, S.E.; Homer, H.; Jiang, S.; Askenazi, M.; Karakousi, T.R.; Pass, H.I.; Bhutkar, A.J.; Tsirigos, A.; Ueberheide, B.; et al. NRF2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell 2019, 178, 316–329.e18. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Ye, X.; Zhang, L.; Wang, Z.; Xu, X.; Shen, Y.; Zheng, C. Loss-of-function mutations in KEAP1 drive lung cancer progression via KEAP1/NRF2 pathway activation. Cell Commun. Signal. 2020, 18, 98. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, X.; Meng, D.; Zeng, L.; Zhuang, R.; Huang, S.; Lv, W.; Hu, J. NRF2 Mediates Metabolic Reprogramming in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 578315. [Google Scholar] [CrossRef]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebuzzi, S.E.; Zullo, L.; Rossi, G.; Grassi, M.; Murianni, V.; Tagliamento, M.; Prelaj, A.; Coco, S.; Longo, L.; Dal Bello, M.G.; et al. Novel Emerging Molecular Targets in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 2625. [Google Scholar] [CrossRef] [PubMed]
- Dholaria, B.; Hammond, W.; Shreders, A.; Lou, Y. Emerging therapeutic agents for lung cancer. J. Hematol. Oncol. 2016, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Piperdi, B.; Perez-Soler, R. Role of erlotinib in the treatment of non-small cell lung cancer: Clinical outcomes in wild-type epidermal growth factor receptor patients. Drugs 2012, 72 (Suppl. S1), 11–19. [Google Scholar] [CrossRef] [Green Version]
- Nurwidya, F.; Takahashi, F.; Takahashi, K. Gefitinib in the treatment of nonsmall cell lung cancer with activating epidermal growth factor receptor mutation. J. Nat. Sci. Biol. Med. 2016, 7, 119–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Antonicelli, A.; Cafarotti, S.; Indini, A.; Galli, A.; Russo, A.; Cesario, A.; Lococo, F.M.; Russo, P.; Mainini, A.F.; Bonifati, L.G. EGFR-targeted therapy for non-small cell lung cancer: Focus on EGFR oncogenic mutation. Int. J. Med. Sci. 2013, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Scagliotti, G.; Moro-Sibilot, D.; Kollmeier, J.; Favaretto, A.; Cho, E.K.; Grosch, H.; Kimmich, M.; Girard, N.; Tsai, C.-M.; Hsia, T.-C. A randomized-controlled phase 2 study of the MET antibody emibetuzumab in combination with erlotinib as first-line treatment for EGFR mutation–positive NSCLC patients. J. Thorac. Oncol. 2020, 15, 80–90. [Google Scholar] [CrossRef]
- Boolell, V.; Alamgeer, M.; Watkins, D.N.; Ganju, V. The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers 2015, 7, 1815–1846. [Google Scholar] [CrossRef]
- Golding, B.; Luu, A.; Jones, R.; Viloria-Petit, A.M. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol. Cancer 2018, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Galiacy, S.; Allouche, M. Targeting ALK in cancer: Therapeutic potential of proapoptotic peptides. Cancers 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, H.; Kim, J.-H.; Lim, H.J.; Kim, J.-H.; Kim, M.; Koh, J.; Im, J.-Y.; Kim, B.-K.; Won, M.; Park, J.-H.; et al. DNA methylome and single-cell transcriptome analyses reveal CDA as a potential druggable target for ALK inhibitor–resistant lung cancer therapy. Exp. Mol. Med. 2022, 54, 1236–1249. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Yu, Z.; Lu, Y.; Fan, J.; Ni, Y.; Ma, L. microRNA-148a-3p inhibited the proliferation and epithelial-mesenchymal transition progression of non-small-cell lung cancer via modulating Ras/MAPK/Erk signaling. J. Cell. Physiol. 2019, 234, 12786–12799. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X. miR-148a promotes cell sensitivity through downregulating SOS2 in radiation-resistant non-small cell lung cancer cells. Oncol. Lett. 2022, 23, 135. [Google Scholar] [CrossRef]
- Yoder, L.H. An overview of lung cancer symptoms, pathophysiology, and treatment. Medsurg Nurs. 2006, 15, 231. [Google Scholar]
- Mornex, F.; Girard, N. Gemcitabine and radiation therapy in non-small cell lung cancer: State of the art. Ann. Oncol. 2006, 17, 1743–1747. [Google Scholar] [CrossRef]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Rajeshkumar, S.; Tambuwala, M.; Bakshi, H.A.; et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem. Biol. Interact. 2019, 309, 108720. [Google Scholar] [CrossRef]
- Daffrè, E.; Prieto, M.; Huang, H.; Janet-Vendroux, A.; Blanc, K.; N’Guyen, Y.-L.; Fournel, L.; Alifano, M. Normalized Pulmonary Artery Diameter Predicts Occurrence of Postpneumonectomy Respiratory Failure, ARDS, and Mortality. Cancers 2020, 12, 1515. [Google Scholar] [CrossRef]
- Belluomini, L.; Calvetti, L.; Inno, A.; Pasello, G.; Roca, E.; Vattemi, E.; Veccia, A.; Menis, J.; Pilotto, S. SCLC Treatment in the Immuno-Oncology Era: Current Evidence and Unmet Needs. Front. Oncol. 2022, 12, 840783. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, S.; Zhao, Z.; Liu, P.; Ke, C.; Xu, S. New insights into small-cell lung cancer development and therapy. Cell Biol. Int. 2020, 44, 1564–1576. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.A.S.; Cerqueira, N.; Fernandes, P.A.; Ramos, M.J. Ribonucleotide reductase: A mechanistic portrait of substrate analogues inhibitors. Curr. Med. Chem. 2010, 17, 2854–2872. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Understanding ribonucleotide reductase inactivation by gemcitabine. Chem. Eur. J. 2007, 13, 8507–8515. [Google Scholar] [CrossRef]
- Toschi, L.; Cappuzzo, F. Gemcitabine for the treatment of advanced nonsmall cell lung cancer. Onco Targets Ther. 2009, 2, 209. [Google Scholar]
- Yang, J.; Solimando, D.A., Jr.; Waddell, J.A. Docetaxel and Cisplatin regimen for non-small-cell lung cancer. Hosp. Pharm. 2013, 48, 550–557. [Google Scholar] [CrossRef]
- Gubens, M.A.; Wakelee, H.A. Docetaxel in the treatment of non-small cell lung carcinoma: An update and analysis. Lung Cancer Targets Ther. 2010, 1, 63. [Google Scholar]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, H.; Watanabe, K.; Kunikane, H.; Yokoyama, A.; Kudoh, S.; Asakawa, T.; Shibata, T.; Kunitoh, H.; Tamura, T.; Saijo, N. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br. J. Cancer 2007, 97, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Odogwu, L.; Mathieu, L.; Blumenthal, G.; Larkins, E.; Goldberg, K.B.; Griffin, N.; Bijwaard, K.; Lee, E.Y.; Philip, R.; Jiang, X.; et al. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. Oncologist 2018, 23, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Khunger, A.; Khunger, M.; Velcheti, V. Dabrafenib in combination with trametinib in the treatment of patients with BRAF V600-positive advanced or metastatic non-small cell lung cancer: Clinical evidence and experience. Ther. Adv. Respir. Dis. 2018, 12, 1753466618767611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Morrissey, K.M.; Marchand, M.; Patel, H.; Zhang, R.; Wu, B.; Chan, H.P.; Mecke, A.; Girish, S.; Jin, J.Y.; Winter, H.R.; et al. Alternative dosing regimens for atezolizumab: An example of model-informed drug development in the postmarketing setting. Cancer Chemother. Pharmacol. 2019, 84, 1257–1267. [Google Scholar] [CrossRef] [Green Version]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.H.; Keam, B.; Kim, M.; Kim, S.H.; Kim, Y.J.; Kim, T.M.; Kim, D.-W.; Lee, J.S.; Heo, D.S. Low-dose nivolumab can be effective in non-small cell lung cancer: Alternative option for financial toxicity. ESMO Open 2018, 3, e000332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sale, M.J.; Cook, S.J. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem. J. 2013, 450, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Levchenko, E.V.; Kuligina, E.S.; Orlov, S.V. Treating non-small cell lung cancer with selumetinib: An up-to-date drug evaluation. Expert Opin. Pharmacother. 2020, 21, 1943–1953. [Google Scholar] [CrossRef]
- Gandhi, L.; Camidge, D.R.; de Oliveira, M.R.; Bonomi, P.; Gandara, D.; Khaira, D.; Hann, C.L.; McKeegan, E.M.; Litvinovich, E.; Hemken, P.M.; et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 2011, 29, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Bradford, D.; Larkins, E.; Mushti, S.L.; Rodriguez, L.; Skinner, A.M.; Helms, W.S.; Price, L.S.L.; Zirkelbach, J.F.; Li, Y.; Liu, J. FDA approval summary: Selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin. Cancer Res. 2021, 27, 2130–2135. [Google Scholar] [CrossRef]
- Bebb, D.G.; Agulnik, J.; Albadine, R.; Banerji, S.; Bigras, G.; Butts, C.; Couture, C.; Cutz, J.C.; Desmeules, P.; Ionescu, D.N. Crizotinib inhibition of ROS1-positive tumours in advanced non-small-cell lung cancer: A Canadian perspective. Curr. Oncol. 2019, 26, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Desai, S. Crizotinib: A comprehensive review. South Asian J. Cancer 2013, 2, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Cho, B.C.; Obermannova, R.; Bearz, A.; McKeage, M.; Kim, D.-W.; Batra, U.; Borra, G.; Orlov, S.; Kim, S.-W.; Geater, S.L. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)–positive NSCLC: Primary efficacy results from the ASCEND-8 study. J. Thorac. Oncol. 2019, 14, 1255–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, A.T.; Kim, D.-W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T. Ceritinib in ALK-rearranged non–small-cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Horn, L.; Wakelee, H.A.; Reckamp, K.L.; Blumenschein, G.R.; Infante, J.R.; Carter, C.A.; Waqar, S.N.; Neal, J.W.; Gockerman, J.P.; Harrow, K. Plasma genotyping of patients enrolled on the expansion phase I/II trial of X-396 in patients (pts) with ALK+ non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2016, 34, 9056. [Google Scholar] [CrossRef]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase−Positive Non–Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef]
- Horn, L.; Infante, J.R.; Reckamp, K.L.; Blumenschein, G.R.; Leal, T.A.; Waqar, S.N.; Gitlitz, B.J.; Sanborn, R.E.; Whisenant, J.G.; Du, L.; et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin. Cancer Res. 2018, 24, 2771–2779. [Google Scholar] [CrossRef] [Green Version]
- Russo, A.E.; Priolo, D.; Antonelli, G.; Libra, M.; McCubrey, J.A.; Ferraù, F. Bevacizumab in the treatment of NSCLC: Patient selection and perspectives. Lung Cancer 2017, 8, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Reck, M.; von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL). Ann. Oncol. 2010, 21, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.F.; Canon, J.-L.; De Braud, F.; Grossi, F.; De Pas, T.; Gray, J.E.; Su, W.-C.; Felip, E.; Yoshioka, H.; Gridelli, C.; et al. Safety and Efficacy of Buparlisib (BKM120) in Patients with PI3K Pathway-Activated Non-Small Cell Lung Cancer: Results from the Phase II BASALT-1 Study. J. Thorac. Oncol. 2015, 10, 1319–1327. [Google Scholar] [CrossRef] [Green Version]
- McGowan, D.R.; Skwarski, M.; Bradley, K.M.; Campo, L.; Fenwick, J.D.; Gleeson, F.V.; Green, M.; Horne, A.; Maughan, T.S.; McCole, M.G. Buparlisib with thoracic radiotherapy and its effect on tumour hypoxia: A phase I study in patients with advanced non-small cell lung carcinoma. Eur. J. Cancer 2019, 113, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Cochin, V.; Gross-Goupil, M.; Ravaud, A.; Godbert, Y.; Le Moulec, S. Cabozantinib: Mechanism of action, efficacy and indications. Bull. Cancer 2017, 104, 393–401. [Google Scholar] [CrossRef]
- Wang, G.; Gao, J.; Lv, J.; Chen, X.; Wu, J.; Wang, R.; Jiang, J. Effective Treatment with Cabozantinib in an Advanced Non-Small-Cell Lung Cancer Patient Harboring a CD74-ROS1 Fusion: A Case Report. Onco Targets Ther. 2020, 13, 1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, T.; Hirai, F.; Makiyama, A.; Seto, T.; Bando, H.; Naito, Y.; Yoh, K.; Ishihara, K.; Kakizume, T.; Natsume, K. Phase I dose-escalation study of capmatinib (INC 280) in Japanese patients with advanced solid tumors. Cancer Sci. 2019, 110, 1340–1351. [Google Scholar] [CrossRef]
- Yeo, W.-L.; Riely, G.J.; Yeap, B.Y.; Lau, M.W.; Warner, J.L.; Bodio, K.; Huberman, M.S.; Kris, M.G.; Tenen, D.G.; Pao, W. Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations. J. Thorac. Oncol. 2010, 5, 1048–1053. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Reckamp, K.L.; Kim, Y.-C.; Novello, S.; Smit, E.F.; Lee, J.-S.; Su, W.-C.; Akerley, W.L.; Blakely, C.M.; Groen, H.J.M.; et al. Efficacy and Safety of Rociletinib Versus Chemotherapy in Patients With EGFR-Mutated NSCLC: The Results of TIGER-3, a Phase 3 Randomized Study. JTO Clin. Res. Rep. 2021, 2, 100114. [Google Scholar] [CrossRef]
- Sequist, L.V.; Soria, J.-C.; Goldman, J.W.; Wakelee, H.A.; Gadgeel, S.M.; Varga, A.; Papadimitrakopoulou, V.; Solomon, B.J.; Oxnard, G.R.; Dziadziuszko, R. Rociletinib in EGFR-mutated non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Pirker, R.; Filipits, M. Cetuximab in non-small-cell lung cancer. Transl. Lung Cancer Res. 2012, 1, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Becerra, C.; Spira, A.I.; Conkling, P.R.; Richey, S.L.; Hanna, W.T.; Cote, G.M.; Heist, R.S.; Langleben, A.; Laurie, S.A.; Edenfield, W.J. A phase Ib/II study of cancer stemness inhibitor napabucasin (BB608) combined with weekly paclitaxel in advanced non-small cell lung cancer. J. Clin. Oncol. 2016, 34, 9093. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Wei, X. Napabucasin, a novel inhibitor of STAT3, inhibits growth and synergises with doxorubicin in diffuse large B-cell lymphoma. Cancer Lett. 2020, 491, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.V.N.; Brabender, J.; Gandara, D.; Alberola, V.; Camps, C.; Domine, M.; Cardenal, F.; Sánchez, J.M.; Gumerlock, P.H.; Tarón, M.; et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin. Cancer Res. 2002, 8, 2286–2291. [Google Scholar]
- de Sousa, G.F.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular mechanisms of action associated with chemoresistance. Braz. J. Pharm. Sci. 2014, 50, 693–701. [Google Scholar] [CrossRef]
- Schneider, B.J.; Saxena, A.; Downey, R.J. Surgery for early-stage small cell lung cancer. J. Natl. Compr. Cancer Netw. 2011, 9, 1132–1139. [Google Scholar] [CrossRef]
- Chatterjee, D.; Roy, S.; Hazra, A.; Dasgupta, P.; Ganguly, S.; Das, A.K. Variation of adverse drug reaction profile of platinum-based chemotherapy with body mass index in patients with solid tumors: An observational study. Indian J. Pharmacal. 2014, 46, 222–224. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Surendiran, A.; Balamurugan, N.; Gunaseelan, K.; Akhtar, S.; Reddy, K.S.; Adithan, C. Adverse drug reaction profile of cisplatin-based chemotherapy regimen in a tertiary care hospital in India: An evaluative study. Indian J. Pharmacol. 2010, 42, 40–43. [Google Scholar] [CrossRef] [Green Version]
- Barlesi, F.; Scherpereel, A.; Rittmeyer, A.; Pazzola, A.; Tur, N.F.; Kim, J.-H.; Ahn, M.-J.; Aerts, J.G.; Gorbunova, V.; Vikström, A.; et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J. Clin. Oncol. 2013, 31, 3004–3011. [Google Scholar] [CrossRef]
- Barlesi, F.; Scherpereel, A.; Gorbunova, V.; Gervais, R.; Vikström, A.; Chouaid, C.; Chella, A.; Kim, J.H.; Ahn, M.J.; Reck, M.; et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann. Oncol. 2014, 25, 1044–1052. [Google Scholar] [CrossRef]
- Ricciardi, S.; Tomao, S.; de Marinis, F. Efficacy and safety of erlotinib in the treatment of metastatic non-small-cell lung cancer. Lung Cancer 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M., Jr.; Zhang, P.; Mookerjee, B. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Casaluce, F.; Sgambato, A.; Maione, P.; Sacco, P.C.; Santabarbara, G.; Gridelli, C. Selumetinib for the treatment of non-small cell lung cancer. Expert Opin. Investig. Drugs 2017, 26, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, E.C.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Liu, Y.; Zhao, H.; Bi, Y.; Liu, J.; Rahman, A.; Wearne, E.; et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin. Cancer Res. 2022, 28, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F. Sotorasib for lung cancers with KRAS p. G12C mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Tie, Y.; Yang, H.; Zhao, R.; Zheng, H.; Yang, D.; Zhao, J.; Liu, M. Safety and efficacy of atezolizumab in the treatment of cancers: A systematic review and pooled-analysis. Drug Des. Dev. Ther. 2019, 13, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Tomasini, P.; Egea, J.; Souquet-Bressand, M.; Greillier, L.; Barlesi, F. Alectinib in the treatment of ALK-positive metastatic non-small cell lung cancer: Clinical trial evidence and experience with a focus on brain metastases. Ther. Adv. Respir. Dis. 2019, 13, 1753466619831906. [Google Scholar] [CrossRef]
- Vuong, H.G.; Nguyen, T.Q.; Nguyen, H.C.; Nguyen, P.T.; Ho, A.T.N.; Hassell, L. Efficacy and safety of crizotinib in the treatment of advanced non-small-cell lung cancer with ROS1 rearrangement or MET alteration: A systematic review and meta-analysis. Target. Oncol. 2020, 15, 589–598. [Google Scholar] [CrossRef]
- Besse, B.; Salgia, R.; Solomon, B.; Shaw, A.; Kim, D.; Schachar, R.; Wilner, K.; Reisman, A.; Bartlett, C.H.; Iyer, S. Visual disturbances in patients (pts) with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) treated with crizotinib. Ann. Oncol. 2012, 23, ix416. [Google Scholar] [CrossRef]
- Schnell, P.; Safferman, A.Z.; Bartlett, C.H.; Tang, Y.; Wilner, K.D. Clinical presentation of hepatotoxicity-associated crizotinib in ALK-positive (ALK+) advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2012, 30, 7598. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Azada, M.; Dy, J.; Stiber, J.A. Asymptomatic profound sinus bradycardia (heart rate ≤ 45) in non-small cell lung cancer patients treated with crizotinib. J. Thorac. Oncol. 2011, 6, 2135–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weickhardt, A.J.; Rothman, M.S.; Salian-Mehta, S.; Kiseljak-Vassiliades, K.; Oton, A.B.; Doebele, R.C.; Wierman, M.E.; Camidge, D.R. Rapid-onset hypogonadism secondary to crizotinib use in men with metastatic nonsmall cell lung cancer. Cancer 2012, 118, 5302–5309. [Google Scholar] [CrossRef]
- Song, J.; Fan, X.; Zhao, Z.; Chen, M.; Chen, W.; Wu, F.; Zhang, D.; Chen, L.; Tu, J.; Ji, J. 125I brachytherapy of locally advanced non-small-cell lung cancer after one cycle of first-line chemotherapy: A comparison with best supportive care. Onco Targets Ther. 2017, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Hilaris, B.S.; Mastoras, D.A. Contemporary brachytherapy approaches in non–small-cell lung cancer. J. Surg. Oncol. 1998, 69, 258–264. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action BT—Plant Toxins; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–15. ISBN 978-94-007-6728-7. [Google Scholar]
- Chen, Q.; Peng, W.; Qi, S.; Xu, A. Apoptosis of human highly metastatic lung cancer cell line 95-D induced by acutiaporberine, a novel bisalkaloid derived from Thalictrum acutifolium. Planta Med. 2002, 68, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Yamada, H.; Moriya, S.; Miyazawa, K. The β-carboline alkaloid harmol induces cell death via autophagy but not apoptosis in human non-small cell lung cancer A549 cells. Biol. Pharm. Bull. 2011, 34, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. P53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Liu, Y.; Zhang, R.; Zhang, B.; Wang, T.; Zhu, X.; Mei, L.; Chen, H.; Zhang, H.; Ming, P.; et al. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer cells. Sci. Rep. 2015, 5, 8477. [Google Scholar] [CrossRef] [Green Version]
- Weng, T.Y.; Wu, H.F.; Li, C.Y.; Hung, Y.H.; Chang, Y.W.; Chen, Y.L.; Hsu, H.P.; Chen, Y.H.; Wang, C.Y.; Chang, J.Y.; et al. Homoharringtonine induced immune alteration for an Efficient Anti-tumor Response in Mouse Models of Non-small Cell Lung Adenocarcinoma Expressing Kras Mutation. Sci. Rep. 2018, 8, 8216. [Google Scholar] [CrossRef]
- Lim, H.M.; Park, S.H.; Nam, M.J. Induction of apoptosis in indole-3-carbinol-treated lung cancer H1299 cells via ROS level elevation. Hum. Exp. Toxicol. 2021, 40, 812–825. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, H.; Yuan, C.M.; Zhang, Y.; Cao, M.M.; Zhang, H.Y.; Feng, Y.; Ding, X.; Zhou, Q.; Zhao, Q.; et al. Cytotoxic indole alkaloids from the fruits of Melodinus cochinchinensis. Phytochemistry 2015, 116, 367–373. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Liao, H.; Li, L.; Pan, L. Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumor Biol. 2014, 35, 3305–3310. [Google Scholar] [CrossRef]
- Marques da Fonseca, L.; Jacques da Silva, L.R.; Santos Dos Reis, J.; Rodrigues da Costa Santos, M.A.; de Sousa Chaves, V.; Monteiro da Costa, K.; Sa-Diniz, J.N.; Freire de Lima, C.G.; Morrot, A.; Nunes Franklim, T.; et al. Piperine Inhibits TGF-β Signaling Pathways and Disrupts EMT-Related Events in Human Lung Adenocarcinoma Cells. Medicines 2020, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Tang, Q.; Xiao, Q.; Yang, L.J.; Hann, S.S. Targeting EP4 downstream c-Jun through ERK1/2-mediated reduction of DNMT1 reveals novel mechanism of solamargine-inhibited growth of lung cancer cells. J. Cell. Mol. Med. 2017, 21, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.P.; Khan, M.A.; Yadav, D.K.; Rawat, A.K.; Singh, R.K.; Ahamad, T.; Hussain, M.K.; Saquib, M.; Khan, M.F. Monoterpene Indole Alkaloids from Anthocephalus cadamba Fruits Exhibiting Anticancer Activity in Human Lung Cancer Cell Line H1299. ChemistrySelect 2018, 3, 8468–8472. [Google Scholar] [CrossRef]
- Chien, S.T.; Lin, S.S.; Wang, C.K.; Lee, Y.B.; Chen, K.S.; Fong, Y.; Shih, Y.W. Acacetin inhibits the invasion and migration of human non-small cell lung cancer A549 cells by suppressing the p38α MAPK signaling pathway. Mol. Cell. Biochem. 2011, 350, 135–148. [Google Scholar] [CrossRef]
- Paul, S.; Chakrabarty, S.; Ghosh, S.; Nag, D.; Das, A.; Dastidar, D.G.; Dasgupta, M.; Dutta, N.; Kumari, M.; Pal, M.; et al. Targeting cellular microtubule by phytochemical apocynin exhibits autophagy-mediated apoptosis to inhibit lung carcinoma progression and tumorigenesis. Phytomedicine 2020, 67, 153152. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Q.; Zhai, Y.; Lu, D.; Li, C.; Fu, Y.; Zheng, Z.; Song, Y.; Guo, J. Baicalein inhibits non-small-cell lung cancer invasion and metastasis by reducing ezrin tension in inflammation microenvironment. Cancer Sci. 2020, 111, 3802–3812. [Google Scholar] [CrossRef]
- Pinkhien, T.; Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Batatasin III inhibits migration of human lung cancer cells by suppressing epithelial to mesenchymal transition and FAK-AKT signals. Anticancer Res. 2017, 37, 6281–6289. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Jaziri, S.K.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Hu, Q.; Shi, L.; Lö, M.; Deng, M.; Luo, G. Cardamonin inhibits the progression of oesophageal cancer by inhibiting the PI3K/AKT signalling pathway. J. Cancer 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, Q.; Shi, D.; Niu, P.; Chen, Y.; Deng, J. MTOR inhibition of cardamonin on antiproliferation of A549 cells is involved in a FKBP12 independent fashion. Life Sci. 2014, 99, 44–51. [Google Scholar] [CrossRef]
- He, W.; Jiang, Y.; Zhang, X.; Zhang, Y.; Ji, H.; Zhang, N. Anticancer cardamonin analogs suppress the activation of NF-kappaB pathway in lung cancer cells. Mol. Cell. Biochem. 2014, 389, 25–33. [Google Scholar] [CrossRef]
- Ramchandani, S.; Naz, I.; Lee, J.H.; Khan, M.R.; Ahn, K.S. An overview of the potential antineoplastic effects of casticin. Molecules 2020, 25, 1287. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Cao, X.; Liu, Z.; Guo, H.; Ren, K.; Quan, M.; Zhou, Y.; Xiang, H.; Cao, J. Casticin suppresses self-renewal and invasion of lung cancer stem-like cells from A549 cells through down-regulation of pAkt. Acta Biochim. Biophys. Sin. 2014, 46, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, S.; Suresh, S.; Rahul, B.S.; Saikant, R.; Maya, V.; Gopi, M.; Padmaja, G.; Remani, P. In vitro and in vivo studies of 5,7-dihydroxy flavones isolated from Alpinia galanga (L.) against human lung cancer and ascetic lymphoma. Med. Chem. Res. 2019, 28, 39–51. [Google Scholar] [CrossRef]
- Sak, K. Radiosensitizing Potential of Curcumin in Different Cancer Models. Nutr. Cancer 2020, 72, 1276–1289. [Google Scholar] [CrossRef]
- Lin, S.-S.; Huang, H.-P.; Yang, J.-S.; Wu, J.-Y.; Hsai, T.-C.; Lin, C.-C.; Lin, C.-W.; Kuo, C.-L.; Wood, W.G.; Chung, J.-G. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett. 2008, 272, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Hang, L.W.; Yang, J.S.; Chen, H.Y.; Lin, H.Y.; Chiang, J.H.; Lu, C.C.; Yang, J.L.; Lai, T.Y.; Ko, Y.C.; et al. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res. 2010, 30, 2125–2133. [Google Scholar]
- Chen, H.W.; Lee, J.Y.; Huang, J.Y.; Wang, C.C.; Chen, W.J.; Su, S.F.; Huang, C.W.; Ho, C.C.; Chen, J.J.W.; Tsai, M.F.; et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008, 68, 7428–7438. [Google Scholar] [CrossRef] [Green Version]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Fujii, A.; Suganuma, M.; Fujiki, H. Apoptosis of human lung cancer cells by curcumin mediated through up-regulation of “growth arrest and DNA damage inducible genes 45 and 153”. Biol. Pharm. Bull. 2010, 33, 1291–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Liu, J.; Zhu, L.; Chen, Q.; Meng, Z.; Sun, L.; Hu, J.; Ni, Z.; Wang, X. Curcumin inhibits growth of human NCI-H292 lung squamous cell carcinoma cells by increasing FOXA2 expression. Front. Pharmacol. 2018, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Wu, J.-G.; Jiang, Y.-B.; Liu, Y.-J.; Sun, T.; Wu, N.; Wu, C.-J. Antitumor activity of 4-O-(2″-O-acetyl-6″-O-p-coumaroyl-β-d-glucopyranosyl)-p-coumaric acid against lung cancers via mitochondrial-mediated apoptosis. Chem. Biol. Interact. 2015, 233, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Phan, A.N.H.; Choi, J.W. Anti-cancer effects of polyphenolic compounds in epidermal growth factor receptor tyrosine kinase inhibitor-resistant non-small cell lung cancer. Pharmacogn. Mag. 2017, 13, 595–599. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. Synergistic inhibition of lung cancer cell lines by (−)-epigallocatechin-3-gallate in combination with clinically used nitrocatechol inhibitors of catechol-O-methyltransferase. Carcinogenesis 2014, 35, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Liu, F.; Zhang, W.; Liu, X.; Lin, B.; Tang, X. Epigallocatechin-3-gallate inhibits nicotine-induced migration and invasion by the suppression of angiogenesis and epithelial-mesenchymal transition in non-small cell lung cancer cells. Oncol. Rep. 2015, 33, 2972–2980. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Chen, S.; Shi, Y.; Li, C.H.; Wang, X.J.; Li, F.J.; Wang, C.H.; Meng, Q.H.; Zhong, J.N.; Liu, M.; et al. Epigallocatechin gallate from green tea exhibits potent anticancer effects in A-549 non-small lung cancer cells by inducing apoptosis, cell cycle arrest and inhibition of cell migration. J. BUON 2017, 22, 1422–1427. [Google Scholar] [PubMed]
- Datta, S.; Sinha, D. Low dose epigallocatechin-3-gallate revives doxorubicin responsiveness by a redox-sensitive pathway in A549 lung adenocarcinoma cells. J. Biochem. Mol. Toxicol. 2022, 36, e22999. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, D. EGCG maintained NRF2-mediated redox homeostasis and minimized etoposide resistance in lung cancer cells. J. Funct. Foods 2019, 62, 103553. [Google Scholar] [CrossRef]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.J.; Qiao, K.S.; Sun, P.; Chen, P.; Li, Q. Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4557–4563. [Google Scholar]
- Amin, A.R.M.R.; Wang, D.; Zhang, H.; Peng, S.; Shin, H.J.C.; Brandes, J.C.; Tighiouart, M.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: Potential role of p53. J. Biol. Chem. 2010, 285, 34557–34565. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, C.; Saha, P.; Panda, C.K.; Das, S. Inhibition of growth, induction of apoptosis and alteration of gene expression by tea polyphenols in the highly metastatic human lung cancer cell line NCI-H460. Asian Pac. J. Cancer Prev. 2005, 6, 326–331. [Google Scholar] [PubMed]
- Khan, N.; Afaq, F.; Khusro, F.H.; Mustafa Adhami, V.; Suh, Y.; Mukhtar, H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer 2012, 130, 1695–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.A.; Piao, M.J.; Madduma Hewage, S.R.K.; Ryu, Y.S.; Oh, M.C.; Kwon, T.K.; Chae, S.; Hyun, J.W. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the MAPK signaling pathway. Tumor Biol. 2016, 37, 9615–9624. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hyun, J.W. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Zhuo, W.; Zhu, Y.; Zhu, B.; Chen, Z. Fisetin, a dietary phytochemical, overcomes erlotinib-resistance of lung adenocarcinoma cells through inhibition of MAPK and AKT pathways. Am. J. Transl. Res. 2016, 8, 4857–4868. [Google Scholar]
- You, B.R.; Park, W.H. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol. Vitr. 2010, 24, 1356–1362. [Google Scholar] [CrossRef]
- Phan, A.N.; Hua, T.N.; Kim, M.-K.; Vo, V.T.; Choi, J.-W.; Kim, H.-W.; Rho, J.K.; Kim, K.W.; Jeong, Y. Gallic acid inhibition of Src-Stat3 signaling overcomes acquired resistance to EGF receptor tyrosine kinase inhibitors in advanced non-small cell lung cancer. Oncotarget 2016, 7, 54702–54713. [Google Scholar] [CrossRef] [Green Version]
- Shiau, R.-J.; Chen, K.-Y.; Wen, Y.-D.; Chuang, C.-H.; Yeh, S.-L. Genistein and β-carotene enhance the growth-inhibitory effect of trichostatin A in A549 cells. Eur. J. Nutr. 2010, 49, 19–25. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Ali, S.; Philip, P.A.; Wozniak, A.; Sarkar, F.H. Retraction to: Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines (Cancer, 10.1002/cncr.24250). Cancer 2016, 122, 3248. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Zhan, S.; Cao, K. Apoptotic activity of genistein on human lung adenocarcinoma SPC-A-1 cells and preliminary exploration of its mechanisms using microarray. Biomed. Pharmacother. 2008, 62, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ahmed, F.; Ali, S.; Philip, P.A.; Kucuk, O.; Sarkar, F.H. Inactivation of nuclear factor κB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005, 65, 6934–6942. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Hao, Y.; Xu, H.; Chen, K.; Ren, B. Gigantol inhibits cell proliferation and induces apoptosis by regulating DEK in non-small cell lung cancer. Exp. Ther. Med. 2021, 22, 1317. [Google Scholar] [CrossRef]
- Unahabhokha, T.; Chanvorachote, P.; Sritularak, B.; Kitsongsermthon, J.; Pongrakhananon, V. Gigantol Inhibits Epithelial to Mesenchymal Process in Human Lung Cancer Cells. Evid. Based Complement. Altern. Med. 2016, 2016, 4561674. [Google Scholar] [CrossRef] [Green Version]
- Losuwannarak, N.; Maiuthed, A.; Kitkumthorn, N.; Leelahavanichkul, A.; Roytrakul, S.; Chanvorachote, P. Gigantol targets cancer stem cells and destabilizes tumors via the suppression of the PI3K/AKT and JAK/STAT pathways in ectopic lung cancer xenografts. Cancers 2019, 11, 2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell. Oncol. 2015, 38, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Xu, S.; Gao, W.; Feng, J.; Zhao, G. Honokiol induces endoplasmic reticulum stress-mediated apoptosis in human lung cancer cells. Life Sci. 2019, 221, 204–211. [Google Scholar] [CrossRef]
- Rauf, A.; Olatunde, A.; Imran, M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Khan, S.A.; Uddin, M.S.; Mitra, S.; Emran, T.B.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, C.F.; Yang, L.; Ma, L. Formylated honokiol analogs showed antitumor activity against lung carcinoma. Anticancer Drugs 2019, 30, 795–802. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, C.S.; Li, S.; Jin, H.; Ho, C.T.; Patel, T. Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol. Nutr. Food Res. 2009, 53, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, W.L.; Gao, Y.D.; Shin, D.S.; Ye, Q.; Su, L.; Jiang, F.; Zhao, B.X.; Miao, J.Y. Novel indolyl-chalcone derivatives inhibit A549 lung cancer cell growth through activating Nrf-2/HO-1 and inducing apoptosis in vitro and in vivo. Sci. Rep. 2017, 7, 3919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.; Hu, K.; Chen, H. Autophagy inhibition enhances isorhamnetin.induced mitochondria.dependent apoptosis in non-small cell lung cancer cells. Mol. Med. Rep. 2015, 12, 5796–5806. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.Y.; Wang, Y.M.; Gong, H.; Zhao, H.; Lv, X.Y.; Yuan, G.H.; Han, S.R. Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cis-platin and carboplatin in non-small cell lung carcinoma (NSCLC). Int. J. Clin. Exp. Pathol. 2015, 8, 25–37. [Google Scholar]
- Hang, M.; Zhao, F.; Chen, S.B.; Sun, Q.; Zhang, C.X. Kaempferol modulates the metastasis of human non-small cell lung cancer cells by inhibiting epithelial-mesenchymal transition. Bangladesh J. Pharmacol. 2015, 10, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.K.; Kim, B.C. Kaempferol Suppresses Transforming Growth Factor-β1-Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, H.W.C.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol. 2007, 45, 2005–2013. [Google Scholar] [CrossRef]
- Kumar, S.; Prajapati, K.S.; Shuaib, M.; Kushwaha, P.P.; Tuli, H.S.; Singh, A.K. Five-Decade Update on Chemopreventive and Other Pharmacological Potential of Kurarinone: A Natural Flavanone. Front. Pharmacol. 2021, 12, 737137. [Google Scholar] [CrossRef]
- Chung, T.W.; Lin, C.C.; Lin, S.C.; Chan, H.L.; Yang, C.C. Antitumor effect of kurarinone and underlying mechanism in small cell lung carcinoma cells. Onco Targets Ther. 2019, 12, 6119–6131. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. Vitr. 2011, 25, 1385–1391. [Google Scholar] [CrossRef]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Li, M.H.; Qin, Y.M.; Jiang, H.Y.; Zhang, X.; Wu, M.H. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of microRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Peng, H.; Li, K.; Zhao, R.; Li, L.; Yu, Y.; Wang, X.; Han, Z. Luteolin exerts an anticancer effect on NCI-H460 human non-small cell lung cancer cells through the induction of Sirt1-mediated apoptosis. Mol. Med. Rep. 2015, 12, 4196–4202. [Google Scholar] [CrossRef] [Green Version]
- Busaranon, K.; Plaimee, P.; Sritularak, B.; Chanvorachote, P. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J. Nat. Med. 2016, 70, 18–27. [Google Scholar] [CrossRef]
- Chang, H.L.; Chang, Y.M.; Lai, S.C.; Chen, K.M.; Wang, K.C.; Chiu, T.T.; Chang, F.H.; Hsu, L.S. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and-9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Luo, X.; Chen, T.; Guo, W.; Liang, C.; Tang, S.; Mo, J. Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J. Cell. Mol. Med. 2021, 25, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Hung, L.V.M.; Unno, T.; Cho, S.K. Nobiletin enhances chemosensitivity to adriamycin through modulation of the Akt/GSK3β/β–catenin/ MYCN/MRP1 signaling pathway in A549 human non-small-cell lung cancer cells. Nutrients 2018, 10, 1829. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, Y.; Qu, D.; Jiang, T.; Li, S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011, 30, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.M.; Zhang, Y.; Qu, D.; Feng, X.W.; Chen, Y.; Zhao, L. Osthole suppresses migration and invasion of A549 human lung cancer cells through inhibition of matrix metalloproteinase-2 and matrix metallopeptidase-9 in vitro. Mol. Med. Rep. 2012, 6, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential anticancer properties of osthol: A comprehensive mechanistic review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Lu, J.J.; Wang, Y.; Pei, L.; Chen, X. Osthole inhibited TGF β-induced epithelial–mesenchymal transition (EMT) by suppressing NF-κB mediated Snail activation in lung cancer A549 cells. Cell Adhes. Migr. 2017, 11, 464–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Wang, R.; Nan, Y.; Li, W.; Wang, Q.; Jin, F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int. J. Oncol. 2016, 48, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Li, X.; Huang, K.; Tang, H.; Ding, X.; Qi, C.; Qin, X.; Xu, Z. Phloretin induces apoptosis of non-small cell lung carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol. Rep. 2015, 34, 2871–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhuang, Z.; Meng, Q.; Jiao, Y.; Xu, J.; Fan, S. Polydatin inhibits growth of lung cancer cells by inducing apoptosis and causing cell cycle arrest. Oncol. Lett. 2014, 7, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.G.; Alosi, J.A.; McDonald, D.E.; McFadden, D.W. Pterostilbene Inhibits Lung Cancer through Induction of Apoptosis1. J. Surg. Res. 2010, 161, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Li, Y.; Jiang, D.; Zhao, J.; Ge, J.F. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol. Med. Rep. 2012, 5, 822–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef]
- Yin, H.T.; Tian, Q.Z.; Guan, L.; Zhou, Y.; Huang, X.E.; Zhang, H. In vitro and in vivo evaluation of the antitumor efficiency of resveratrol against lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1703–1706. [Google Scholar] [CrossRef] [Green Version]
- Vejselova, D.; Kutlu, H.M. Inhibitory effects of salicylic acid on A549 human lung adenocarcinoma cell viability. Turk. J. Biol. 2015, 39, 1–5. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Ho, C.T.; Chang, Y.H.; Tan, K.T.; Chung, T.W.; Wang, B.Y.; Chen, Y.K.; Lin, C.C. Tangeretin derivative, 5-acetyloxy-6,7,8,4′-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol. Ther. 2016, 17, 48–64. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, C.; Ma, X.; Peng, C.; Zhang, H.; Qin, L. Cytotoxic phenylpropanoid glycosides from Fagopyrum tataricum (L.) Gaertn. Food Chem. 2012, 132, 433–438. [Google Scholar] [CrossRef]
- Pandey, N.; Tyagi, G.; Kaur, P.; Pradhana, S.; Rajam, M.V.; Srivastava, T. Allicin Overcomes Hypoxia Mediated Cisplatin Resistance in Lung Cancer Cells through ROS Mediated Cell Death Pathway and by Suppressing Hypoxia Inducible Factors. Cell. Physiol. Biochem. 2020, 54, 748–766. [Google Scholar] [CrossRef]
- Wang, D.-X.; Zou, Y.-J.; Zhuang, X.-B.; Chen, S.-X.; Lin, Y.; Li, W.-L.; Lin, J.-J.; Lin, Z.-Q. Sulforaphane suppresses EMT and metastasis in human lung cancer through miR-616-5p-mediated GSK3β/β-catenin signaling pathways. Acta Pharmacol. Sin. 2017, 38, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.L.; Zhou, S.J.; Zhang, X.M.; Chen, H.Q.; Liu, W. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed. Pharmacother. 2016, 78, 74–80. [Google Scholar] [CrossRef]
- Gao, L.; Cheng, D.; Yang, J.; Wu, R.; Li, W.; Kong, A.N. Sulforaphane epigenetically demethylates the CpG sites of the miR-9-3 promoter and reactivates miR-9-3 expression in human lung cancer A549 cells. J. Nutr. Biochem. 2018, 56, 109–115. [Google Scholar] [CrossRef]
- Garcia, C.; Silva, C.O.; Monteiro, C.M.; Nicolai, M.; Viana, A.; Andrade, J.M.; Barasoain, I.; Stankovic, T.; Quintana, J.; Hernández, I.; et al. Anticancer properties of the abietane diterpene 6, 7-dehydroroyleanone obtained by optimized extraction. Future Med. Chem. 2018, 10, 1177–1189. [Google Scholar] [CrossRef]
- Rajavel, T.; Mohankumar, R.; Archunan, G.; Ruckmani, K.; Devi, K.P. Beta sitosterol and Daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci. Rep. 2017, 7, 3418. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Huifu, H.; Kumari, A.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Induction of Senescence in Cancer Cells by a Novel Combination of Cucurbitacin B and Withanone: Molecular Mechanism and Therapeutic Potential. J. Gerontol. Ser. A 2020, 75, 1031–1041. [Google Scholar] [CrossRef]
- Mu, D.; Zhang, W.; Chu, D.; Liu, T.; Xie, Y.; Fu, E.; Jin, F. The role of calcium, P38 MAPK in dihydroartemisinin-induced apoptosis of lung cancer PC-14 cells. Cancer Chemother. Pharmacol. 2008, 61, 639–645. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.; Wang, J.; Yang, B.; Zhao, Y.; Rao, Z.; Gao, J. Dihydroartemisinin sensitizes lewis lung carcinoma cells to carboplatin therapy via p38 mitogen-activated protein kinase activation. Oncol. Lett. 2018, 15, 7531–7536. [Google Scholar] [CrossRef]
- Li, Y.; Luan, G.; Guo, P. The inhibitory effect of dihydroartemisinin on non-small cells lung cancer. Pharmacol. Res. Mod. Chin. Med. 2021, 1, 100006. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Md Hashim, N.F.; Ammar, A.; Zakuan, N.M. An insight into the anti-angiogenic and anti-metastatic effects of oridonin: Current knowledge and future potential. Molecules 2021, 26, 775. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, Y.; Fan, X.; Liu, X.; Peng, L.; Ci, X. Oridonin sensitizes cisplatin-induced apoptosis via AMPK/Akt/mTOR-dependent autophagosome accumulation in A549 cells. Front. Oncol. 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, A.; Kalra, R.S.; Putri, J.; Elwakeel, A.; Kaul, S.C.; Wadhwa, R. Soyasapogenol-A targets CARF and results in suppression of tumor growth and metastasis in p53 compromised cancer cells. Sci. Rep. 2020, 10, 6323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attoub, S.; Sperandio, O.; Raza, H.; Arafat, K.; Al-Salam, S.; Al Sultan, M.A.; Al Safi, M.; Takahashi, T.; Adem, A. Thymoquinone as an anticancer agent: Evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Jiménez, N.S.; Leyva-Paredes, K.; Baltierra-Uribe, S.L.; Castillo-Cruz, J.; Campillo-Navarro, M.; Hernández-Pérez, A.D.; Luna-Angulo, A.B.; Chacón-Salinas, R.; Coral-Vázquez, R.M.; Estrada-García, I.; et al. Ursolic and oleanolic acids induce mitophagy in a549 human lung cancer cells. Molecules 2019, 24, 3444. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.S.; Zhou, H.; Yang, L.; Wang, L.; Jiang, Z.S.; Sun, H.; Wang, S.M. Ursolic acid attenuates TGF-b1-induced epithelial-mesenchymal transition in NSCLC by targeting integrin Avb5/MMPs signaling. Oncol. Res. 2019, 27, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, H.; Wu, R.; Chen, Z.Y.; Hu, Q.; Zhang, Y.F.; Gao, S.H.; Zhou, G.B. Autophagy inhibition enhances the inhibitory effects of ursolic acid on lung cancer cells. Int. J. Mol. Med. 2020, 46, 1816–1826. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Liang, T.; Tian, X.D.; Liu, Y.; Zhang, T. Withaferin A induces mitochondrial-dependent apoptosis in non-small cell lung cancer cells via generation of reactive oxygen species. J. BUON 2017, 22, 244–250. [Google Scholar]
- Haustein, M.; Ramer, R.; Linnebacher, M.; Manda, K.; Hinz, B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014, 92, 312–325. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From plant to human body: A promising bioactive molecule with multi-target effects in cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Treesuwan, S.; Sritularak, B.; Chanvorachote, P.; Pongrakhananon, V. Cypripedin diminishes an epithelial-to-mesenchymal transition in non-small cell lung cancer cells through suppression of Akt/GSK-3β signalling. Sci. Rep. 2018, 8, 8009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattanathamsan, O.; Treesuwan, S.; Sritularak, B.; Pongrakhananon, V. Cypripedin, a phenanthrenequinone from Dendrobium densiflorum, sensitizes non-small cell lung cancer H460 cells to cisplatin-mediated apoptosis. J. Nat. Med. 2018, 72, 503–513. [Google Scholar] [CrossRef]
- Su, J.; Yan, Y.; Qu, J.; Xue, X.; Liu, Z.; Cai, H. Emodin induces apoptosis of lung cancer cells through ER stress and the TRIB3/NF-κB pathway. Oncol. Rep. 2017, 37, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.F.; Houng, J.Y.; Kuo, C.F.; Tsao, N.; Wu, Y.C. Glossogin, a novel phenylpropanoid from Glossogyne tenuifolia, induced apoptosis in A549 lung cancer cells. Food Chem. Toxicol. 2008, 46, 3785–3791. [Google Scholar] [CrossRef]
- Trenti, A.; Grumati, P.; Cusinato, F.; Orso, G.; Bonaldo, P.; Trevisi, L. Cardiac glycoside ouabain induces autophagic cell death in non-small cell lung cancer cells via a JNK-dependent decrease of Bcl-2. Biochem. Pharmacol. 2014, 89, 197–209. [Google Scholar] [CrossRef]
- Zhu, F.; Dai, C.; Fu, Y.; Loo, J.F.C.; Xia, D.; Gao, S.P.; Ma, Z.; Chen, Z. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget 2016, 7, 9462–9476. [Google Scholar] [CrossRef] [Green Version]
- Hsia, T.C.; Yang, J.S.; Chen, G.W.; Chiu, T.H.; Lu, H.F.; Yang, M.D.; Yu, F.S.; Liu, K.C.; Lai, K.C.; Lin, C.C.; et al. The roles of endoplasmic reticulum stress and Ca2+ on rhein-induced apoptosis in A-549 human lung cancer cells. Anticancer Res. 2009, 29, 309–318. [Google Scholar]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Naidu, V.G.M.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules 2020, 25, 2278. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Wang, C.; Jin, H.; Meng, Q.; Huo, X.; Sun, H.; Sun, P.; Wu, J.; Ma, X.; Liu, Z.; et al. Organic anion transporters and PI3K–AKT–mTOR pathway mediate the synergistic anticancer effect of pemetrexed and rhein. J. Cell. Physiol. 2020, 235, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Xu, L.; Lin, S.; Xiang, Y.; Dai, X.; Liang, G.; Huang, X.; Zhu, J.; Zhao, C. Rhein shows potent efficacy against non-small-cell lung cancer through inhibiting the STAT3 pathway. Cancer Manag. Res. 2019, 11, 1167–1176. [Google Scholar] [CrossRef] [Green Version]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-carbolines in food: A review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The anti-cancer mechanisms of berberine: A review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, N.; Khatik, G.L.; Suttee, A. The possible role of Saponin in Type-II Diabetes—A review. Curr. Diabetes Rev. 2020, 16, 107–121. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Zhao, Y.; Zhang, L.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Cui, L. Pretreatment by evodiamine is neuroprotective in cerebral ischemia: Up-regulated pAkt, pGSK3β, down-regulated NF-κB expression, and ameliorated BBB permeability. Neurochem. Res. 2014, 39, 1612–1620. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakano, Y.; Kizaki, M.; Hoshikuma, K.; Yokoo, Y.; Kamiya, T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 2001, 67, 628–633. [Google Scholar] [CrossRef]
- Iwaoka, E.; Wang, S.; Matsuyoshi, N.; Kogure, Y.; Aoki, S.; Yamamoto, S.; Noguchi, K.; Dai, Y. Evodiamine suppresses capsaicin-induced thermal hyperalgesia through activation and subsequent desensitization of the transient receptor potential V1 channels. J. Nat. Med. 2016, 70, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, C.; Huang, C.; Tian, X.; Sun, W.; Jiang, S. Research Advances in Antitumor Mechanism of Evodiamine. J. Chem. 2022, 2022, 2784257. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Huang, J.M.; Xie, Y.J.; Zhang, Y.Z.; Chang, C.; Lai, H.L.; Wang, W.; Yao, X.J.; Fan, X.X.; Wu, Q.B.; et al. Evodiamine suppresses non-small cell lung cancer by elevating CD8+ T cells and downregulating the MUC1-C/PD-L1 axis. J. Exp. Clin. Cancer Res. 2020, 39, 249. [Google Scholar] [CrossRef]
- Lou, C.; Takahashi, K.; Irimura, T.; Saiki, I.; Hayakawa, Y. Identification of Hirsutine as an anti-metastatic phytochemical by targeting NF-ΚB activation. Int. J. Oncol. 2014, 45, 2085–2091. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhou, R.; Li, Q.; Jie, X.; Hong, J.; Zong, Y.; Dong, X.; Zhang, S.; Li, Z.; Wu, G. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anticancer Drugs 2019, 30, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Wang, X.; Yang, M.; Shi, X.; Huang, W.; Feng, Q. Combination of low concentration of (−)-epigallocatechin gallate (EGCG) and curcumin strongly suppresses the growth of non-small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int. J. Mol. Sci. 2013, 14, 12023–12036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimoto, J.; Kiura, K.; Matsuo, K.; Yoshino, T.; Takata, I.; Ueoka, H.; Kataoka, M.; Harada, M. (−)-Epigallocatechin gallate can prevent cisplatin-induced lung tumorigenesis in A/J mice. Carcinogenesis 2000, 21, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Lee, Y.; Cheng, G.; Zielonka, J.; Zhang, Q.; Bajzikova, M.; Xiong, D.; Tsaih, S.W.; Hardy, M.; Flister, M.; et al. Mitochondria-Targeted Honokiol Confers a Striking Inhibitory Effect on Lung Cancer via Inhibiting Complex I Activity. iScience 2018, 3, 192–207. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, J.-Q.; Ge, M.-M.; Zhang, Q.; Wang, X.-Q.; Zhu, J.-Y.; Xie, C.-F.; Li, X.-T.; Zhong, C.-Y.; Han, H.-Y. Sulforaphane inhibits epithelial–mesenchymal transition by activating extracellular signal-regulated kinase 5 in lung cancer cells. J. Nutr. Biochem. 2019, 72, 108219. [Google Scholar] [CrossRef] [PubMed]
- He, D.H.; Chen, Y.F.; Zhou, Y.L.; Zhang, S.B.; Hong, M.; Yu, X.; Wei, S.F.; Fan, X.Z.; Li, S.Y.; Wang, Q.; et al. Phytochemical library screening reveals betulinic acid as a novel Skp2-SCF E3 ligase inhibitor in non–small cell lung cancer. Cancer Sci. 2021, 112, 3218–3232. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Ren, H.; Yan, Q.-L.; Li, Y.-L.; Liu, Q.; Yao, G.-D.; Song, S.-J. SCP-7, a germacrane-type sesquiterpene lactone derivative, induces ROS-mediated apoptosis in NSCLC cells in vitro and in vivo. Eur. J. Pharmacol. 2022, 925, 174989. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, B.; Shen, Y.; Ouahab, A. Co-delivery of siRNA and hypericin into cancer cells by hyaluronic acid modified PLGA-PEI nanoparticles. Drug Dev. Ind. Pharm. 2016, 42, 737–746. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, D.; Feng, Y.; Li, Y.; Huang, D.; Jiang, C.; Gao, M.; Peng, F.; Wang, X.; Jing, S.; et al. Biodistribution and anti-tumor efficacy of intratumorally injected necrosis-avid theranostic agent radioiodinated hypericin in rodent tumor models. J. Drug Target. 2015, 23, 371–379. [Google Scholar] [CrossRef]
- Hishiki, T.; Kato, F.; Tajima, S.; Toume, K.; Umezaki, M.; Takasaki, T.; Miura, T. Hirsutine, an indole alkaloid of uncaria rhynchophylla, inhibits late step in dengue virus lifecycle. Front. Microbiol. 2017, 8, 1674. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Yokoyama, S.; Saiki, I.; Hayakawa, Y. Selective anticancer activity of hirsutine against HER2-positive breast cancer cells by inducing DNA damage. Oncol. Rep. 2015, 33, 2072–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Su, R.; Wang, L.; Yuan, B.; Li, L. Inhibitory effect and mechanism of action (MOA) of hirsutine on the proliferation of T-cell leukemia Jurkat clone E6-1 cells. PeerJ 2021, 9, e10692. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.J.; Wang, Z.H.; Meng, W.; Li, C.C.; Hu, Y.X.; Zhou, L.; Wang, X.J. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses 2018, 10, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-B.; Wang, D.-N.; Wu, L.-G.; Cao, J.; Tian, J.-H.; Liu, R.; Ma, R.; Yu, J.-J.; Wang, J.; Huang, Q.; et al. Homoharringtonine inhibited breast cancer cells growth via mir-18a-3p/akt/mtor signaling pathway. Int. J. Biol. Sci. 2021, 17, 995–1009. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the target-based design of anticancer agents: A versatile scaffold with diverse mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C. Anticancer activity of bisindole alkaloids derived from natural sources and synthetic bisindole hybrids. Arch. Pharm. 2020, 353, e2000092. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Anil Kumar, N.V.; Salehi, B.; Cho, W.C.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A review of its bioactivity and studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Wang, X.; Hu, Y.; Du, H.; Dong, B.; Ao, S.; Zhang, L.; Sun, Z.; Zhang, L.; Lv, G.; et al. Solamargine inhibits gastric cancer progression by regulating the expression of lncNEAT1_2 via the MAPK signaling pathway. Int. J. Oncol. 2019, 54, 1545–1554. [Google Scholar] [CrossRef] [Green Version]
- Dhuguru, J.; Skouta, R. Role of indole scaffolds as pharmacophores in the development of anti-lung cancer agents. Molecules 2020, 25, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and cancer: Potential for natural polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Yang, T.; Zang, D.W.; Shan, W.; Guo, A.C.; Wu, J.P.; Wang, Y.J.; Wang, Q. Synthesis and evaluations of novel apocynin derivatives as anti-glioma agents. Front. Pharmacol. 2019, 10, 951. [Google Scholar] [CrossRef]
- Huang, Y.; Tsang, S.Y.; Yao, X.; Chen, Z.Y. Biological properties of baicalein in cardiovascular system. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 177–184. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.P.; Cao, G.D.; Zhu, J.H.; Li, J.Z.; Liu, X.H. Naturally occurring Batatasins and their derivatives as α-glucosidase inhibitors. RSC Adv. 2015, 5, 82153–82158. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.C.R.S.; Da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging cellular and molecular mechanisms underlying anticancer indications of chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Jang, M.G.; Ko, H.C.; Kim, S.J. Effects of p-coumaric acid on microRNA expression profiles in SNU-16 human gastric cancer cells. Genes Genom. 2020, 42, 817–825. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2022, 80, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Yu, H.; Guo, W.; Kong, Y.; Gu, L.; Li, Q.; Yang, S.; Zhang, Y.; Wang, Y. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018, 18, 102. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Singh, A.K.; Loka, M.; Pandey, A.K.; Bishayee, A. Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv. Protein Chem. Struct. Biol. 2021, 125, 215–257. [Google Scholar]
- Sundarraj, K.; Raghunath, A.; Perumal, E. A review on the chemotherapeutic potential of fisetin: In vitro evidences. Biomed. Pharmacother. 2018, 97, 928–940. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A potent anti-breast cancer agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Z.; Su, Z.; Song, J.; Zhou, L.; Sun, Q.; Liu, S.; Li, S.; Li, Y.; Wang, M.; et al. Gigantol inhibits Wnt/β-catenin signaling and exhibits anticancer activity in breast cancer cells. BMC Complement. Altern. Med. 2018, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Yap, K.M.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Rani, N.N.I.M.; Seow, L.J.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Lum, P.T. Hesperidin and its aglycone hesperetin in breast cancer therapy: A review of recent developments and future prospects. Saudi J. Biol. Sci. 2021, 28, 6730–6747. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A review of its anticancer potential and mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Hano, C.; Giglioli-Guivarc’H, N.; Abbasi, B.H. Mechanistic evaluation of phytochemicals in breast cancer remedy: Current understanding and future perspectives. RSC Adv. 2018, 8, 29714–29744. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Inoue, Y.; Hori, Y.; Miyajima, C.; Morishita, D.; Ohoka, N.; Hida, S.; Makino, T.; Hayashi, H. Anti-inflammatory activity of kurarinone involves induction of ho-1 via the keap1/NRF2 pathway. Antioxidants 2020, 9, 842. [Google Scholar] [CrossRef]
- Taheri, Y.; Sharifi-Rad, J.; Antika, G.; Yilmaz, Y.B.; Tumer, T.B.; Abuhamdah, S.; Chandra, S.; Saklani, S.; Kiliç, C.S.; Sestito, S.; et al. Paving Luteolin Therapeutic Potentialities and Agro-Food-Pharma Applications: Emphasis on in vivo Pharmacological Effects and Bioavailability Traits. Oxid. Med. Cell. Longev. 2021, 2021, 1987588. [Google Scholar] [CrossRef]
- Choudhary, N.; Tewari, D.; Nabavi, S.F.; Kashani, H.R.K.; Lorigooini, Z.; Filosa, R.; Khan, F.B.; Masoudian, N.; Nabavi, S.M. Plant based food bioactives: A boon or bane for neurological disorders. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Yu, C.L.; Weng, M.S.; Chen, W.C.; Chien, K.T.; Chi, C.W.; Chung, C.H.; Huang, C.W.; Wang, P.C.; Chen, C.C.; Tsai, A.C.; et al. Moscatilin inhibits metastatic behavior of human hepatocellular carcinoma cells: A crucial role of upa suppression via akt/nf-κb-dependent pathway. Int. J. Mol. Sci. 2021, 22, 2930. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.H.; Jang, H.; Park, S.Y.; Seol, J.W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Saberifar, S.; Hashemi, F.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Mohammadinejad, R.; Najafi, M.; Garg, M. Nobiletin in cancer therapy: How this plant derived-natural compound targets various oncogene and onco-suppressor pathways. Biomedicines 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological Aspects and Potential Use of Phloretin: A Systemic Review. Mini-Rev. Med. Chem. 2019, 19, 1060–1067. [Google Scholar] [CrossRef]
- Zhao, G.; Yang, L.; Zhong, W.; Hu, Y.; Tan, Y.; Ren, Z.; Ban, Q.; Yang, C.S.; Wang, Y.; Wang, Z. Polydatin, A Glycoside of Resveratrol, Is Better Than Resveratrol in Alleviating Non-alcoholic Fatty Liver Disease in Mice Fed a High-Fructose Diet. Front. Nutr. 2022, 9, 857879. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J.; et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol. Res. 2019, 145, 104265. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, E.B.; Tzvetkov, N.T.; Jóźwik, M.S.; Strzałkowska, N.; Horbańczuk, A.G. Quercetin: Total-scale literature landscape analysis of a valuable nutraceutical with numerous potential applications in the promotion of human and animal health—A review. Anim. Sci. Pap. Rep. 2021, 39, 199–212. [Google Scholar]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Venkata, K.C.N.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vender, R.B.; Andriessen, A.; Barankin, B.; Freiman, A.; Kyritsis, D.; Mistos, L.M.; Salsberg, J.; Amar, L. Cohort Using a Ceramides Containing Cleanser and Cream with Salicylic Acid for Dry, Flaking, and Scaling Skin Conditions. J. Drugs Dermatol. 2019, 18, 80–85. [Google Scholar]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Afshar, E.G. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190191. [Google Scholar] [CrossRef]
- Jing, R.; Li, H.Q.; Hu, C.L.; Jiang, Y.P.; Qin, L.P.; Zheng, C.J. Phytochemical and pharmacological profiles of three Fagopyrum buckwheats. Int. J. Mol. Sci. 2016, 17, 589. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, M.; Huang, D. Dietary Organosulfur-Containing Compounds and Their Health-Promotion Mechanisms. Annu. Rev. Food Sci. Technol. 2022, 13, 287–313. [Google Scholar] [CrossRef]
- Miekus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A Review of Their Anti-inflammatory Effects in Human Health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer potential of garlic and its bioactive constituents: A systematic and comprehensive review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Attanzio, A.; Tesoriere, L.; Garcia-Llatas, G.; Barberá, R.; Cilla, A. Apoptotic effect of a phytosterol-ingredient and its main phytosterol (β-sitosterol) in human cancer cell lines. Int. J. Food Sci. Nutr. 2019, 70, 323–334. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic acid in the treatment of tumour diseases: Application and research progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef]
- Jing, S.; Zou, H.; Wu, Z.; Ren, L.; Zhang, T.; Zhang, J.; Wei, Z. Cucurbitacins: Bioactivities and synergistic effect with small-molecule drugs. J. Funct. Foods 2020, 72, 104042. [Google Scholar] [CrossRef]
- Yu, R.; Jin, G.; Fujimoto, M. Dihydroartemisinin: A Potential Drug for the Treatment of Malignancies and Inflammatory Diseases. Front. Oncol. 2021, 11, 722331. [Google Scholar] [CrossRef]
- Bajalia, E.M.; Azzouz, F.B.; Chism, D.A.; Giansiracusa, D.M.; Wong, C.G.; Plaskett, K.N.; Bishayee, A. Phytochemicals for the Prevention and Treatment of Renal Cell Carcinoma: Preclinical and Clinical Evidence and Molecular Mechanisms. Cancers 2022, 14, 3278. [Google Scholar] [CrossRef]
- Gao, Y.; Nie, Z.; Cao, H.; Huang, D.; Chen, M.; Xiang, Y.; Yu, X.; Zhang, S. Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer in vitro. Cancers 2022, 14, 5976. [Google Scholar] [CrossRef]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.Z.; Yang, S.Z.; Ying, X.Z.; Liao, W.; Liu, H.X.; Lin, Z.Q.; Chen, Q.Y.; et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, S.; Mannan, A. A Review on Nigella sativa: A Marvel Herb. J. Drug Deliv. Ther. 2020, 10, 213–219. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Wang, L.; Goh, B.C.; Ahn, K.S.; Bishayee, A.; Sethi, G. Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol. Res. 2018, 129, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular Mechanisms of Thymoquinone as Anticancer Agent. Comb. Chem. High Throughput Screen. 2020, 24, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atteeq, M. Evaluating anticancer properties of Withaferin A—A potent phytochemical. Front. Pharmacol. 2022, 13, 975320. [Google Scholar] [CrossRef]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Choi, B.Y. Withaferin-A—A natural anticancer agent with pleitropic mechanisms of action. Int. J. Mol. Sci. 2016, 17, 290. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga, R.; Concha, G.; Cayo, A.; Cikutović-Molina, R.; Arevalo, B.; González, W.; Catalán, M.A.; Zúñiga, L. Withaferin A suppresses breast cancer cell proliferation by inhibition of the two-pore domain potassium (K2P9) channel TASK-3. Biomed. Pharmacother. 2020, 129, 110383. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz, A.; Tomala, A.; Podolak, I.; Cielecka-Piontek, J. Cannabis sativa L. As a natural drug meeting the criteria of a multitarget approach to treatment. Int. J. Mol. Sci. 2021, 22, 778. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A. Phenanthrenes from Orchidaceae and Their Biological Activities. In Orchids Phytochemistry, Biology and Horticulture; Merillon, J.M., Kodja, H., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2021; pp. 1–41. [Google Scholar]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Li, Q.; Gao, J.; Pang, X.; Chen, A.; Wang, Y. Molecular Mechanisms of Action of Emodin: As an Anti-Cardiovascular Disease Drug. Front. Pharmacol. 2020, 11, 559607. [Google Scholar] [CrossRef]
- Hsu, H.F.; Houng, J.Y.; Chang, C.L.; Wu, C.C.; Chang, F.R.; Wu, Y.C. Antioxidant activity, cytotoxicity, and DNA information of Glossogyne tenuifolia. J. Agric. Food Chem. 2005, 53, 6117–6125. [Google Scholar] [CrossRef]
- Osborn, H.M.I.; Evans, P.G.; Gemmell, N.; Osborne, S.D. Carbohydrate-based therapeutics. J. Pharm. Pharmacol. 2010, 56, 691–702. [Google Scholar] [CrossRef]
- Wu, J.; Li, D.; Du, L.; Baldawi, M.; Gable, M.E.; Askari, A.; Liu, L. Ouabain prevents pathological cardiac hypertrophy and heart failure through activation of phosphoinositide 3-kinase α in mouse. Cell Biosci. 2015, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Meira, C.S.; Soares, J.W.C.; dos Reis, B.P.Z.C.; Pacheco, L.V.; Santos, I.P.; Silva, D.K.C.; de Lacerda, J.C.; Daltro, S.R.T.; Guimarães, E.T.; Soares, M.B.P. Therapeutic Applications of Physalins: Powerful Natural Weapons. Front. Pharmacol. 2022, 13, 864714. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, J.K.; Huang, H.; Park, S.; Park, S.; Kwon, W.; Kim, E.; Jang, S.; Kim, S.Y.; Choi, S.K.; et al. Rhein suppresses colorectal cancer cell growth by inhibiting the mtor pathway in vitro and in vivo. Cancers 2021, 13, 2176. [Google Scholar] [CrossRef]
- Wadegaonkar, V.P.; Wadegaonkar, P.A. Withanone as an inhibitor of survivin: A potential drug candidate for cancer therapy. J. Biotechnol. 2013, 168, 229–233. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Singh, H.; Joshi, M.; Mulay, V.P.; Haldar, S.; Varshney, A. Withanone from Withania somnifera attenuates SARS-CoV-2 rbd and host ace2 interactions to rescue spike protein induced pathologies in humanized zebrafish model. Drug Des. Devel. Ther. 2021, 15, 1111–1133. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.H.; Chen, H.A.; Chen, P.S.; Cheng, Y.J.; Hsu, W.H.; Chang, Y.W.; Chen, Y.H.; Jan, Y.; Hsiao, M.; Chang, T.Y.; et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 2013, 32, 431–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar] [PubMed]
- MacCallum, D.E.; Melville, J.; Frame, S.; Watt, K.; Anderson, S.; Gianella-Borradori, A.; Lane, D.P.; Green, S.R. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005, 65, 5399–5407. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Jiang, T.; Li, Q.; Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res. 2017, 7, 2350–2394. [Google Scholar]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1131–1135. [Google Scholar]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.E.; Sarkar, K.K.; Bachar, S.C.; Ahmed, F.; Monjur-Al-hossain, A.S.M.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef]
- Mann, J.; Yang, N.; Montpetit, R.; Kirschenman, R.; Lemieux, H.; Goping, I.S. BAD sensitizes breast cancer cells to docetaxel with increased mitotic arrest and necroptosis. Sci. Rep. 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, P.A.; Kris, M.G.; Rigas, J.R.; Grant, S.C.; Miller, V.A. Paclitaxel (Taxol) and Docetaxel (Taxotere): Active chemotherapeutic agents in lung cancer. Lung Cancer 1995, 12, S163–S172. [Google Scholar] [CrossRef]
- Gatzemeier, U.; Heckmayr, M.; Neuhauss, R.; Schlüter, I.; Pawel, J.V.; Wagner, H.; Dreps, A. Phase II study with paclitaxel for the treatment of advanced inoperable non-small cell lung cancer. Lung Cancer 1995, 12, S101–S106. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.K.; Fossella, F.V.; Winn, R.J.; Shin, D.M.; Hynes, H.E.; Gross, H.M.; Davilla, E.; Leimert, J.; Dhingra, H.; Raber, M.N.; et al. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J. Natl. Cancer Inst. 1993, 85, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shambhwani, D.; Pandey, S.; Singh, J.; Lalhlenmawia, H.; Kumarasamy, M.; Singh, S.K.; Chellappan, D.K.; Gupta, G.; Prasher, P.; et al. Advances in Lung Cancer Treatment Using Nanomedicines. ACS Omega 2022, 8, 10–41. [Google Scholar] [CrossRef]
- Wei, Z.; Zou, H.; Liu, G.; Song, C.; Tang, C.; Chen, S.; Zhang, G.; Ran, J.; Wang, Y.; Yin, X. Peroxidase-mimicking evodiamine/indocyanine green nanoliposomes for multimodal imaging-guided theranostics for oral squamous cell carcinoma. Bioact. Mater. 2021, 6, 2144–2157. [Google Scholar] [CrossRef] [PubMed]

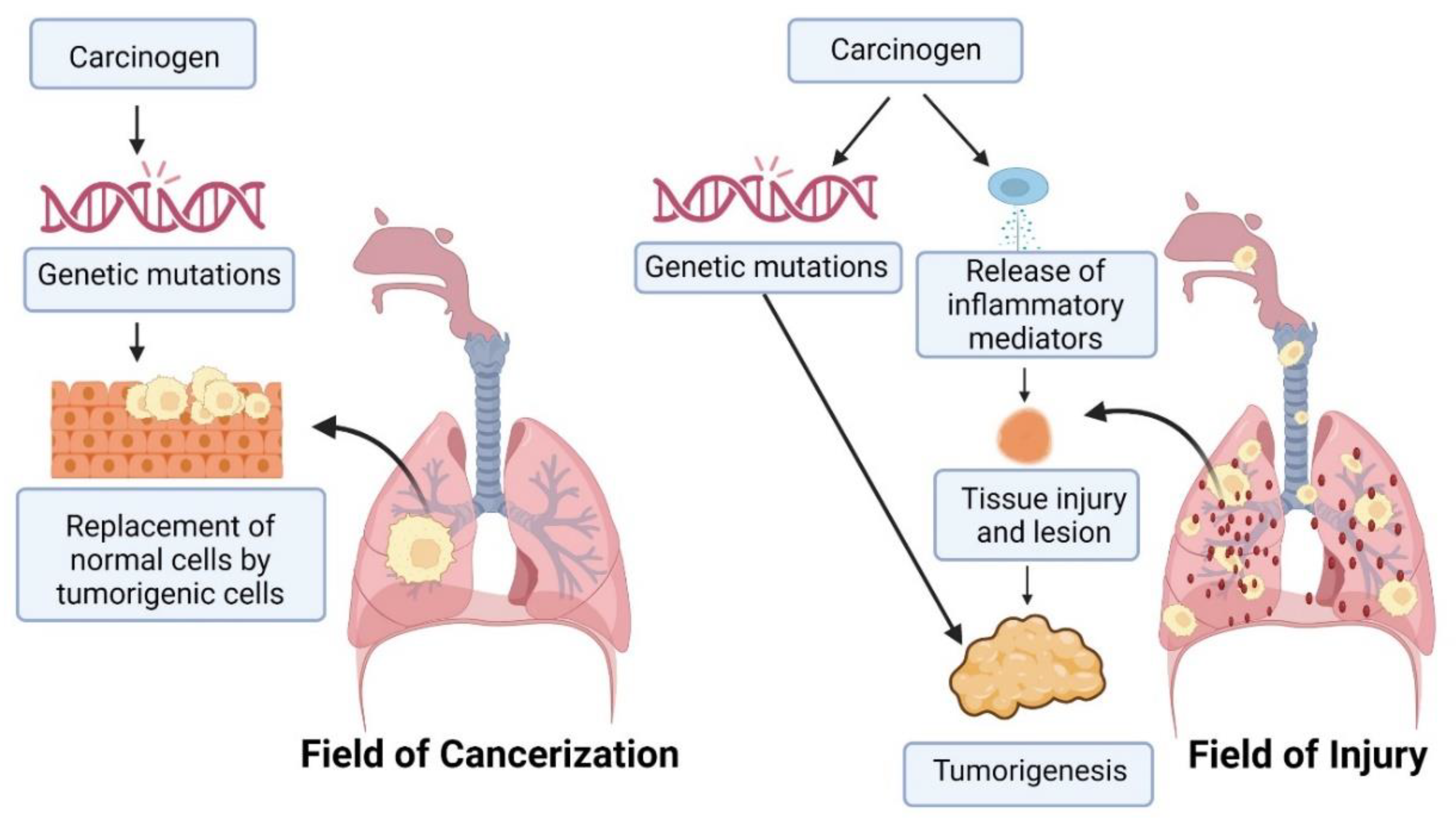
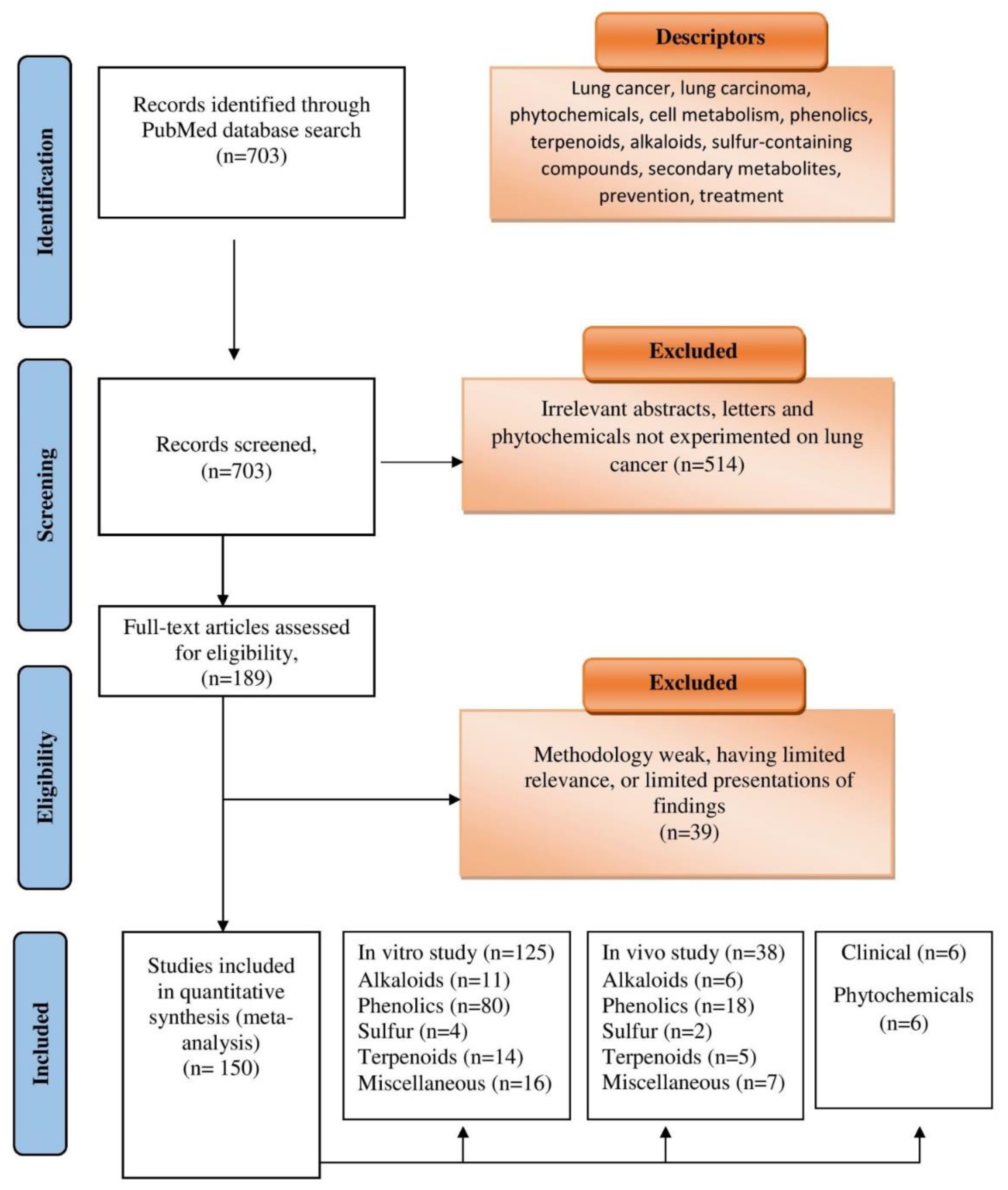
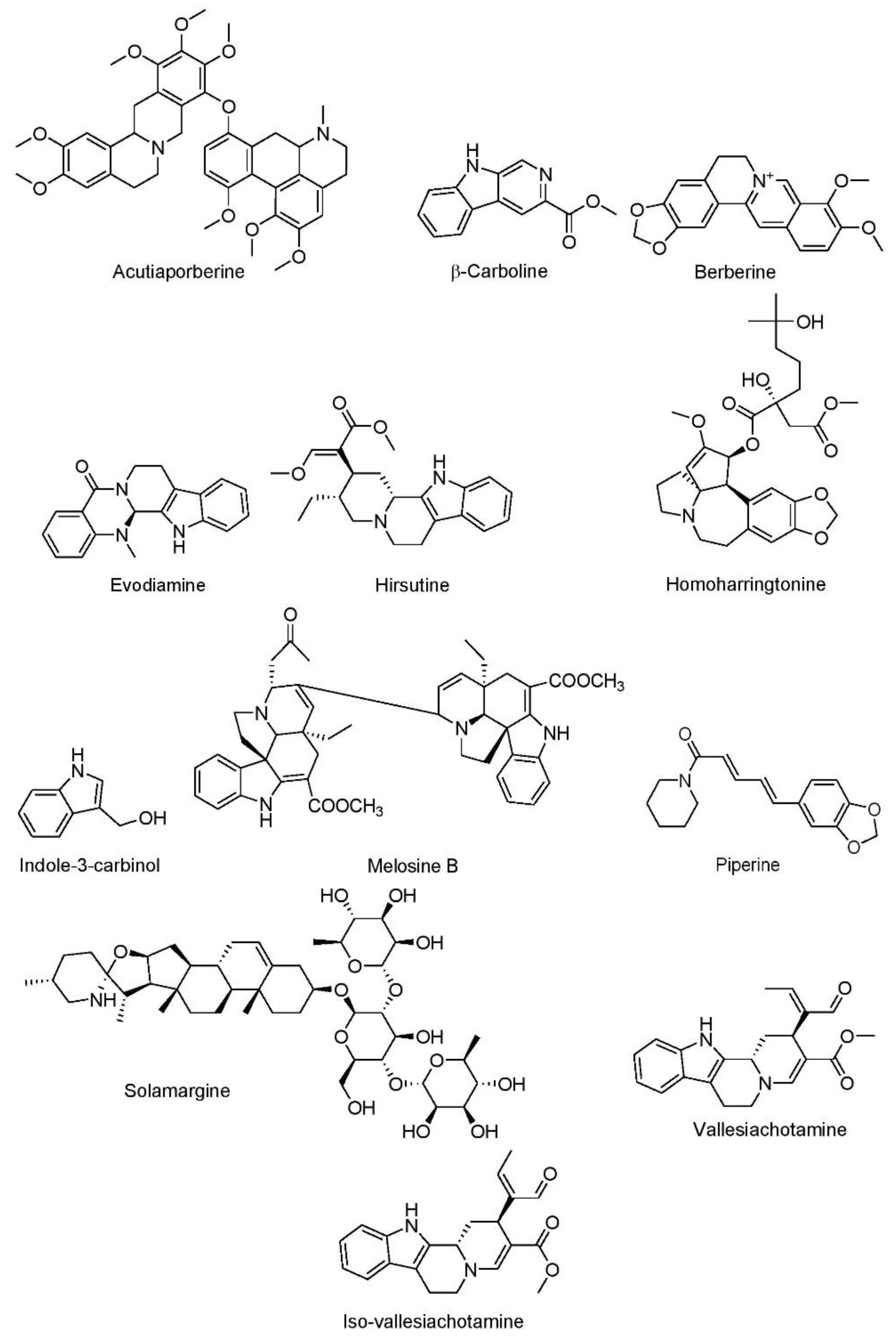
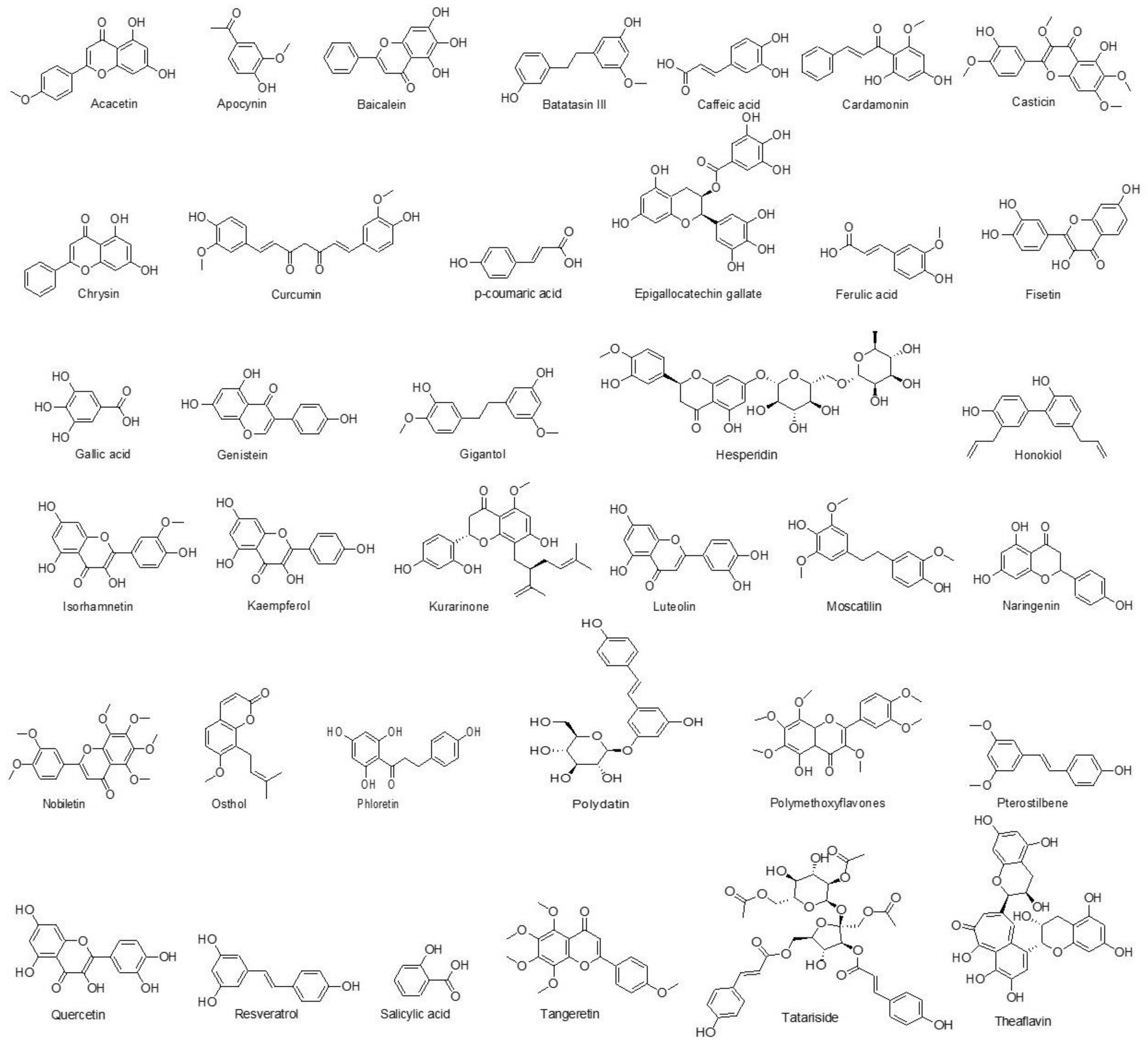
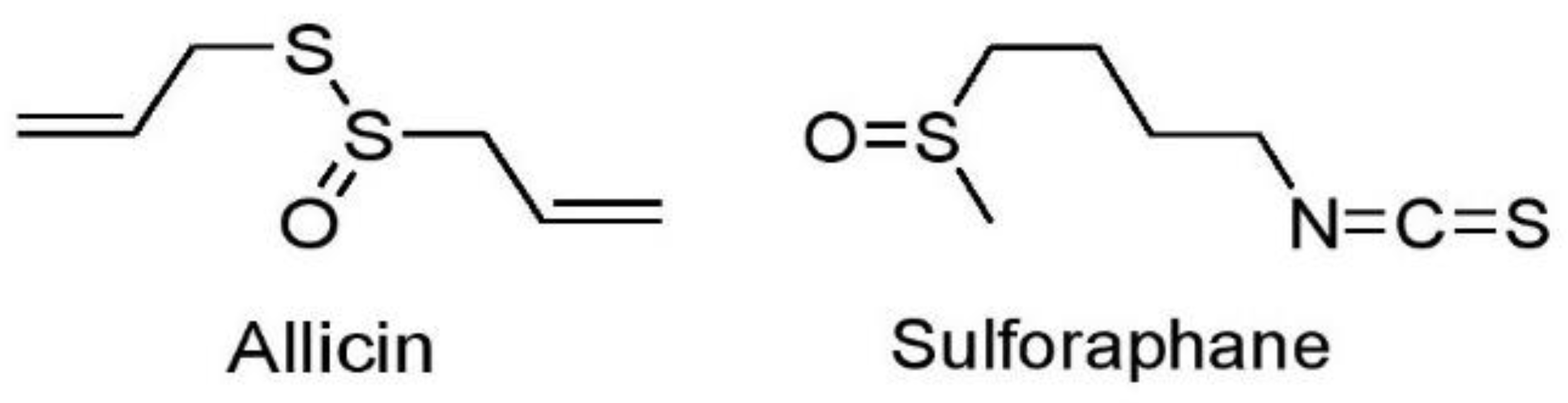
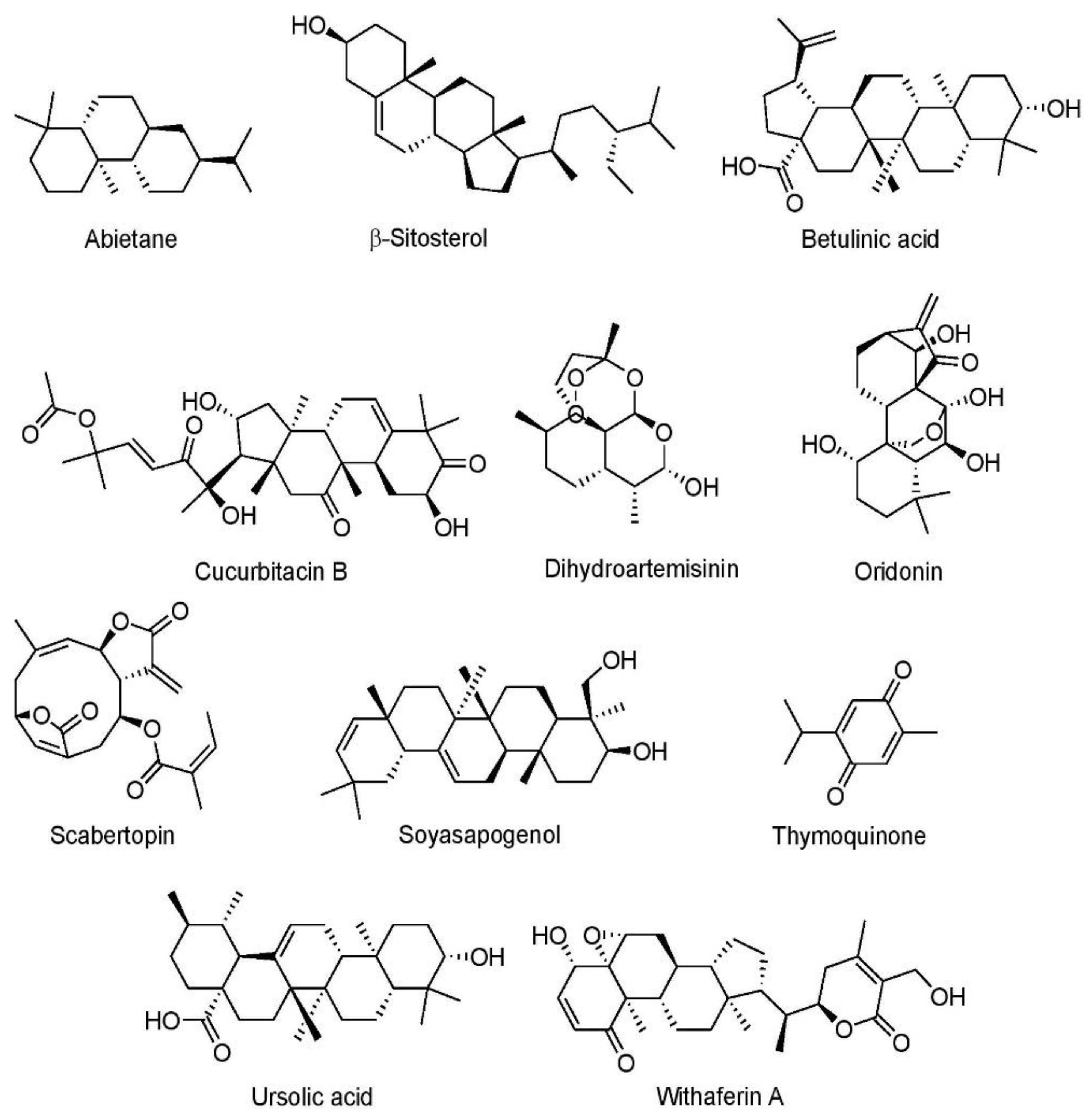
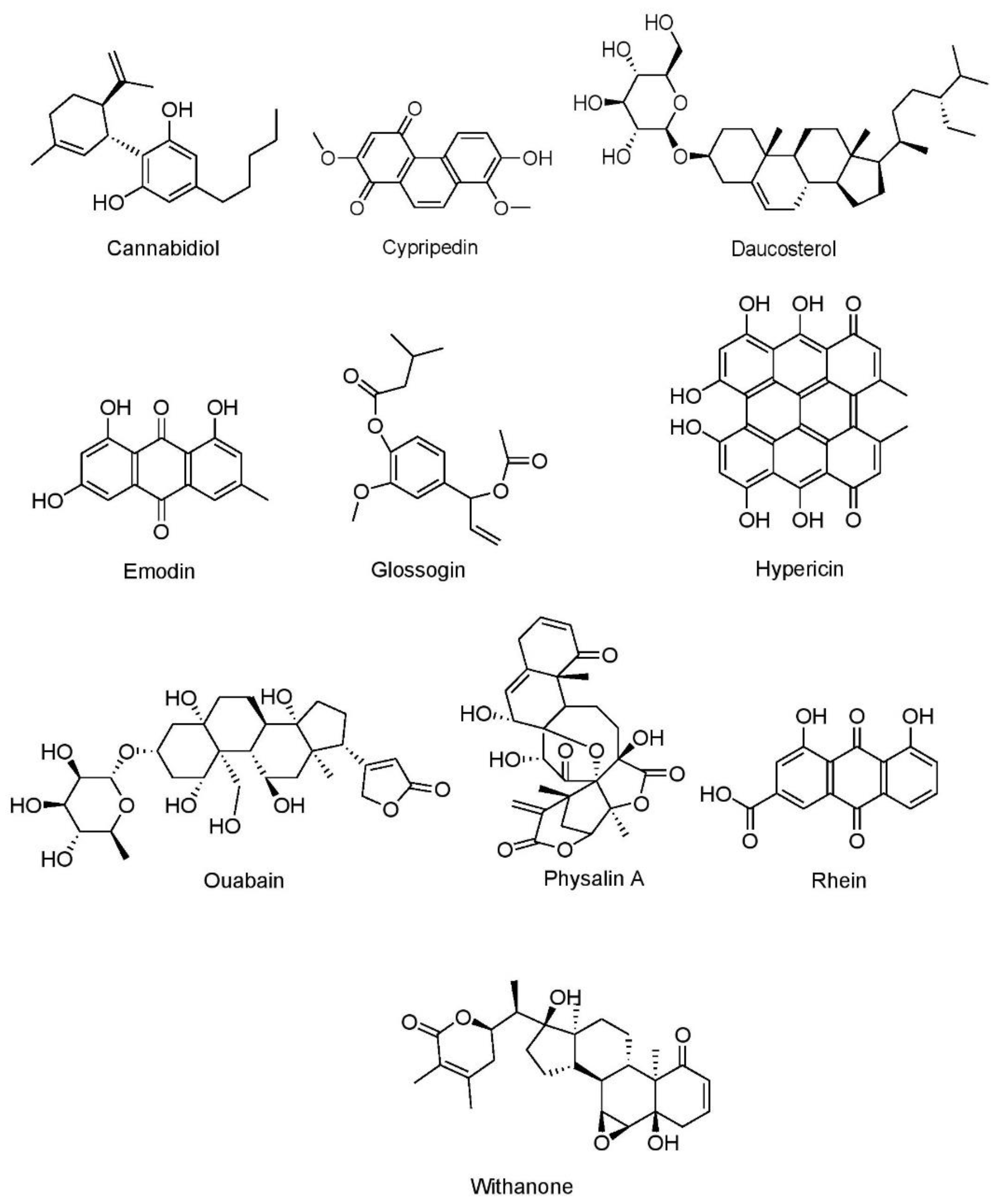

| Drug/Chemical Moiety | Mechanism of Action | Dose, Frequency, and Route | References |
|---|---|---|---|
| Gemcitabine | Inhibits ribonucleotide reductase resulting in inhibition of DNA synthesis | 1000 mg/m2, weekly, i.v. | [105,106,107] |
| Docetaxel | Binds to tubulin protein of microtubules, promotes its polymerization and stabilization, resulting in cell arrest in G2/M phase | 75 mg/m2, daily, i.v. | [108,109] |
| Carboplatin | Forms adducts with purine bases, resulting in inhibition of DNA replication and subsequent apoptosis of cancer cells owing to damaged DNA | 25 mg/m2, daily, i.v. | [110,111] |
| Cisplatin | Forms adducts with purine, resulting in inhibition of DNA replication and subsequent apoptosis of cancer cells owing to damaged DNA | 75 mg/m2, daily, i.v. | [108,110] |
| Trametinib | Inhibits MEK 1/2, resulting in obstruction of the RAS/RAF/MEK/ERK oncogenic pathway and cell cycle arrest | 2 mg, daily, orally | [112,113] |
| Dabrafenib | Inhibits of RAF, resulting in obstruction of the RAS/RAF/MEK/ERK oncogenic pathway and cell cycle arrest | 150 mg, twice daily, orally | [112,113] |
| Atezolizumab | Reverses immunosuppression within the tumor by blocking PD-L1 by binding to its receptor | 1200 mg, every 3 weeks, orally | [114,115] |
| Pembrolizumab | Reverses immunosuppression within the tumor by blocking PD-L1 by binding to its receptor | 250 mg, every 3 weeks, orally | [116] |
| Nivolumab | Reverses immunosuppression within the tumor by blocking PD-L1 by binding to its receptor | 240 mg, every 2 weeks, orally | [117] |
| Selumitinib | Inhibits MEK 1/2, resulting in obstruction of the RAS/RAF/MEK/ERK oncogenic pathway and cell cycle arrest | 75 mg, twice daily, orally | [118,119] |
| Navitoclax (ABT-263) | Blocks binding of Bcl-2 and BCL-XL to BIM, halting the antiapoptotic outcome | 150 mg, daily, orally | [37,118,120] |
| Selpercatinib | Inhibits multiple altered RET kinase isoforms, thus inhibiting oncogenic signaling | 20 mg, twice daily, orally | [94,121] |
| Crizotinib | Induces apoptosis in tumor cells and produces G1/S phase arrest by inhibiting ALK, MET, and ROS1 and downregulating JAK and STAT | 250 mg, twice daily, orally | [66,122,123] |
| Alectinib | Induces apoptosis in tumor cells by inhibiting ALK | 300 mg, twice daily, orally | [124] |
| Ceritinib | Inhibits ALK tyrosine kinase | 400 mg, daily orally | [125,126] |
| Ensartinib | Inhibits ALK tyrosine kinase and oncogenic triggers from MET, ROS1, SLK, ABL, LTK, anexelekto (Axl), and EPHA2 | 225 mg, daily orally | [127,128,129] |
| Bevacizumab | Inhibits VEGF, resulting in angiogenesis | 15 mg/kg, every three weeks, i.v. | [130,131] |
| Buparlisib | Inhibits PI3K, resulting in downregulation of PI3K/Akt/mTOR signaling and downstream cancer cell proliferation and angiogenesis | 100 mg/day, orally | [132,133] |
| Cabozantinib | Inhibits c-MET, RET, and VEGFR2 tyrosine kinase receptors, thus obstructing the stimulation of downstream signaling molecules involved in tumor proliferation and angiogenesis | 60 mg, daily, orally | [134,135] |
| Capmatinib | Inhibits c-MET, thus obstructing the stimulation of downstream signaling molecules involved in tumor proliferation | 400 mg, twice daily, orally | [136] |
| Erlotinib | Inhibits EGFR tyrosine kinase, resulting in obstruction of cancer cell proliferation by arresting cells in G0/G1 phase of cell cycle | 150 mg, daily, orally | [84,137] |
| Gefitinib | Inhibits EGFR tyrosine kinase blocking oncogenic signals from EGFR-activating mutations | 250 mg, daily, orally | [137] |
| Afatinib | Inhibits EGFR tyrosine kinase blocking oncogenic signals from EGFR-activating mutations | 40 mg, daily, orally | [138] |
| Rociletinib | Inhibits EGFR tyrosine kinase blocking oncogenic signals from EGFR-activating mutations | 625 mg, twice daily, orally | [139,140] |
| Cetuximab | EGFR inhibition and downregulation, antibody-mediated and complement-mediated cytotoxicity in lung cancer cells | 400 mg/m2 loading dose followed by 250 mg/m2 dose weekly, i.v. | [141] |
| Emibetuzumab | Inhibits ligand-dependent and ligand-independent MET oncogenic signaling | 750 mg, every two weeks, orally | [88] |
| Napabucasin | Inhibits STAT3 and promotes its downregulation, resulting in the inhibition of oncogenic transducer signaling and triggering apoptosis | 240 mg, twice daily, orally | [142,143] |
| Phytochemicals | Cell Lines | Conc. | IC50 | Anticancer Effect | Mechanisms | References |
|---|---|---|---|---|---|---|
| Alkaloids | ||||||
| Acutiaporberine | 95-D | 0.003 µM | Not reported | Increased cell death | ↑Bak/Bcl-2 ratio | [174] |
| β-Carboline | A549 | 1.80 μM | Not reported | Showed cytotoxic activity | ↑ERK1/2; ↓Akt/mTOR | [175] |
| Berberine | A549 and H1299 | 25, 50, 75, and 100 µM | Not reported | Suppressed tumor cell growth and increased cell death | ↓Bcl-2; ↑caspase-3; ↑Bax | [176] |
| Homoharringtonine | A549 and H1975 | 2–4 μM | 3.7 μM(A549) and 0.7 μM (H1975) | Inhibited tumor cell metastasis | ↓JAK1/STAT3 | [177] |
| A549 and H1299 | 2 µM | Not reported | Inhibited tumor cell growth and metastasis | ↓KRAS; ↓ERK; ↓Akt; ↓STAT3; ↓CDK4; ↓CDK6; ↓p21; ↓RB | [178] | |
| Indole-3-carbinol | H1299 | 400 μM | 449.5 μM | Increased cell death and oxidative stress | ↑ROS; ↑caspase-3; ↑caspase-7; ↑caspase-9; ↓Bcl-2 | [179] |
| Melosine B | A549 | 0.064, 0.32, 1.6, 8, and 40 µM | 8.1 μM | Exhibited cytotoxicity and increased cell death | Not reported | [180] |
| Piperine | A549 | 50, 100, and 200 μg/mL | 122 μg/mL | Inhibited tumor cell growth | ↑Bax/Bcl-2 ratio; ↑caspase-3; ↑caspase-9 | [181] |
| A549 | 20, 40, 80, 160, and 320 µM | 198 µM | Inhibited tumor cell migration and invasion | ↓ERK 1/2; ↓SMAD 2; ↓TGF-β | [182] | |
| Solamargine | H1650, H1975, PC9, A549, and H1299 | 2, 4, and 6 µM | Not reported | Reduced tumor cell growth and increased DNA damage | ↑ERK1/2; ↓prostaglandin E2; ↓DNMT1; ↓c-Jun | [183] |
| Vallesiachotamine and iso-vallesiachotamine | H1299 | 12.5, 25, 50, 100, and 200 μM | 4.24 μM (vallesiachotamine) and 3.79 μM (iso-vallesiachotamine) | Suppressed tumor cell growth and caused DNA damage | ↑Apoptosis | [184] |
| Phenolics | ||||||
| Acacetin | A549 | 1–5 μM | Not reported | Decreased tumor cell growth and viability | ↓Activator protein-1; ↓NF-κB; ↓MLK3; ↓MAPK3/6; ↓p38a; ↓MAPK | [185] |
| Apocynin | A549 | 50–1000 μM | 890 μM | Decreased tumor cell growth and enhanced cell death | ↓Cellular microtubule network | [186] |
| Baicalein | A549 and H1299 | 2.5, 10, and 40 μM | Not reported | Reduced tumor cell growth, metastasis, and invasion | ↓Cellular ezrin S-nitrosylation | [187] |
| Batatasin III | H460 | 25–100 μM | Not reported | Inhibited tumor cell migration and invasion | ↓EMT; ↓N-cadherin; ↓vimentin; ↓Akt; ↑E-cadherin | [188] |
| Caffeic acid | A549 | 50–1000 μM | Not reported | Reduced tumor cell growth | ↓Superoxide level | [189] |
| Cardamonin | A549 and H460 | 40 μM | Not reported | Decreased tumor cell growth and increased cell death | ↑Caspase-3; ↑Bcl-2; ↑Bax; ↑cyclin D1; ↓CDK4; ↓PI3K; ↓Akt; ↓mTOR | [190] |
| Cardamonin | A549 | 0.1, 1, 10, and 30 μM | Not reported | Reduced tumor cell growth and enhanced cell death | ↓mTOR; ↓DNA synthesis; ↓p70S6K | [191] |
| Cardamonin analogs | A549 and NCI-H460 | 0.05–100 µM | 0.445 µM (DHC) and 0.166 µM (DHMC) | Inhibited tumor cell growth | ↓NF-κB | [192] |
| Casticin | A549 | 1, 5, 10 µM | 14.3 µM | Suppressed tumor cell growth and enhanced cell death | ↓IL-6; ↓COX-2; ↓MAPK; ↓NF-κB; ↓p65; ↓chemokine gene | [193,194] |
| Chrysin | A549 | 25, 50, and 75 µg/mL | 55.72 μg/mL | Inhibited tumor cell growth and increased cell death | ↑Bax; ↓Bcl-2; ↑caspase-3 | [195] |
| Curcumin | A549 | 10 µM | Not reported | Decreased tumor cell growth and enhanced cell death | ↓Prosurvival antiapoptotic factors; ↓EGFR | [196] |
| A-549 | 10–50 µM | Not reported | Caused DNA damage and G2/M phase cell cycle arrest | ↑Caspase-3-induced apoptosis; ↑DNA damage; ↑ER stress | [197] | |
| NCI-H460 | 30 μM | Not reported | Suppressed tumor cell growth and enhanced cell death | ↑Caspase-3; ↑caspase-8; ↓cyclin-dependent kinase 1 | [198] | |
| CL1–5 | 1–20 μM | Not reported | Inhibited tumor cell growth and metastasis | ↑Activator protein-1; ↓E-cadherin; | [199] | |
| PC-9 | 50 μM | Not reported | Enhanced DNA damage, cell death and suppressed tumor cell growth | ↑DNA damage; ↓Bcl-2; ↓cyclin D1; ↓CDK2; ↓CDK4; ↓CDK6 | [200] | |
| NCI-H292 | 5–40 μM | 15 μM | Increased cell death and inhibited tumor cell growth | ↑Bax; ↑caspase-3; ↑caspase-7 | [201] | |
| p-Coumaric acid | A549, NCI-H1299, and HCC827 | 10–100 µg/mL | 37.73 μg/mL (A549); 50.6 μg/mL (H1299); 62.0 μg/mL (HCC827) | Increased cell death | ↑Bax; ↓Bcl-2; ↑caspase-3; ↑caspase-9 | [202] |
| H1993 | 50–100 μM | Not reported | Reduced tumor cell growth and viability | ↓Resistance of tyrosine kinase inhibitor | [203] | |
| EGCG | A549 and H1299 | 20–300 μM | 86.4 µM (A549) and 80.6 µM (H1299) | Inhibited tumor cell proliferation and induced apoptosis | ↓NF-κB | [204] |
| H1299 and CL-13 | 10–100 µM | 174.9 µM(H1299) and 181.5 µM (CL-13) | Reduced tumor cell proliferation | ↑ROS; ↓ NF-κB | [205] | |
| A549 | 10, 25, 50, and 100 µM | Not reported | Decreased tumor cell growth | ↓Nicotine-induced Akt; ↓ERK1/2 | [206] | |
| A549 | 12.5, 25, and 50 μM | 25 μM | Suppressed tumor cell growth, invasion, migration and increased G2/M phase cell cycle arrest | ↑Bax/Bcl-2 ratio | [207] | |
| A549 | 0.5 μM | Not reported | Decreased tumor cell growth and increased oxidative stress | ↑ Nrf2; ↑ROS | [208] | |
| A549 and NCI-H23 | 0.05–500 µM | Not reported | Reduced etoposide resistance and tumor cell growth | ↑Nrf2; ↑ROS; | [209] | |
| H1299, H460 and A549 | 40 µM | Not reported | Decreased tumor cell growth | ↑miR-210 | [210] | |
| H1299 and A549 | 10, 20, and 40 µM | Not reported | Induced apoptosis | ↓PI3K/Akt | [211] | |
| EGCG and luteolin | A549 and H460 | 30 µM (EGCG) and 10 µM (luteolin) | Not reported | Induced apoptosis | ↑p53 mitochondrial translocation; ↑DNA damage | [212] |
| EGCG and theaflavins | NCI-H460 | 100µM | Not reported | Inhibited tumor cell proliferation and promoted apoptosis | ↑p53; ↓Bcl-2 | [213] |
| Ferulic acid | A549 | 50–1000 μM | Not reported | Enhanced oxidative stress and decreased cell viability | ↓Superoxide anion | [189] |
| Fisetin | A549 | 5–20 μM | Not reported | Decreased cell viability and increased cell death | ↓PI3K/Akt; ↓mTOR | [214] |
| NCI-H460 | 75 µg/mL | Not reported | Inhibited tumor cell growth and viability | ↓β-cell lymphoma-2; ↑Bcl-2; ↑caspase-9; ↑caspase-3 | [215,216] | |
| HCC827-ER | 10, 20, 40, 60, 80, 100, 120 μM | Not reported | Inhibited tumor cell growth, viability and increased cell death | ↓Axl; ↓MAPK; ↓Akt | [217] | |
| Gallic acid | Calu-6 and A549 | 10–200 μM | 10–50 µM (Calu-6); 100–200 µM (A549) | Inhibited tumor cell growth and enhanced oxidative stress | ↓GSH; ↑ROS | [218] |
| H1975 and H1993 | 50 μM | Not reported | Increased cell death | ↓Src-mediated STAT3; ↓Bcl-2; ↓cyclin D; ↓NF-κB; ↓IL-6 | [219] | |
| Genistein | A549 | 10 μM | Not reported | Inhibited tumor cell growth and enhanced cell death | ↑Caspase-3 | [220] |
| H3255, H1650, and H1781 | 25 μM | Not reported | Decreased tumor cell growth and increased cell death | ↓DNA binding of NF-κB; ↓COX-2; ↓pAkt; ↓EGFR; ↓PGE2 | [221] | |
| SPC-A-1 | 20–40 μM | Not reported | Reduced tumor cell growth and increased cell death | ↓Bcl-2 | [222] | |
| H460 | 15–30 μM | Not reported | Suppressed tumor cell growth and increased cell death | ↓NF-κB | [223] | |
| Gigantol | A549 | 25, 50, and 100 µM | Not reported | Inhibited tumor cell growth and increased cell death | ↓Ki-67; ↓Bcl-2; ↑Bax; ↑Wnt/β-catenin | [224] |
| H460 | 50 μM | Not reported | Increased tumor cell death | ↓EMT | [225] | |
| H460 | 20–200 µM | Not reported | Reduced tumor cell proliferation, migration, and invasion | ↓PI3K/Akt/mTOR; ↓JAK/STAT | [226] | |
| Hesperidin | A549 and NCI-H358 | 5–50 μM | 50 μM | Increased cell death | ↑Apoptosis; ↑mitochondrial disruption; ↑caspase-3; ↑NF-κB | [227] |
| H1993 | 5–100 μM | Not reported | Decreased cell viability and enhanced cell death | ↓Resistance of tyrosine kinase inhibitor | [203] | |
| Honokiol | A549 and 95-D | 5, 10, or 20 μM | Not reported | Increased cell death | ↑Bax; ↑caspase-9; ↑PERK; ↑ER stress; ↓Bcl-2 | [228,229] |
| A549 and LL/2 | 10–50 μM | 21.1 μM | Reduced tumor cell growth and increased cell death | ↓VEGF-A | [230] | |
| Mono-demethylated polymethoxyflavones | H1299 | 1–30 µM | 16.5 μM | Increased cell death | ↓iNOS; ↓COX-2; ↓Mcl-1; ↑caspase-3; ↑PARP cleavage | [231] |
| Indolyl-chalcone derivatives | A549 | 2.5 μM and 5 μM | 2.46 μM | Suppressed tumor cell growth | ↑Nrf-2/HO-1 | [232] |
| Isorhamnetin | A549 | 8 μM and 16 μM | Not reported | Increased cell death and mitochondrial dysfunction | ↑Caspase-3 | [233] |
| A549 | 25 μM | Not reported | Enhanced mitochondrial dysfunction, cell death and decreased tumor cell growth | ↑Caspase-3; ↑PARP | [234] | |
| Kaempferol | A549 | 10–140 μM | 72 μM | Inhibited epithelial–mesenchymal transition and increased cell death | ↑EMT; ↓E-cadherin; ↓vimentin | [235] |
| A549 | 25 μM | Not reported | Inhibited tumor cell growth and viability | ↓E-Cadherin; ↓vimentin ↓Akt1-mediated phosphorylation; ↓TGF-β1 | [236] | |
| H460 | 30, 50, and 80 μM | 50 μM | Enhanced oxidative stress and cell death | ↑Caspase-3; ↑AIF | [237] | |
| Kurarinone | H460 | 2 µg/mL | 5.8 µg/mL | Inhibited tumor cell growth | ↓NF-κB; ↓tyrosine kinase | [238] |
| H1688 and H146 | 6.25, 12.5, and 25 μM | 12.5 µM (H1688) and 30.4 µM (H146) | Enhanced cell death | ↓EMT; ↓MMP-2 | [239] | |
| Luteolin | A549 | 20–80 μM | 40.2 μM | Increased G2/M phase cell cycle arrest and cell death | ↑Bax; ↑procaspase-9; ↑caspase-3; ↓NF-κB; ↑JNK | [240] |
| A549 | 25–100 μM | 42.8 µM | Decreased cell viability and increased cell death | ↑Bax; ↑caspase-3; ↑caspase-9; ↑MEK/ERK; ↓Bcl-2 | [241] | |
| A549 and H460 | 10–100 μM | 40 μM | Inhibited tumor cell growth and increased cell death | ↑miR-34a-5p via targeting MDM4 | [242,243] | |
| NCI-H460 | 20–160 μM | Not reported | Decreased cell viability and increased cell death | ↓Bad; ↓Bcl-2; ↑caspase-3; ↓Sirt1 | [244] | |
| Moscatilin | H460 | 1 μM | Not reported | Reduced tumor cell growth | ↓ERK; ↓EMT; ↓Akt; ↓Cav-1 | [245] |
| Naringenin | A549 | 25, 50, 100, 200, and 300 µM | Not reported | Reduced tumor cell growth | ↓MMP-2; ↓MMP-9; ↓Akt | [246] |
| A549 | 10, 100, and 200 µM | Not reported | Decreased tumor cell growth and enhanced cell death | ↑Caspase-3; ↓MMP-3; ↓MMP-9; ↑p38 | [247] | |
| Nobiletin | A549 (adriamycin resistant) | 50 µM | Not reported | Enhanced cell death | ↑Caspase-3; ↓Akt; ↓GSK-3β; ↓β-catenin; ↓MRP1 | [248] |
| Osthol | A549 | 25, 50, 100, 150, and 200 μM | Not reported | Increased G2/M phase cell cycle arrest and cell death | ↑Bax; ↓cyclin B1; ↓p-Cdc2 ↓Bcl-2; ↓PI3K/Akt | [249] |
| A549 | 40 and 80 µM | Not reported | Inhibited tumor cell growth, migration, and invasion | ↓MMP-2; ↓MMP-9 | [250,251] | |
| A549 | 5–80 μM | Not reported | Inhibited tumor cell growth and metastasis | ↓TGF-β-induced EMT; ↓NF-κB; ↓Snail | [252] | |
| Phloretin | A549, Calu-1 H838, and H520 | 25–75 μg/mL | Not reported | Enhanced cell death | ↓Bcl-2; ↓MMP-2; ↓MMP-9; ↑caspase-3; ↑caspase-9 | [253] |
| A549 | 25, 50, 100, and 200 μM | Not reported | Inhibited tumor cell growth and increased cell death | ↑Bax; ↓Bcl-2; ↑caspase-3; ↑caspase-9; ↑ERK; ↑JNK; ↑p38; ↑MAPK; ↑JNK1/2; ↓NF-κB | [254] | |
| Polydatin | A549 and NCI-H1975 | 50 µM | 2.95 µM (A549) and 3.23 µM (NCI-H1975) | Reduced tumor cell growth and increased cell cycle arrest | ↑Bak/Bcl-2 ratio | [255] |
| Pterostilbene | NCI-H460 and SK-MES-1 | 10–100 μM | Not reported | Decreased cell viability and increased cell death | ↑Caspase-3; ↑caspase-7 | [256] |
| Quercetin | A549 | 0.74–4.40 μM | 1.41 μM | Decreased cell growth and increased cell death | ↑Bax; ↓Bc1-2 | [257] |
| Resveratrol | A549 | 20 μM | Not reported | Inhibited tumor cell growth and invasion | ↓TGF-β1-induced EMT | [258] |
| A549 | 4–64 μM | 8.9 μM | Reduced tumor cell growth and increased cell death | ↑Caspase-3 | [259] | |
| H1993 | 1–10 μM | Not reported | Decreased cell viability and increased cell death | ↓Resistance of tyrosine kinase inhibitor | [203] | |
| Salicylic acid | A549 | 1.5–9.5 mM | 6.0 mM | Showed cytotoxicity and suppressed tumor cell growth | Not reported | [260] |
| Tangeretin derivative | CL1-5, H1299, H226, and A549 | 2.5 and 5 µM | 3.2 µM (CL1-5), 6.7 µM (H1299), 10.2 µM (H226), and 9.8 µM (A549) | Enhanced G2/M phase cell cycle arrest, cell death, mitochondrial dysfunction and reduced tumor cell growth | ↑Caspase-3; ↓Bcl-2; ↓survivin; ↓PI3K/Akt/mTOR | [261] |
| Tatariside B, C, and D | A549 | 0.001, 0.01, 0.1, 1, 10, and 100 µg/mL | 18.31 µg/mL (Tatariside B), 6.44–7.49 μg/mL (Tatariside C), and 2.83 μg/mL (Tatariside D) | Enhanced cytotoxicity, oxidative stress, cell death and reduced tumor cell growth | Not reported | [262] |
| Sulfur-containing compounds | ||||||
| Allicin | A549 and NCI-H460 | 10–60 μg/mL | 25 µg/mL (A549) and 15 µg/mL (NCI-H460) | Inhibited tumor cell growth | ↓Cadherin 2; ↑cadherin 1 | [263] |
| Sulforaphane | H1299, 95-C and 95-D | 1–5 μM | 9.52 μM (H1299), 9.04 μM (95-C), and 17.35 μM (95-D) | Reduced tumor cell growth and increased S/G2–M phase cell cycle arrest | ↓miR-616-5p levels; ↓GSK3β/β-catenin | [264] |
| A549 and H1299 | 0, 5, 10, and 15 mM | Not reported | Inhibited tumor cell growth and enhanced G2/M cell cycle arrest | ↑Apoptosis; ↓histone deacetylase | [265] | |
| A549 | 2.5 and 5 μM | Not reported | Suppressed tumor cell growth and increased G1/S cell cycle arrest | ↓miR-21; ↓CDH1; ↓DNMTs | [266] | |
| Terpenoids | ||||||
| Abietane diterpene | NCI-H460, and A549 | 10 and 30 µg/mL | 14 µM (NCI-H460) and 30 µM (A549) | Enhanced cell death | ↑Caspase-3; ↓caspase-9 | [267] |
| β-Sitosterol | A549 | 50–200 μg/mL | 95.19 μg/mL | Enhanced G2/M phase cell cycle arrest | ↑Apoptosis | [268] |
| Cucurbitacin B | A549 | 10 µM | Not reported | Reduced tumor cell growth and increased cell death | ↓CDK2; ↓CDK4; ↓cyclin D; ↓cyclin E; ↓mortalin; ↓hnRNP-K; ↓MMP-2; ↓fibronectin; ↑p53; ↑CARF | [269] |
| Dihydroartemisinin | PC-14 | 1 μg/mL | Not reported | Increased cell death | ↑p38 MAPK; ↑Ca2+ | [270] |
| LLC cells | 5, 10, 20, and 40 µg/mL | 26.98 µg/mL | Enhanced G0/G1 phase cell cycle arrest | ↑p38 MAPK | [271] | |
| A549 and H1299 | 0.23–749.90 µM | 80.89 uM | Reduced tumor cell growth | ↓Transferrin receptor | [272] | |
| Oridonin | H1975 | 10 µM | Not reported | Decreased tumor cell metastasis and angiogenesis | ↓Mesenchymal transition; ↑proapoptotic activity | [273] |
| A549 | 10, 20, and 30 μM | Not reported | Reduced tumor cell metastasis and angiogenesis | ↑Bax; ↑cisplatin-induced apoptosis via AMPK/Akt/mTOR; ↑PARP expression | [274] | |
| Soyasapogenol | H-1299 | 2–10 µM | 6 µM | Reduced tumor cell growth, metastasis and increased cell death | ↓CDK2; ↓CDK4; ↓cyclin A; ↓cyclin D1; ↓pATR-Chk1 ↓catenin/vimentin/hnRNPK-mediated EMT | [275] |
| Thymoquinone | LNM35 | 1–100 µM | 50–78 µM | Suppressed tumor cell growth and increased cell death | ↑Caspase-3 | [276] |
| Ursolic acid | A549 | 11, 22, 44, and 88 µM | Not reported | Decreased cell viability and enhanced autophagy | ↑LC3-II/LC3-I ratio; ↑p62; ↑PINK1; ↑Nrf2; ↑ROS; ↓p-Akt/mTOR | [277] |
| H1975 | 0.001–0.1 µM | Not reported | Reduced tumor cell growth and angiogenesis | ↓N-cadherin; ↓MMP-2; ↓MMP-9; ↓TGF-β1; ↑E-cadherin | [278] | |
| A549, H460, H1975, H1299, H520, H82, LLC, and H446 | 5–40 µM | Not reported | Inhibited tumor cell growth and angiogenesis | ↓Bcl-2; ↑cleaved PARP; ↑LC3-II; ↓p-S6K T389; ↓p-Akt | [279] | |
| Withaferin A | A549 | 10 µM | Not reported | Increased cell death, oxidative stress and decreased cell viability | ↑ROS | [280] |
| Miscellaneous compounds | ||||||
| Cannabidiol | A549 and H460 | 3 µM | Not reported | Increased cell death | ↑ICAM-1 | [281,282] |
| A549 and H460 | 1–10 µM | 3.47 µM (A549) and 2.80 µM (H460) | Increased cell death | ↑ICAM-1; ↑COX-2; ↑PPAR-γ | [283] | |
| A549 | 3 µM | Not reported | Enhanced cell death and reduced tumor cell growth | ↑MMP-1 | [284] | |
| Cypripedin | H23 | 50 μM | Not reported | Suppressed tumor cell growth | ↓N-cadherin; ↓vimentin; ↓Akt/GSK-3β | [285] |
| H460 | 50 μM | Not reported | Inhibited tumor cell growth | ↓Bcl-2 | [286] | |
| Daucosterol | A549 | 50–200 μg/mL | 17.46 μg/mL | Reduced tumor cell growth and enhanced G2/M phase cell cycle arrest | ↓Bcl-2; ↑Bax; ↑caspase-3 | [268] |
| Emodin | A549 and H1299 | 20, 40, 60, and 80 μM | Not reported | Enhanced cell death | ↑ER stress; ↑TRIB3/NF-κB | [287] |
| Glossogin | A549 | 12.5 μg/mL | Not reported | Suppressed tumor cell growth | ↑Cyt c; ↑caspase-9; ↑caspase-3; ↑Bak/Bcl-2 ratio | [288] |
| Ouabain | A549 and H1975 | 25 nM | Not reported | Inhibited tumor cell growth | ↑JNK; ↓Bcl-2 | [289] |
| Physalin A | H292, H358, and H1975 | 5, 10, and 15 μM | Not reported | Decreased tumor cell growth and enhanced cell death | ↓JAK/STAT3 | [290] |
| Rhein | A549 | 25, 50, and 100 μM | 45 μM | Enhanced G0/G1 phase cell cycle arrest and cell death | ↑ER stress; ↑p53; ↑p21; ↑Bax; ↓Bcl-2; ↓GADD153; ↓cyt c | [291,292] |
| A549 | 25, 50, and 100 μM | 100 μM | Inhibited tumor cell growth | ↓Bcl-2; ↓p-PI3K; ↓Akt; ↓mTOR | [293] | |
| PC-9, H460, and A549 | 30, 60, and 100 µM | 24.59 µM (PC-9), 52.88 µM(H460), and 23.9 µM (A549) | Increased G2/M phase cell cycle arrest and cell death | ↓Bcl-2; ↑Bax; ↓STAT3 | [294] | |
| Withanone | A549 | 2.5–10 µM | Not reported | Reduced tumor cell growth and increased cell death | ↓CDK2; ↓CDK4; ↓cyclin D; ↓cyclin E ↓mortalin; ↓hnRNP-K; ↓MMP-2; ↓fibronectin; ↑p53; ↑CARF | [269] |
| Phytochemicals | Anticancer Model | Dose (Route) | Anticancer Effects | Mechanisms | References |
|---|---|---|---|---|---|
| Alkaloids | |||||
| Berberine | Xenograft athymic nude mouse model | 50, 100, and 200 mg/kg (p.o.) | Increased cell death and decreased tumor weight | ↓Bcl-2; ↑Bax; ↑caspase-3 | [176] |
| Evodiamine | Xenograft nude mouse model and Lewis lung carcinoma model | 10, 20, and 30 mg/kg (p.o.) | Reduction in tumor volume | ↑CD8+ T cells; ↓MUC1-C/PD-L1 | [302] |
| Hirsutine | Lung metastasis model in BALB/c mice | 25 µM (i.p.) | Decreased tumor weight | ↓NF-κB | [303] |
| Homoharringtonine | Xenograft tumor mouse model | 10 mg/kg (p.o.) | Suppressed tumor growth | ↓IL-6; ↓JAK1/STAT3 | [177] |
| Xenograft tumor mouse mode and transgenic carrying the KRAS mutation model | 1.25 and 2.5 mg/kg (i.p.) | Inhibited tumor growth | ↓Bcl-2; ↑caspase-3; ↑caspase-9 | [178] | |
| Solamargine | Xenograft mouse model | 4 and 8 mg/kg (p.o.) | Decreased tumor growth | ↑ERK1/2; ↓prostaglandin E2; ↓DNMT1; ↓c-Jun | [183] |
| Phenolics | |||||
| Apocynin | Xenograft BALB/c mouse model | 50 and 100 mg/kg (i.p.) | Suppressed tumor growth | ↓Microtubule network | [186] |
| Baicalein | Xenograft BALB/c nude mice | 2.5, 10, and 40 mg/kg (i.g.) | Reduction in tumor volume | ↓Cellular ezrin S-nitrosylation | [187] |
| Cardamonin | Xenograft nude mouse model | 5 mg/kg (i.p.) | Enhanced cell death and inhibited tumor cell metastasis | ↑Bax; ↓Bcl-2; ↑caspase-3; ↓cyclin D1; ↓CDK4; ↓PI3K; ↓Akt/mTOR | [304] |
| Chrysin | Tumor reduction model in BALB/c mice | 1.3 mg/kg (p.o.) | Increased cell death | Caspase-3 | [195] |
| Curcumin + neoadjuvant radiotherapy | Lung carcinoma model in C57BL/6J mice | 100 µg (i.v.) | Inhibited angiogenesis and increased cell death | ↓Prosurvival antiapoptotic factors | [196] |
| p-Coumaric acid | Xenograft model in nude mice | 50 mg/kg (i.p.) | Enhanced cell death | ↑Bax; ↓Bcl-2; ↑caspase-3; ↑caspase-9 | [202] |
| EGCG | Xenograft BALB/c athymic nude mouse model | 20 mg/kg (i.p.) | Inhibited tumor size and induced apoptosis | ↓NF-κB | [305] |
| Xenograft BALB/c athymic nude mouse model | 100 µM (s.c.) | Inhibited tumor number | ↓Nicotine-induced Akt; ↓ERK1/2; ↓ HIF-1α; ↓VEGF | [206] | |
| Xenograft nude mouse model | 1.62 mg/kg (i.p.) | Inhibited tumor number and size | ↓Cisplatin-induced lung tumorigenesis | [306] | |
| EGCG and luteolin | Xenograft nude mouse model | 125 mg/kg (EGCG) and 10 mg/kg (luteolin) (p.o.) | Decreased tumor size, volume and induced tumor cell apoptosis | ↑p53 mitochondrial translocation; ↑DNA damage | [212] |
| Gallic acid | Xenograft tumor mouse model | 200 mg/kg (i.p.) | Increased cell death and G2/M phase cell cycle arrest | ↓Src-mediated STAT3; ↓Bcl-2; ↓cyclin D | [219] |
| Gigantol | Xenograft tumor mouse model | Pretreated 20 µM (i.p.) | Inhibited tumor cell growth, migration, and invasion | ↓PI3K/Akt/mTOR; ↓JAK/STAT | [226] |
| Honokiol | Orthotopic model of lung cancer in NOD/SCID mice | 7.5, 37.5, and 75 μmol/kg (p.o.) | Decrease in tumor volume | ↑ ROS; ↑mitochondrial Prx3 oxidation; ↑AMPK; ↓STAT3 | [307] |
| Kurarinone | Xenograft in BALB/c nude mouse model | 100 mg/kg (i.p.) | Increased cell death | ↓Bcl-2; ↑caspase-8; ↑caspase-3 | [238] |
| Nobiletin | Xenograft in athymic BALB/c nude mouse model | 40 mg/kg (s.c.) | Inhibited tumor growth and enhanced cell death | ↑Caspase-3; ↓Akt; ↓GSK3β, β-catenin; ↓MRP1 | [248] |
| Quercetin | Xenograft BALB/c nude mouse model | 8 mg/kg (i.v.) | Decreased tumor growth, viability and promoted cell death | ↑Bax; ↓Bc1-2 | [257] |
| Resveratrol | Xenograft BALB/c nude mouse model | 15, 30, and 60 mg/kg (i.v.) | Inhibited tumor growth and increased cell death | ↑Caspase-3 | [259] |
| Tangeretin derivatives | Xenograft BALB/c athymic nude mouse model | 20 mg/kg (i.p.) | Increased G2/M cell cycle arrest, mitochondrial disruption, cell death and decreased tumor growth | ↓Bcl-2; ↑caspase-3; ↓phophoatidylinositol 3-kinase/Akt/mTOR | [261] |
| Sulfur-containing compounds | |||||
| Sulforaphane | Xenograft nude mouse model | 9 µM (p.o.) | Suppressed tumor growth and enhanced G2/M cell cycle arrest | ↑Apoptosis; ↓histone deacetylase | [265] |
| Sulforaphane | Xenograft nude mouse model | 25 and 50 mg/kg (i.p.) | Reduction in tumor volume | ↑E-cadherin; ↑ZO-1; ↑ERK5; ↓N-cadherin; ↓Snail 1 | [308] |
| Terpenoids | |||||
| Thymoquinone | Xenograft nude mouse model | 10 mg/kg (i.p.) | Inhibited tumor growth | ↑Caspase-3 | [276] |
| Betulinic acid | Xenograft nude mouse model | 50 and 75 mg/kg (i.p.) | Suppressed tumor growth | ↓Skp2; ↑p27; ↑E-cadherin | [309] |
| Scabertopin | Xenograft nude mouse model | 20 mg/kg (i.p.) | Inhibited tumor growth | ↑Apoptosis ↑Bax; ↑ROS | [310] |
| Soyasapogenol | Xenograft immune-deficient mouse model | 15 mg/kg (i.v.) | Suppressed tumor growth and metastasis | ↓CDK2; ↓CDK4; ↓cyclin A; ↓cyclin D1; ↓catenin/vimentin/hnRNPK | [275] |
| Miscellaneous compounds | |||||
| Cannabidiol | Xenograft nude mouse model | 5 mg/kg (i.p.) | Increased cell death and inhibited tumor proliferation | ↑ICAM-1; ↑COX-2; ↑PPAR-γ | [283] |
| Emodin | Xenograft model in nude mice | 20 and 50 mg/kg (i.p.) | Induced cell death | ↑ER stress; ↑TRIB3/NF-κB | [287] |
| Hypericin | Rodent tumor model in BALB/c nude mice | 0.1 mg/kg (i.p.) | Displayed antiproliferative effects | ↑siRNA; ↓HIF-1α | [168,311] |
| Rodent tumor model/W256 tumor rats and mice | 2 mg/kg (intra tumor) | Inhibited tumor proliferation and induced cell death | ↑Apoptosis | [168,312] | |
| Physalin A | Xenograft mouse model | 40 and 80 mg/kg (i.p.) | Decreased tumor growth and increased cell death | ↓STAT3; ↓JAK/STAT3 | [290] |
| Rhein | Xenograft mouse model | 60 and 100 mg/kg (i.p.) | Increased G2/M phase cell cycle arrest, cell death and reduction in tumor volume | ↓Bcl-2; ↑Bax; ↓STAT3 | [294] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, N.; Bawari, S.; Burcher, J.T.; Sinha, D.; Tewari, D.; Bishayee, A. Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds. Cancers 2023, 15, 3980. https://doi.org/10.3390/cancers15153980
Choudhary N, Bawari S, Burcher JT, Sinha D, Tewari D, Bishayee A. Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds. Cancers. 2023; 15(15):3980. https://doi.org/10.3390/cancers15153980
Chicago/Turabian StyleChoudhary, Neeraj, Sweta Bawari, Jack T. Burcher, Dona Sinha, Devesh Tewari, and Anupam Bishayee. 2023. "Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds" Cancers 15, no. 15: 3980. https://doi.org/10.3390/cancers15153980
APA StyleChoudhary, N., Bawari, S., Burcher, J. T., Sinha, D., Tewari, D., & Bishayee, A. (2023). Targeting Cell Signaling Pathways in Lung Cancer by Bioactive Phytocompounds. Cancers, 15(15), 3980. https://doi.org/10.3390/cancers15153980










