Chronic Periodontitis and the Potential Likelihood of Gastric Cancer: A Nested Case-Control Study in the Korean Population Utilizing a National Health Sample Cohort
Abstract
Simple Summary
Abstract
1. Introduction
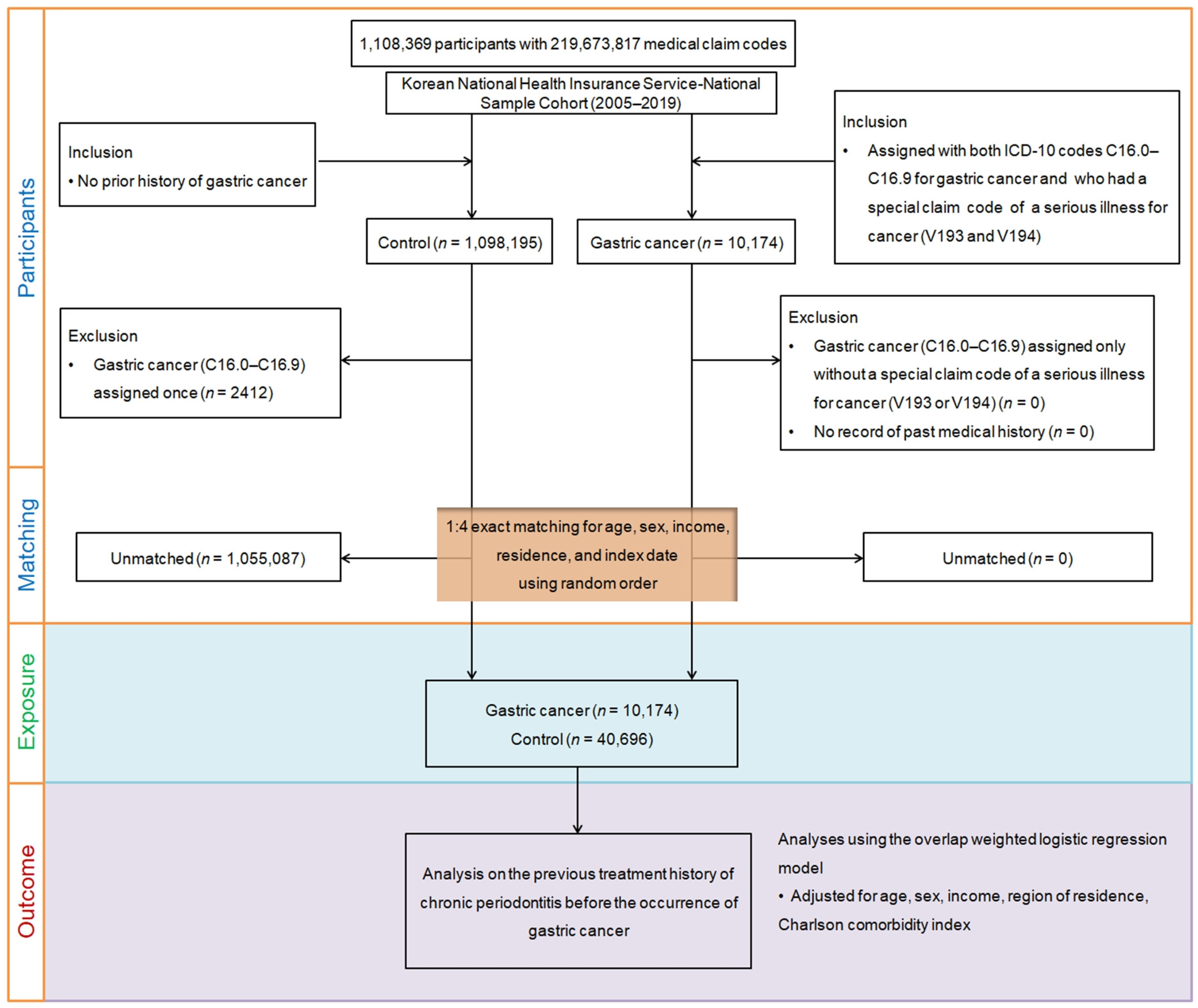
2. Materials and Methods
2.1. Exposure (Chronic Periodontitis)
2.2. Outcome (Gastric Cancer)
2.3. Covariates
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, K.W.; Won, Y.J.; Hong, S.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res. Treat. 2021, 53, 316–322. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Tahamtan, S.; Shirban, F.; Bagherniya, M.; Johnston, T.P.; Sahebkar, A. The effects of statins on dental and oral health: A review of preclinical and clinical studies. J. Transl. Med. 2020, 18, 155. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder model: The good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol. 2000 2017, 75, 116–151. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Abnet, C.C.; Qiao, Y.L.; Mark, S.D.; Dong, Z.W.; Taylor, P.R.; Dawsey, S.M. Prospective study of tooth loss and incident esophageal and gastric cancers in China. Cancer Causes Control 2001, 12, 847–854. [Google Scholar] [CrossRef]
- Abnet, C.C.; Kamangar, F.; Dawsey, S.M.; Stolzenberg-Solomon, R.Z.; Albanes, D.; Pietinen, P.; Virtamo, J.; Taylor, P.R. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand. J. Gastroenterol. 2005, 40, 681–687. [Google Scholar] [CrossRef]
- Shakeri, R.; Malekzadeh, R.; Etemadi, A.; Nasrollahzadeh, D.; Abedi-Ardekani, B.; Khoshnia, M.; Islami, F.; Pourshams, A.; Pawlita, M.; Boffetta, P.; et al. Association of tooth loss and oral hygiene with risk of gastric adenocarcinoma. Cancer Prev. Res. 2013, 6, 477–482. [Google Scholar] [CrossRef]
- Watabe, K.; Nishi, M.; Miyake, H.; Hirata, K. Lifestyle and gastric cancer: A case-control study. Oncol. Rep. 1998, 5, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Demirer, T.; Icli, F.; Uzunalimoglu, O.; Kucuk, O. Diet and stomach cancer incidence. A case-control study in Turkey. Cancer 1990, 65, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, N.; Ploner, A.; Liu, Z.; Roosaar, A.; Axell, T.; Ye, W. Association between poor oral health and gastric cancer: A prospective cohort study. Int. J. Cancer 2018, 143, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Kwon, S.; Wang, L.; Polychronidis, G.; Knudsen, M.D.; Zhong, R.; Cao, Y.; Wu, K.; Ogino, S.; Giovannucci, E.L.; et al. Periodontal disease, tooth loss, and risk of oesophageal and gastric adenocarcinoma: A prospective study. Gut 2021, 70, 620–621. [Google Scholar] [CrossRef]
- Lopes, C.; Almeida, T.C.; Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Pereira, C. Linking dysbiosis to precancerous stomach through inflammation: Deeper than and beyond imaging. Front. Immunol. 2023, 14, 1134785. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Chou, S.H.; Tung, Y.C.; Wu, L.S.; Chang, C.J.; Kung, S.; Chu, P.H. Severity of chronic periodontitis and risk of gastrointestinal cancers: A population-based follow-up study from Taiwan. Medicine 2018, 97, e11386. [Google Scholar] [CrossRef]
- Michaud, D.S.; Liu, Y.; Meyer, M.; Giovannucci, E.; Joshipura, K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol. 2008, 9, 550–558. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; Weiss, N.S. An exploration of the periodontitis-cancer association. Ann. Epidemiol. 2003, 13, 312–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Song, E.J.; Liang, M.; Shi, T.; Min, M.; Sun, Y. Is periodontitis a risk indicator for gastrointestinal cancers? A meta-analysis of cohort studies. J. Clin. Periodontol. 2020, 47, 134–147. [Google Scholar] [CrossRef]
- Yin, X.H.; Wang, Y.D.; Luo, H.; Zhao, K.; Huang, G.L.; Luo, S.Y.; Peng, J.X.; Song, J.K. Association between Tooth Loss and Gastric Cancer: A Meta-Analysis of Observational Studies. PLoS ONE 2016, 11, e0149653. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kim, J.H.; Kang, H.S.; Lim, H.; Kim, M.J.; Kim, N.Y.; Kim, S.H.; Choi, H.G.; Kim, E.S. Possible Incidental Parkinson’s Disease following Asthma: A Nested Case-Control Study in Korea. J. Pers. Med. 2023, 13, 718. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Kwon, M.J.; Byun, S.H.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Park, H.R.; Choi, H.G. Longitudinal follow-up study of the association between statin use and chronic periodontitis using national health screening cohort of Korean population. Sci. Rep. 2022, 12, 5504. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.; Sundararajan, V. Practice of epidemiology: Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Li, F.; Morgan, K.L.; Zaslavsky, A.M. Balancing Covariates via Propensity Score Weighting. J. Am. Stat. Assoc. 2018, 113, 390–400. [Google Scholar] [CrossRef]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef]
- Borrell, L.N.; Crawford, N.D. Socioeconomic position indicators and periodontitis: Examining the evidence. Periodontol. 2000 2012, 58, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Vermaire, J.H.; Chen, Y.; van der Sluis, L.W.M.; Thomas, R.Z.; Tjakkes, G.E.; Schuller, A.A. Trends in socioeconomic inequality of periodontal health status among Dutch adults: A repeated cross-sectional analysis over two decades. BMC Oral. Health 2021, 21, 346. [Google Scholar] [CrossRef]
- Sarkar, S.; Dauer, M.J.; In, H. Socioeconomic Disparities in Gastric Cancer and Identification of a Single SES Variable for Predicting Risk. J. Gastrointest. Cancer 2022, 53, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Hsu, T.W.; Chang, C.M.; Yu, C.H.; Wang, Y.F.; Lee, C.C. The effect of individual and neighborhood socioeconomic status on gastric cancer survival. PLoS ONE 2014, 9, e89655. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, A.; Matsuo, K.; Suzuki, T.; Kawase, T.; Tajima, K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1222–1227. [Google Scholar] [CrossRef]
- Vanoli, A.; Parente, P.; Fassan, M.; Mastracci, L.; Grillo, F. Gut inflammation and tumorigenesis: Every site has a different tale to tell. Intern. Emerg. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, M.; Salazar, C.R.; Hays, R.; Bedi, S.; Chen, Y.; Li, Y. Chronic Periodontal Disease, Periodontal Pathogen Colonization, and Increased Risk of Precancerous Gastric Lesions. J. Periodontol. 2017, 88, 1124–1134. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, A.S.; Choi, H.I.; Jung, J.H.; Park, J.Y.; Ko, H.J. Inflammatory markers for predicting overall survival in gastric cancer patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236445. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.F.; Miguel, C.B.; Lazo-Chica, J.E.; Trindade da Silva, C.A.; Vieira, C.U.; Clemente-Napimoga, J.T.; Freire Oliveira, C.J.; Napimoga, M.H. Interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and hematological parameters in experimental periodontal disease after beta-adrenergic blockade. J. Indian Soc. Periodontol. 2019, 23, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Lan, C.; Pei, H.; Zhu, Z. Expression of interleukin 1beta in gastric cancer tissue and its effects on gastric cancer. OncoTargets Ther. 2016, 9, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Saremi, L.; Shafizadeh, M.; Esmaeilzadeh, E.; Ghaffari, M.E.; Mahdavi, M.H.; Amid, R.; Kadkhodazadeh, M. Assessment of IL-10, IL-1ss and TNF-alpha gene polymorphisms in patients with peri-implantitis and healthy controls. Mol. Biol. Rep. 2021, 48, 2285–2290. [Google Scholar] [CrossRef]
- Bao, J.; Li, L.; Zhang, Y.; Wang, M.; Chen, F.; Ge, S.; Chen, B.; Yan, F. Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral. Sci. 2022, 14, 32. [Google Scholar] [CrossRef]
- Kunath, B.J.; Hickl, O.; Queiros, P.; Martin-Gallausiaux, C.; Lebrun, L.A.; Halder, R.; Laczny, C.C.; Schmidt, T.S.B.; Hayward, M.R.; Becher, D.; et al. Alterations of oral microbiota and impact on the gut microbiome in type 1 diabetes mellitus revealed by integrated multi-omic analyses. Microbiome 2022, 10, 243. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.H.; Wu, F.F.; Wang, X.J. Alterations in the Gut Microbiota and Their Metabolites in Colorectal Cancer: Recent Progress and Future Prospects. Front. Oncol. 2022, 12, 841552. [Google Scholar] [CrossRef]
- Kendall, B.E.; Fox, G.A.; Fujiwara, M.; Nogeire, T.M. Demographic heterogeneity, cohort selection, and population growth. Ecology 2011, 92, 1985–1993. [Google Scholar] [CrossRef]
| Characteristics | Before PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment | |||||
|---|---|---|---|---|---|---|---|
| Gastric Cancer | Control | Standardized Difference | Gastric Cancer | Control | Standardized Difference | ||
| Age (y), n (%) | 0.00 | 0.00 | |||||
| 5–9 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 10–14 | 3 (0.03) | 12 (0.03) | 2 (0.02) | 2 (0.02) | |||
| 20–24 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 25–29 | 19 (0.19) | 76 (0.19) | 13 (0.17) | 13 (0.17) | |||
| 30–34 | 95 (0.93) | 380 (0.93) | 63 (0.85) | 63 (0.85) | |||
| 35–39 | 205 (2.01) | 820 (2.01) | 140 (1.91) | 140 (1.91) | |||
| 40–44 | 466 (4.58) | 1864 (4.58) | 334 (4.56) | 334 (4.56) | |||
| 45–49 | 711 (6.99) | 2844 (6.99) | 505 (6.89) | 505 (6.89) | |||
| 50–54 | 994 (9.77) | 3976 (9.77) | 701 (9.56) | 701 (9.56) | |||
| 55–59 | 1197 (11.77) | 4788 (11.77) | 856 (11.68) | 856 (11.68) | |||
| 60–64 | 1449 (14.24) | 5796 (14.24) | 1041 (14.21) | 1041 (14.21) | |||
| 65–69 | 1463 (14.38) | 5852 (14.38) | 1058 (14.43) | 1058 (14.43) | |||
| 70–74 | 1490 (14.65) | 5960 (14.65) | 1085 (14.80) | 1085 (14.80) | |||
| 75–79 | 1071 (10.53) | 4284 (10.53) | 784 (10.69) | 784 (10.69) | |||
| 80–84 | 693 (6.81) | 2772 (6.81) | 512 (6.99) | 512 (6.99) | |||
| 85+ | 316 (3.11) | 1264 (3.11) | 235 (3.20) | 235 (3.20) | |||
| Sex, n (%) | 0.00 | 0.00 | |||||
| Male | 6834 (67.17) | 27,336 (67.17) | 4927 (67.23) | 4927 (67.23) | |||
| Female | 3340 (32.83) | 13,360 (32.83) | 2401 (32.77) | 2401 (32.77) | |||
| Income, n (%) | 0.00 | 0.00 | |||||
| 1 (lowest) | 1959 (19.25) | 7836 (19.25) | 1399 (19.09) | 1399 (19.09) | |||
| 2 | 1260 (12.38) | 5040 (12.38) | 896 (12.23) | 896 (12.23) | |||
| 3 | 1621 (15.93) | 6484 (15.93) | 1165 (15.89) | 1165 (15.89) | |||
| 4 | 2144 (21.07) | 8576 (21.07) | 1535 (20.95) | 1535 (20.95) | |||
| 5 (highest) | 3190 (31.35) | 12,760 (31.35) | 2334 (31.84) | 2334 (31.84) | |||
| Region of residence, n (%) | 0.00 | 0.00 | |||||
| Urban | 4310 (42.36) | 17,240 (42.36) | 3107 (42.40) | 3107 (42.40) | |||
| Rural | 5864 (57.64) | 23,456 (57.64) | 4221 (57.60) | 4221 (57.60) | |||
| CCI score, mean (SD) | 2.40 (2.70) | 0.92 (1.58) | 0.67 | 1.71 (1.88) | 1.71 (0.97) | 0.00 | |
| The number of CP treatments for 1 year before index date, mean (SD) | 0.52 (1.31) | 0.51 (1.36) | 0.01 | 0.54 (1.13) | 0.49 (0.56) | 0.05 | |
| The number of CP treatments for 2 year before index date, mean (SD) | 1.00 (2.09) | 0.98 (2.08) | 0.01 | 1.03 (1.78) | 0.94 (0.86) | 0.06 | |
| Characteristics | Odd Ratios for Gastric Cancer (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Crude | p-Value | Overlap Weighted Model † | p-Value | ||
| From the index date to the before the 1-year period | |||||
| Total participants (n = 50,870) | 1.04 (0.88–1.21) | 0.671 | 1.31 (1.15–1.49) | <0.001 * | |
| Age < 65 years old (n = 25,705) | 1.20 (0.97–1.50) | 0.095 | 1.50 (1.25–1.80) | <0.001 * | |
| Age ≥ 65 years old (n = 25,165) | 0.88 (0.69–1.11) | 0.27 | 1.10 (0.90–1.33) | 0.346 | |
| Male (n = 34,170) | 1.06 (0.88–1.27) | 0.556 | 1.32 (1.13–1.54) | <0.001 * | |
| Female (n = 16,700) | 0.98 (0.72–1.34) | 0.884 | 1.25 (0.97–1.62) | 0.091 | |
| Low-income group (n = 24,200) | 1.01 (0.80–1.28) | 0.936 | 1.33 (1.09–1.62) | 0.005 * | |
| High-income group (n = 26,670) | 1.06 (0.85–1.31) | 0.616 | 1.29 (1.08–1.55) | 0.005 * | |
| Urban residents (n = 21,550) | 1.09 (0.87–1.36) | 0.465 | 1.33 (1.10–1.60) | 0.003 * | |
| Rural residents (n = 29,320) | 0.98 (0.78–1.24) | 0.891 | 1.28 (1.06–1.55) | 0.009 * | |
| From the index date to the before the 2-year period | |||||
| Total participants (n = 50,870) | 1.04 (0.94–1.15) | 0.456 | 1.24 (1.14–1.35) | <0.001 * | |
| Age < 65 years old (n = 25,705) | 1.16 (1.00–1.33) | 0.049 * | 1.38 (1.22–1.55) | <0.001 * | |
| Age ≥ 65 years old (n = 25,165) | 0.93 (0.80–1.08) | 0.357 | 1.09 (0.96–1.23) | 0.168 | |
| Male (n = 34,170) | 1.05 (0.93–1.19) | 0.396 | 1.25 (1.13–1.38) | <0.001 * | |
| Female (n = 16,700) | 1.00 (0.82–1.23) | 0.966 | 1.20 (1.02–1.42) | 0.028 * | |
| Low-income group (n = 24,200) | 1.04 (0.89–1.22) | 0.592 | 1.32 (1.17–1.51) | <0.001 * | |
| High-income group (n = 26,670) | 1.04 (0.90–1.19) | 0.602 | 1.18 (1.05–1.32) | 0.006 * | |
| Urban residents (n = 21,550) | 1.07 (0.93–1.24) | 0.343 | 1.26 (1.11–1.42) | <0.001 * | |
| Rural residents (n = 29,320) | 1.01 (0.87–1.17) | 0.927 | 1.22 (1.08–1.37) | 0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.J.; Kang, H.S.; Kim, M.-J.; Kim, N.Y.; Choi, H.G.; Lim, H. Chronic Periodontitis and the Potential Likelihood of Gastric Cancer: A Nested Case-Control Study in the Korean Population Utilizing a National Health Sample Cohort. Cancers 2023, 15, 3974. https://doi.org/10.3390/cancers15153974
Kwon MJ, Kang HS, Kim M-J, Kim NY, Choi HG, Lim H. Chronic Periodontitis and the Potential Likelihood of Gastric Cancer: A Nested Case-Control Study in the Korean Population Utilizing a National Health Sample Cohort. Cancers. 2023; 15(15):3974. https://doi.org/10.3390/cancers15153974
Chicago/Turabian StyleKwon, Mi Jung, Ho Suk Kang, Min-Jeong Kim, Nan Young Kim, Hyo Geun Choi, and Hyun Lim. 2023. "Chronic Periodontitis and the Potential Likelihood of Gastric Cancer: A Nested Case-Control Study in the Korean Population Utilizing a National Health Sample Cohort" Cancers 15, no. 15: 3974. https://doi.org/10.3390/cancers15153974
APA StyleKwon, M. J., Kang, H. S., Kim, M.-J., Kim, N. Y., Choi, H. G., & Lim, H. (2023). Chronic Periodontitis and the Potential Likelihood of Gastric Cancer: A Nested Case-Control Study in the Korean Population Utilizing a National Health Sample Cohort. Cancers, 15(15), 3974. https://doi.org/10.3390/cancers15153974







