Performance of ACR TI-RADS and the Bethesda System in Predicting Risk of Malignancy in Thyroid Nodules at a Large Children’s Hospital and a Comprehensive Review of the Pediatric Literature
Abstract
Simple Summary
Abstract
1. Introduction
1.1. General Overview
1.2. Toward the Successful Development of a Standardized Way of Reporting FNA Results
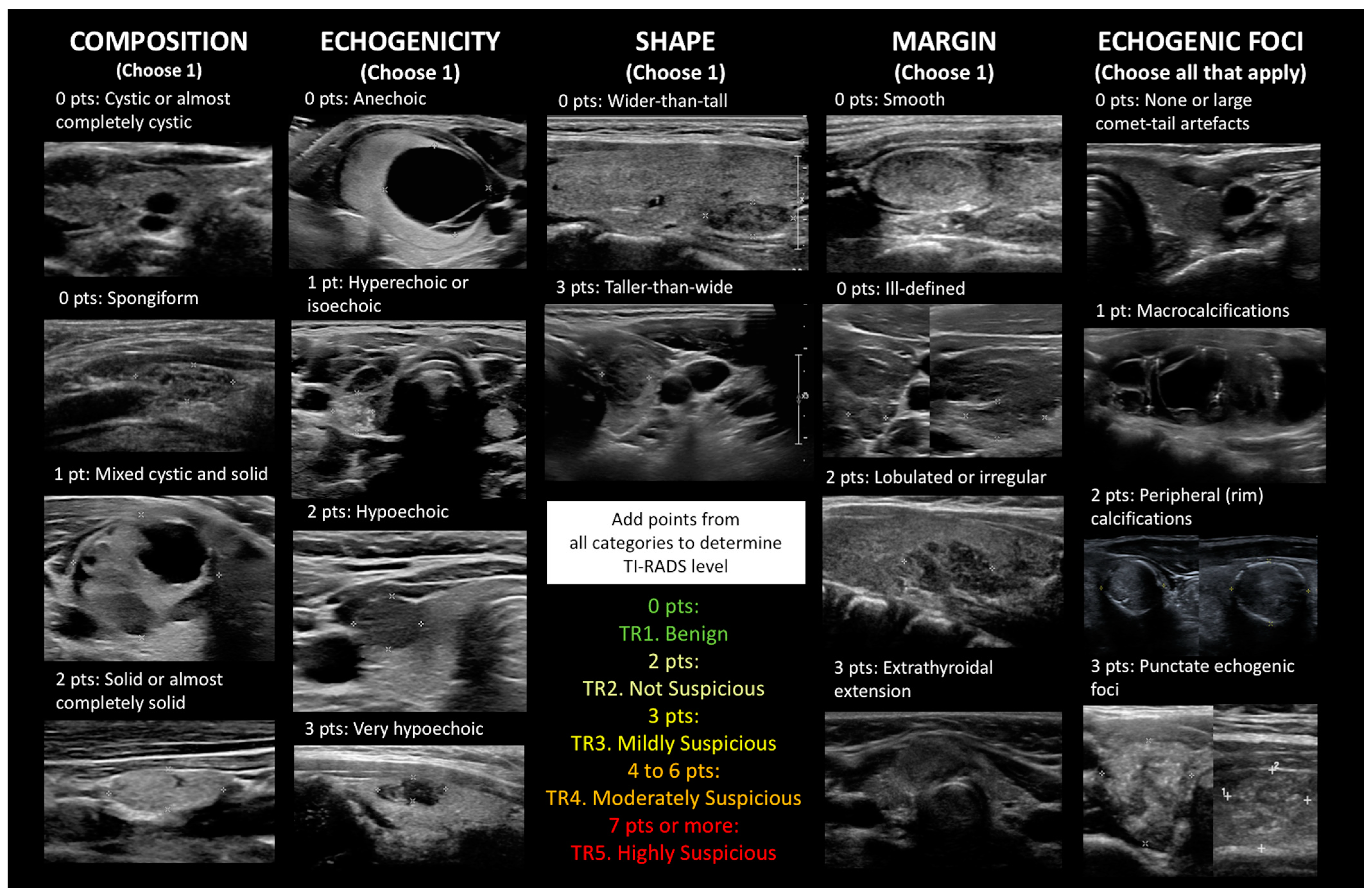
1.3. Building on BI-RADS: The Proliferation of TI-RADS and Many Other Ultrasound Risk Stratification Systems
1.4. Why Is This Study Needed?
2. Materials and Methods
3. Results
3.1. Clinical Characteristics from Our Institution
3.2. ACR TI-RADS Results from Our Institution
3.3. ACR TI-RADS Results from the Pediatric Literature, including Our Cases
3.4. Bethesda Results and Cyto/Histo Correlation from the Pediatric Literature, Including Our Cases
3.5. The Potential Value of a Combined Score That Incorporates TI-RADS and Bethesda
4. Discussion
4.1. Multiple Ultrasound Systems Have Been Applied to Pediatric Thyroid Nodules
4.2. Performance of ACR TI-RADS in Pediatrics
4.3. Comparison of ACR TI-RADS to Other Ultrasound Systems in Pediatrics
4.4. Individual Sonographic Characteristics Associated with Malignancy in Pediatrics
4.5. How Does ACR TI-RADS Perform in the Adult Setting?
4.6. The Application of Artificial Intelligence to Adult and Pediatric Thyroid Ultrasound
4.7. The Frequency, Risk of Malignancy and Risk of Neoplasm in the Various Bethesda System Categories in Pediatrics
4.8. The Bethesda System Does, in Fact, Perform Differently in Children Compared to Adults
4.9. Accounting for Bias
4.10. What if We Add Clinical and Sonographic Results to Bethesda Results?
4.11. Applying the Bethesda System to Frozen Section Diagnosis
4.12. Subtyping AUS by Type of Atypia or Reclassifying AUS by TI-RADS
4.13. Why Rapid On-Site Evaluation Is Important
4.14. Limitations of the Current Study and the Pediatric Literature in General
5. Conclusions
- Crude ROMs for ACR TI-RADS in the pediatric age group based on 1458 cases in the literature (including our cohort) were as follows:
| TR1. Benign | ROM 2.2% |
| TR2. Not Suspicious | ROM 9.3% |
| TR3. Mildly Suspicious | ROM 16.6% |
| TR4. Moderately Suspicious | ROM 27.0% |
| TR5. Highly Suspicious | ROM 76.5% |
- 2.
- It appeared that ultrasound stratification systems performed better for PTC than FTC.
- 3.
- Perhaps the time has come to abandon size cutoffs for recommending FNA in the pediatric age group. A not insubstantial number of malignancies could be missed when pushing adult management guidelines on children and adolescents, whose thyroid glands are smaller.
- 4.
- Crude frequencies, ROMs, and RONs for the Bethesda system in the pediatric age group based on 5911 cases in the literature (including our cohort) were as follows:
| Bethesda I. Unsatisfactory | Frequency 11.4% | ROM 16.8% | RON 26.7% |
| Bethesda II. Benign | Frequency 56.0% | ROM 7.2% | RON 27.5% |
| Bethesda III. AUS | Frequency 9.6% | ROM 29.6% | RON 55.8% |
| Bethesda IV. FN | Frequency 6.4% | ROM 42.3% | RON 86.8% |
| Bethesda V. SFM | Frequency 3.9% | ROM 90.8% | RON 97.6% |
| Bethesda VI. Malignant | Frequency 12.7% | ROM 98.8% | RON 99.7% |
- 5.
- There may be some utility in adding the ACR TI-RADS level and the Bethesda category (excluding Bethesda I) to come up with a combined score to decide whether surgery should be performed. In our cohort, there was a sharp cutoff between 7 and 8: a combined score of 7 or less had a ROM ranging from 0 to 17.6%, whereas 8 or more implied a ROM ranging from 71.4 to 100%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Lei, K.R.; He, Y.P.; Li, X.L.; Ren, W.W.; Zhao, C.K.; Bo, X.W.; Wang, D.; Sun, C.Y.; Xu, H.X. Malignancy risk stratification of thyroid nodules: Comparisons of four ultrasound Thyroid Imaging Reporting and Data Systems in surgically resected nodules. Sci. Rep. 2017, 7, 11560. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.E.; Hackman, S.D.; Francis, G.L. Thyroid nodules in children and adolescents. J. Endocrinol. Sci. 2020, 2, 16–20. [Google Scholar] [CrossRef]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef]
- Gupta, A.; Ly, S.; Castroneves, L.A.; Frates, M.C.; Benson, C.B.; Feldman, H.A.; Wassner, A.J.; Smith, J.R.; Marqusee, E.; Alexander, E.K.; et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J. Clin. Endocrinol. Metab. 2013, 98, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Yoon, H.M.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H.; Lee, J.S.; Jung, A.Y.; Cho, Y.A.; Bak, B.; et al. Diagnostic performance of five adult-based US risk stratification systems in pediatric thyroid nodules. Radiology 2022, 305, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Al-Hadidi, A.; Bobbey, A.; Shah, S.; Stanek, J.; Nicol, K.; Hoffman, R.P.; Aldrink, J.H. Pediatric adaptions are needed to improve the diagnostic accuracy of thyroid ultrasound using TI-RADS. J. Pediatr. Surg. 2021, 56, 1120–1125. [Google Scholar] [CrossRef]
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid cancer in the pediatric population. Genes 2019, 10, 723. [Google Scholar] [CrossRef]
- Ali, S.Z.; Cibas, E.S. (Eds.) The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes; Springer: New York, NY, USA, 2010. [Google Scholar]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Cibas, E.S.; Clark, D.P.; Layfield, L.J.; Ljung, B.M.; Pitman, M.B.; Abati, A. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: A summation. Cytojournal 2008, 5, 6. [Google Scholar] [CrossRef]
- Baloch, Z.W.; LiVolsi, V.A.; Asa, S.L.; Rosai, J.; Merino, M.J.; Randolph, G.; Vielh, P.; DeMay, R.M.; Sidawy, M.K.; Frable, W.J. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 425–437. [Google Scholar] [CrossRef]
- Cibas, E.S.; Alexander, E.K.; Benson, C.B.; de Agustín, P.P.; Doherty, G.M.; Faquin, W.C.; Middleton, W.D.; Miller, T.; Raab, S.S.; White, M.L.; et al. Indications for thyroid FNA and pre-FNA requirements: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ljung, B.M.; Langer, J.; Mazzaferri, E.L.; Oertel, Y.C.; Wells, S.A.; Waisman, J. Training, credentialing and re-credentialing for the performance of a thyroid FNA: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.B.; Abele, J.; Ali, S.Z.; Duick, D.; Elsheikh, T.M.; Jeffrey, R.B.; Powers, C.N.; Randolph, G.; Renshaw, A.; Scoutt, L. Techniques for thyroid FNA: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Filie, A.C.; Asa, S.L.; Geisinger, K.R.; Logani, S.; Merino, M.; Nikiforov, Y.E.; Clark, D.P. Utilization of ancillary studies in thyroid fine needle aspirates: A synopsis of the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 438–441. [Google Scholar] [CrossRef]
- Layfield, L.J.; Abrams, J.; Cochand-Priollet, B.; Evans, D.; Gharib, H.; Greenspan, F.; Henry, M.; LiVolsi, V.; Merino, M.; Michael, C.W.; et al. Post-thyroid FNA testing and treatment options: A synopsis of the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 442–448. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.J.; Jang, H.W.; Kim, H.K.; Yi, J.H.; Lee, W.; Kim, S.H. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid 2009, 19, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef]
- Russ, G.; Bigorgne, C.; Royer, B.; Rouxel, A.; Bienvenu-Perrard, M. The Thyroid Imaging Reporting and Data System (TIRADS) for ultrasound of the thyroid. J. Radiol. 2011, 92, 701–713. [Google Scholar] [CrossRef]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81, 1–122. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, J.H.; Chung, J.; He, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Radiology consensus statement and recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P.; AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for the clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid ultrasound reporting lexicon: White paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Langer, J.E.; Middleton, W.D.; Wu, C.C.; Hammers, L.W.; Cronan, J.J.; Tessler, F.N.; Grant, E.G.; Berland, L.L. Managing incidental thyroid nodules detected on imaging: White paper of the ACR Incidental Thyroid Findings Committee. J. Am. Coll. Radiol. 2015, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, L.; Wei, X.; Zhang, S.; Song, Y.; Luo, B.; Li, J.; Qian, L.; Cui, L.; Chen, W.; et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: The C-TIRADS. Endocrine 2020, 70, 256–279. [Google Scholar] [CrossRef]
- Floridi, C.; Cellina, M.; Buccimazza, G.; Arrichiello, A.; Sacrini, A.; Arrigoni, F.; Pompili, G.; Barile, A.; Carrafiello, G. Ultrasound imaging classifications of thyroid nodules for malignancy risk stratification and clinical management: State of the art. Gland Surg. 2019, 8, S233–S244. [Google Scholar] [CrossRef]
- Kim, P.H.; Yoon, H.M.; Hwang, J.; Lee, J.S.; Jung, A.Y.; Cho, Y.A.; Baek, J.H. Diagnostic performance of adult-based ATA and ACR-TIRADS ultrasound risk stratification systems in pediatric thyroid nodules: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 7450–7463. [Google Scholar] [CrossRef]
- Galuppini, F.; Vianello, F.; Censi, S.; Barollo, S.; Bertazza, L.; Carducci, S.; Colato, C.; Manso, J.; Rugge, M.; Iacobone, M.; et al. Differentiated thyroid carcinoma in pediatric age: Genetic and clinical scenario. Front. Endocrinol. 2019, 10, 552. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Spinelli, C.; Ghionzoli, M.; Oreglio, C.; Sanna, B.; De Napoli, L.; Morganti, R.; Antonelli, A.; Morabito, A.; Miccoli, P. Increased trend of thyroid cancer in childhood over the last 30 years in EU countries: A call for the pediatric surgeon. Eur. J. Pediatr. 2022, 181, 3907–3913. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H. Does iodine cause thyroid cancer? Acta Endocrinol. 2018, 14, 525–526. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Database. Available online: www.seer.cancer.gov (accessed on 13 October 2022).
- Wingard, J.R.; Majhail, N.S.; Brazauskas, R.; Wang, Z.; Sobocinski, K.A.; Jacobsohn, D.; Sorror, M.L.; Horowitz, M.M.; Bolwell, B.; Rizzo, J.D.; et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2011, 29, 2230–2239. [Google Scholar] [CrossRef]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L.; Neglia, J.P. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, S.; Nakamura, J.L. Radiotherapy-induced malignancies: Review of clinical features, pathobiology, and evolving approaches for mitigating risk. Front. Oncol. 2013, 3, 73. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, B.; Ron, E.; Schneider, A.B. Exposing the thyroid to radiation: A review of its current extent, risks, and implications. Endocr. Rev. 2010, 31, 756–773. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, A.J.; Ronckers, C.M.; Mertens, A.C.; Stovall, M.; Smith, S.A.; Liu, Y.; Berkow, R.L.; Hammond, S.; Neglia, J.P.; Meadows, A.T.; et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet 2005, 365, 2014–2023. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-term effects of radiation exposure among adult survivors of childhood cancer: Results from the Childhood Cancer Survivor Study. Pediatr. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2022. [Google Scholar]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Yuan, L.; Jebastin Thangaiah, J.; Chute, D.J. The role of ultrasound-guided fine-needle aspiration of thyroid bed lesions and clinical predictors of recurrent papillary thyroid carcinoma. Am. J. Clin. Pathol. 2021, 155, 389–396. [Google Scholar] [CrossRef]
- Lim-Dunham, J.E.; Toslak, I.E.; Reiter, M.P.; Martin, B. Assessment of the American College of Radiology Thyroid Imaging Reporting and Data System for thyroid nodule malignancy risk stratification in a pediatric population. AJR Am. J. Roentgenol. 2019, 212, 188–194. [Google Scholar] [CrossRef]
- Shapira-Zaltsberg, G.; Miller, E.; Martinez-Rios, C.; Bass, J.; Goldbloom, E.B.; Tang, K.; Hayawi, L.; Highmore, K. Comparison of the diagnostic performance of the 2017 ACR TI-RADS guideline to the Kwak guideline in children with thyroid nodules. Pediatr. Radiol. 2019, 49, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Polat, Y.D.; Öztürk, V.S.; Ersoz, N.; Anık, A.; Karaman, C.Z. Is Thyroid Imaging Reporting and Data System useful as an adult ultrasonographic malignancy risk stratification method in pediatric thyroid nodules? J. Med. Ultrasound 2019, 27, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Richman, D.M.; Benson, C.B.; Doubilet, P.M.; Wassner, A.J.; Asch, E.; Cherella, C.E.; Smith, J.R.; Frates, M.C. Assessment of American College of Radiology Thyroid Imaging Reporting and Data System (TI-RADS) for pediatric thyroid nodules. Radiology 2020, 294, 415–420. [Google Scholar] [CrossRef]
- Uner, C.; Aydin, S.; Ucan, B. Thyroid Imaging Reporting and Data System categorization: Effectiveness in pediatric thyroid nodule assessment. Ultrasound Q. 2020, 36, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Scappaticcio, L.; Maiorino, M.I.; Iorio, S.; Docimo, G.; Longo, M.; Grandone, A.; Luongo, C.; Cozzolino, I.; Piccardo, A.; Trimboli, P.; et al. Exploring the performance of ultrasound risk stratification systems in thyroid nodules of pediatric patients. Cancers 2021, 13, 5304. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.E.; Shaffer, A.D.; Garbin, S.; Squires, J.H.; Vaughan, K.G.; Viswanathan, P.; Witchel, S.F.; Mollen, K.P.; Yip, L.; Monaco, S.E.; et al. Validity of the American College of Radiology Thyroid Imaging Reporting and Data System in children. Laryngoscope, 2022; Online ahead of print. [Google Scholar]
- Tuli, G.; Munarin, J.; Scollo, M.; Quaglino, F.; De Sanctis, L. Evaluation of the efficacy of EU-TIRADS and ACR-TIRADS in risk stratification of pediatric patients with thyroid nodules. Front. Endocrinol. 2022, 13, 1041464. [Google Scholar] [CrossRef]
- Monaco, S.E.; Pantanowitz, L.; Khalbuss, W.E.; Benkovich, V.A.; Ozolek, J.; Nikiforova, M.; Simons, J.P.; Nikiforov, Y. Cytomorphological and molecular genetic findings in pediatric thyroid fine-needle aspiration. Cancer Cytopathol. 2012, 120, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Buryk, M.A.; Simons, J.P.; Picarsic, J.; Monaco, S.E.; Ozolek, J.A.; Joyce, J.; Gurtunca, N.; Nikiforov, Y.E.; Feldman Witchel, S. Can malignant thyroid nodules be distinguished from benign thyroid nodules by clinical characteristics? A review of 89 pediatric patients with thyroid nodules. Thyroid 2015, 25, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Arva, N.C.; Deitch, S.G. Reclassification of cytologically atypical thyroid nodules based on radiologic features in pediatric patients. J. Pediatr. Endocrinol. Metab. 2015, 28, 753–760. [Google Scholar] [CrossRef]
- Norlén, O.; Charlton, A.; Sarkis, L.M.; Henwood, T.; Shun, A.; Gill, A.J.; Delbridge, L. Risk of malignancy for each Bethesda class in pediatric thyroid nodules. J. Pediatr. Surg. 2015, 50, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Lale, S.A.; Morgenstern, N.N.; Chiara, S.; Wasserman, P. Fine needle aspiration of thyroid nodules in the pediatric population: A 12-year cyto-histological correlation experience at North Shore-Long Island Jewish Health System. Diagn. Cytopathol. 2015, 43, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Partyka, K.L.; Huang, E.C.; Cramer, H.M.; Chen, S.; Wu, H.H. Histologic and clinical follow-up of thyroid fine-needle aspirates in pediatric patients. Cancer Cytopathol. 2016, 124, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Pantola, C.; Kala, S.; Khan, L.; Pantola, S.; Singh, M.; Verma, S. Cytological diagnosis of pediatric thyroid nodule in perspective of the Bethesda System for Reporting Thyroid Cytopathology. J Cytol. 2016, 33, 220–223. [Google Scholar] [CrossRef]
- Amirazodi, E.; Propst, E.J.; Chung, C.T.; Parra, D.A.; Wasserman, J.D. Pediatric thyroid FNA biopsy: Outcomes and impact on management over 24 years at a tertiary care center. Cancer Cytopathol. 2016, 124, 801–810. [Google Scholar] [CrossRef]
- Canfarotta, M.; Moote, D.; Finck, C.; Riba-Wolman, R.; Thaker, S.; Lerer, T.J.; Payne, R.J.; Cote, V. McGill Thyroid Nodule Score in differentiating benign and malignant pediatric thyroid nodules: A pilot study. Otolaryngol. Head Neck Surg. 2017, 157, 589–595. [Google Scholar] [CrossRef]
- Rossi, E.D.; Mehrotra, S.; Kilic, A.I.; Lim-Dunham, J.; Martini, M.; Fadda, G.; Lombardi, C.P.; Larocca, L.M.; Barkan, G.A. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in the pediatric age group. Cancer Cytopathol. 2018, 126, 27–35. [Google Scholar] [CrossRef]
- Wang, H.; Mehrad, M.; Ely, K.A.; Liang, J.; Solórzano, C.C.; Neblett, W.W., 3rd; Coogan, A.C.; Weiss, V.L. Incidence and malignancy rates of indeterminate pediatric thyroid nodules. Cancer Cytopathol. 2019, 127, 231–239. [Google Scholar] [CrossRef]
- Kardelen Al, A.D.; Yılmaz, C.; Poyrazoglu, S.; Tunca, F.; Bayramoglu, Z.; Bas, F.; Bundak, R.; Gilse Senyurek, Y.; Ozluk, Y.; Yegen, G.; et al. The role of thyroid fine-needle aspiration cytology in the treatment and follow-up of thyroid nodules in the pediatric population. Acta Endocrinol. 2019, 15, 333–341. [Google Scholar] [CrossRef]
- Cherella, C.E.; Angell, T.E.; Richman, D.M.; Frates, M.C.; Benson, S.B.; Moore, F.D.; Barletta, J.A.; Hollowell, M.; Smith, J.R.; Alexander, E.K.; et al. Differences in thyroid nodule cytology and malignancy risk between children and adults. Thyroid 2019, 29, 1097–1104. [Google Scholar] [CrossRef]
- Hodax, J.K.; Bowerman, K.; Quintos, J.B. Benign thyroid nodules in pediatric patients: Determining best practices for repeat ultrasound evaluations. J. Pediatr. Endocrinol. Metab. 2019, 32, 895–901. [Google Scholar] [CrossRef]
- Suh, J.; Choi, H.S.; Kwon, A.; Chae, H.W.; Kim, H.S. Adolescents with thyroid nodules: Retrospective analysis of factors predicting malignancy. Eur. J. Pediatr. 2020, 179, 317–325. [Google Scholar] [CrossRef]
- Heider, A.; Arnold, S.; Jing, X. Bethesda System for Reporting Thyroid Cytopathology in pediatric thyroid nodules: Experience of a tertiary care referral center. Arch. Pathol. Lab. Med. 2020, 144, 473–477. [Google Scholar] [CrossRef]
- Arora, S.; Khoury, J.; Trout, A.T.; Chuang, J. Improving malignancy prediction in AUS/FLUS pediatric thyroid nodules with the aid of ultrasound. Horm. Res. Paediatr. 2020, 93, 239–244. [Google Scholar] [CrossRef]
- Jiang, W.; Phillips, S.A.; Newbury, R.O.; Naheedy, J.H.; Newfield, R.S. Diagnostic utility of fine needle aspiration cytology in pediatric thyroid nodules based on Bethesda classification. J. Pediatr. Endocrinol. Metab. 2021, 34, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Suzuki, A.; Na, H.Y.; Tuyen, P.V.; Khuy, D.M.; Nguyen, H.C.; Jitpasutham, T.; Abelardo, A.; Amano, T.; Park, S.Y.; et al. Application of the Bethesda System for Reporting Thyroid Cytopathology in the pediatric population. Am. J. Clin. Pathol. 2021, 155, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.R.; Baran, J.A.; Bauer, A.J.; Isaza, A.; Surrey, L.F.; Bhatti, T.; McGrath, C.; Jalaly, J.; Mostoufi-Moab, S.; Adzick, N.S.; et al. Utility of fine-needle aspirations to diagnose pediatric thyroid nodules. Horm. Res. Paediatr. 2021, 94, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ben-Skowronek, I.; Sieniawska, J.; Pach, E.; Wrobel, W.; Skowronek, A.; Tomczyk, Z.; Mlodawska, A.; Makuch, M.; Malka, M.; Cielecki, C.; et al. Thyroid cancer risk factors in children with thyroid nodules: A one-center study. J. Clin. Med. 2021, 10, 4455. [Google Scholar] [CrossRef]
- Yeste Fernández, D.; Vega Amenabar, E.; Coma Muñoz, A.; Arciniegas Vallejo, L.; Clemente León, M.; Planes-Conangla, M.; Iglesias Felip, C.; Sábado Álvarez, C.; Guillén Burrieza, G.; Campos-Martorell, A. Ultrasound criteria (EU-TIRADS) to identify thyroid nodule malignancy risk in adolescents. Correlation with cyto-histological findings. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 728–734. [Google Scholar] [CrossRef]
- Canberk, S.; Barroca, H.; Girão, I.; Aydın, O.; Uguz, A.; Erdogan, K.; Tastekin, E.; Bongiovanni, M.; Soares, P.; Máximo, V.; et al. Performance of the Bethesda System for Reporting Thyroid Cytology in multi-institutional large cohort of pediatric thyroid nodules: A detailed analysis. Diagnostics 2022, 12, 179. [Google Scholar] [CrossRef]
- Baran, J.A.; Halada, S.; Bauer, A.J.; Ricarte-Filho, J.C.; Isaza, A.; Surrey, L.F.; McGrath, C.; Bhatti, T.; Jalaly, J.; Mostoufi-Moab, S.; et al. Indeterminate thyroid fine-needle aspirations in pediatrics: Exploring clinicopathologic features and utility of molecular profiling. Horm. Res. Paediatr. 2022, 95, 430–441. [Google Scholar] [CrossRef]
- Gallant, J.N.; Chen, S.C.; Ortega, C.A.; Rohde, S.L.; Belcher, R.H.; Netterville, J.L.; Baregamian, N.; Wang, H.; Liang, J.; Ye, F.; et al. Evaluation of the molecular landscape of pediatric thyroid nodules and use of a multigene genomic classifier in children. JAMA Oncol. 2022, 8, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Osterbauer, B.; Sahyouni, G.; Paik, C.; Austin, J.; Gomez, G.; Shillingford, N.; Kwon, D. Malignancy rates by Bethesda subcategory in the pediatric population. Pediatr. Dev. Pathol. 2022, 25, 598–603. [Google Scholar] [CrossRef]
- Matalka, L.; Rahman, A.F.; Sparks, S.; Lindeman, B.; Iyer, P. Evaluation and management of pediatric thyroid nodules and thyroid cancer at a single institution after adoption of the American Thyroid Association 2015 guidelines. J. Pediatr Endocrinol Metab. 2023; Online ahead of print. [Google Scholar]
- Lim-Dunham, J.E.; Erdem Toslak, I.; Alsabban, K.; Aziz, A.; Martin, B.; Okur, G.; Longo, K.C. Ultrasound risk stratification for malignancy using the 2015 American Thyroid Association Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Pediatr. Radiol. 2017, 47, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rios, C.; Daneman, A.; Bajno, L.; van der Kaay, D.C.M.; Moineddin, R.; Wasserman, J.D. Utility of adult-based ultrasound malignancy risk stratifications in pediatric thyroid nodules. Pediatr. Radiol. 2018, 48, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Creo, A.; Alhadab, F.; Al Nofal, A.; Thomas, K.; Kolbe, A.; Pittock, S.T. Ultrasonography and the American Thyroid Association ultrasound-based risk stratification tool: Utility in pediatric and adolescent thyroid nodules. Horm. Res. Paediatr. 2018, 90, 93–101. [Google Scholar] [CrossRef]
- Kim, P.H.; Yoon, H.M.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H.; Lee, J.S.; Jung, A.Y.; Cho, Y.A.; Bak, B.; et al. Diagnostic performance of the 2021 Korean thyroid imaging reporting and data system in pediatric thyroid nodules. Eur. Radiol. 2023, 33, 172–180. [Google Scholar] [CrossRef]
- Piccardo, A.; Fiz, F.; Bottoni, G.; De Luca, C.; Massollo, M.; Catrambone, U.; Foppiani, L.; Muraca, M.; Garaventa, A.; Trimboli, P. Facing thyroid nodules in paediatric patients previously treated with radiotherapy for non-thyroidal cancers: Are ultrasound risk stratification systems reliable? Cancers 2021, 13, 4692. [Google Scholar] [CrossRef]
- Al Nofal, A.; Gionfriddo, M.R.; Javed, A.; Haydour, Q.; Brito, J.P.; Prokop, L.J.; Pittock, S.T.; Murad, M.H. Accuracy of thyroid nodule sonography for the detection of thyroid cancer in children: Systematic review and meta-analysis. Clin. Endocrinol. 2016, 84, 423–430. [Google Scholar] [CrossRef]
- Richman, D.M.; Benson, C.B.; Doubilet, P.M.; Peters, H.E.; Huang, S.A.; Asch, E.; Wassner, A.J.; Smith, J.R.; Cherella, C.E.; Frates, M.C. Thyroid nodules in pediatric patients: Sonographic characteristics and likelihood of cancer. Radiology 2018, 288, 591–599. [Google Scholar] [CrossRef]
- Cozzolino, A.; Filardi, T.; Simonelli, I.; Grani, G.; Virili, C.; Stramazzo, I.; Santaguida, M.G.; Locantore, P.; Maurici, M.; Gianfrilli, D.; et al. Diagnostic accuracy of ultrasonographic features in detecting thyroid cancer in the transition age: A meta-analysis. Eur. Thyroid J. 2022, 11, e220039. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Stybayeya, G.; Lee, J.E.; Hwang, S.H. Diagnostic performance of ACR and Kwak TI-RADS for benign and malignant thyroid nodules: An update systematic review and meta-analysis. Cancers 2022, 14, 5961. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhang, L.; Sun, Y. Diagnostic performance of American College of Radiology TI-RADS: A systematic review and meta-analysis. AJR Am. J. Roentgenol. 2021, 216, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Xu, H.; Shang, W.; Dong, A. Systematic review and meta-analysis of American College of Radiology TI-RADS inter-reader reliability for risk stratification of thyroid nodules. Front. Oncol. 2022, 12, 840516. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Na, D.G.; Baek, J.H.; Sung, J.Y.; Kim, J.H.; Kang, S.Y. US fine-needle aspiration biopsy for thyroid malignancy: Diagnostic performance of seven society guidelines applied to 2000 thyroid nodules. Radiology 2018, 287, 893–900. [Google Scholar] [CrossRef]
- Zhao, C.K.; Ren, T.T.; Yin, Y.F.; Shi, H.; Wang, H.X.; Zhou, B.Y.; Wang, X.R.; Li, X.; Zhang, Y.F.; Liu, C.; et al. A comparative analysis of two machine learning-based diagnostic patterns with Thyroid Imaging Reporting and Data System for thyroid nodules: Diagnostic performance and unnecessary biopsy rate. Thyroid 2021, 31, 470–481. [Google Scholar] [CrossRef]
- Radebe, J.; van der Kaay, D.C.M.; Wasserman, J.D.; Goldenberg, A. Predicting malignancy in pediatric thyroid nodules: Early experience with machine learning for clinical decision support. J. Clin. Endocrinol. Metab. 2021, 106, e5236–e5246. [Google Scholar] [CrossRef]
- Yang, J.; Page, L.C.; Wagner, L.; Wildman-Tobriner, B.; Bisset, L.; Frush, D.; Mazurowski, M.A. Thyroid nodules on ultrasound in children and young adults: Comparison of diagnostic performance of radiologists’ impressions, ACR TI-RADS, and a deep learning algorithm. AJR Am. J. Roentgenol. 2023, 220, 408–417. [Google Scholar] [CrossRef]
- Rossi, E.D.; Straccia, P.; Martini, M.; Revelli, L.; Lombardi, C.P.; Pontecorvi, A.; Fadda, G. The role of thyroid fine-needle aspiration cytology in the pediatric population: An institutional experience. Cancer Cytopathol. 2014, 122, 359–367. [Google Scholar] [CrossRef]
- Tuli, G.; Munarin, J.; Agosto, E.; Matarazzo, P.; Quaglino, F.; Mormile, A.; de Sanctis, L. Predictive factors of malignancy in pediatric patients with thyroid nodules and performance of the Italian classification (SIAPEC 2014) in the outcome of the cytological FNA categories. Endocrine 2021, 74, 365–374. [Google Scholar] [CrossRef]
- Chang, S.H.; Joo, M.; Kim, H. Fine needle aspiration biopsy of thyroid nodules in children and adolescents. J. Korean Med. Sci. 2006, 21, 469–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altıncık, A.; Demir, K.; Abacı, A.; Böber, E.; Büyükgebiz, A. Fine-needle aspiration biopsy in the diagnosis and follow-up of thyroid nodules in childhood. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Baş, V.N.; Aycan, Z.; Cetinkaya, S.; Uner, C.; Cavuşoğlu, Y.H.; Arda, N. Thyroid nodules in children and adolescents: A single institution’s experience. J. Pediatr. Endocrinol. Metab. 2012, 25, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Trahan, J.; Reddy, A.; Chang, E.; Gomez, R.; Prasad, P.; Jeyakumar, A. Pediatric thyroid nodules: A single center experience. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 94–97. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda System for Reporting Thyroid Cytopathology: A meta-analysis. Acta Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef]
- Straccia, P.; Rossi, E.D.; Bizzarro, T.; Brunelli, C.; Cianfrini, F.; Damiani, D.; Fadda, G. A meta-analytic review of The Bethesda System for Reporting Thyroid Cytopathology: Has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015, 123, 713–722. [Google Scholar] [CrossRef]
- Ali, S.Z.; Cibas, E.S. (Eds.) The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes, 2nd ed.; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Krauss, E.A.; Mahon, M.; Fede, J.M.; Zhang, L. Application of the Bethesda classification for thyroid fine-needle aspiration: Institutional experience and meta-analysis. Arch. Pathol. Lab. Med. 2016, 140, 1121–1131. [Google Scholar] [CrossRef]
- Vuong, H.G.; Ngo, H.T.T.; Bychkov, A.; Jung, C.K.; Vu, T.H.; Lu, K.B.; Kakudo, K.; Kondo, T. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta-analysis. Cancer Cytopathol. 2020, 128, 238–249. [Google Scholar] [CrossRef]
- Vuong, H.G.; Chung, D.G.B.; Ngo, L.M.; Bui, T.Q.; Hassell, L.; Jung, C.K.; Kakudo, K.; Bychkov, A. The use of The Bethesda System for Reporting Thyroid Cytopathology in pediatric thyroid nodules: A meta-analysis. Thyroid 2021, 31, 1203–1211. [Google Scholar] [CrossRef]
- Cherella, C.E.; Cibas, E.S.; Wassner, A.J. Re: “The use of The Bethesda System for Reporting Thyroid Cytopathology in pediatric thyroid nodules: A meta-analysis” by Vuong et al. Thyroid 2021, 31, 1441. [Google Scholar] [CrossRef]
- Vuong, H.G.; Jung, C.K.; Kakudo, K.; Bychkov, A. Response to Cherella et al. re: “The use of The Bethesda System for Reporting Thyroid Cytopathology in pediatric thyroid nodules: A meta-analysis”. Thyroid 2021, 31, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.W.; Banerjee, A.; Heneghan, C.; Pluddemann, A. Verification bias. BMJ Evid. Based Med. 2018, 23, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Creo, A.; Alahdab, F.; Al Nofal, A.; Thomas, K.; Kolbe, A.; Pittock, S. Diagnostic accuracy of the McGill thyroid nodule score in paediatric patients. Clin. Endocrinol. 2019, 90, 200–207. [Google Scholar] [CrossRef]
- Tan, H.; Li, Z.; Li, N.; Qian, J.; Fan, F.; Zhong, H.; Feng, J.; Xu, H.; Li, Z. Thyroid imaging reporting and data system combined with Bethesda classifiction in qualitative thyroid nodule diagnosis. Medicine 2019, 98, e18320. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Nicol, K.K. The Bethesda System for Reporting Thyroid Cytopathology is applicable to frozen section diagnosis in children. Pediatr. Dev. Pathol. 2015, 18, 139–145. [Google Scholar] [CrossRef]
- Kim, S.J.; Roh, J.; Baek, J.H.; Hong, S.J.; Shong, Y.K.; Kim, W.B.; Song, D.E. Risk of malignancy according to sub-classification of the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) category in the Bethesda System for Reporting Thyroid Cytopathology. Cytopathology 2017, 28, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.R.; Nga, M.E.; Lum, J.H.; Wong, W.M.; Tan, W.B.; Parameswaran, R.; Ngiam, K.Y. Thyroid cytology-nuclear versus architectural atypia within the “atypia of undetermined significance/follicular lesion of undetermined significance” Bethesda category have significantly different rates of malignancy. Cancer Cytopathol. 2017, 125, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.N.; Cavallo, A.B.; Uraizee, I.; Tanager, K.; Lastra, R.R.; Antic, T.; Cipriani, N.A. A proposal for separation of nuclear atypia and architectural atypia in Bethesda category III (AUS/FLUS) based on differing rates of thyroid malignancy. Am. J. Clin. Pathol. 2019, 151, 86–94. [Google Scholar] [CrossRef]
- Thakur, A.; Sarin, H.; Kaue, D.; Sarin, D. Risk of malignancy in thyroid “atypia of undetermined significance/follicular lesion of undetermined significance” and its subcategories—A 5-year experience. Indian J. Pathol. Microbiol. 2019, 62, 544–548. [Google Scholar] [CrossRef]
- Cherella, C.E.; Hollowell, M.L.; Smith, J.R.; Zendejas, B.; Modi, B.P.; Cibas, E.S.; Wassner, A.J. Subtype of atypia on cytology and risk of malignancy in pediatric thyroid nodules. Cancer Cytopathol. 2022, 130, 330–335. [Google Scholar] [CrossRef]
- Gild, M.L.; Chan, M.; Gajera, J.; Lurie, B.; Gandomkar, Z.; Clifton-Bligh, R.J. Risk stratification of indeterminate thyroid nodules using ultrasound and machine learning algorithms. Clin. Endocrinol. 2022, 96, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, C.; Eppenberger-Castori, S.; Zechmann, S.; Hanke, J.; Herzog, M.; Savic Prince, S.; Christ, E.R.; Ebrahimi, F. Effects of rapid on-site evaluation on diagnostic accuracy of thyroid fine-needle aspiration. Acta Cytol. 2022, 66, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.S.; Baek, J.H.; Lim, H.K.; Ha, E.J.; Kim, J.K.; Song, D.E.; Kim, T.Y.; Lee, J.H. Thyroid nodules with initially nondiagnostic cytologic results: The role of core-needle biopsy. Radiology 2013, 268, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Baek, J.H.; Lee, J.H.; Choi, Y.J.; Hong, M.J.; Song, D.E.; Kim, J.K.; Yoon, J.H.; Kim, W.B. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: Comparison of core-needle biopsy and repeated fine-needle aspiration. Eur. Radiol. 2014, 24, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Baek, J.H.; Park, C.; Choi, Y.J.; Lee, J.H. The role of core needle biopsy for thyroid nodules with initially indeterminate results on previous fine-needle aspiration: A systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 2017, 38, 1421–1426. [Google Scholar] [CrossRef]
- Na, D.G.; Kim, J.H.; Sung, J.Y.; Baek, J.H.; Jung, K.C.; Lee, H.; Yoo, H. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid 2012, 22, 468–475. [Google Scholar] [CrossRef]
- Abram, M.; Huhtamella, R.; Kalfert, D.; Hakso-Mäkinen, H.; Ludvíková, M.; Kholová, I. The role of cell blocks and immunohistochemistry in thyroid atypia of undetermined significance/follicular lesion of undetermined significance Bethesda category. Acta Cytol. 2021, 65, 257–263. [Google Scholar] [CrossRef]
- Liu, H.; Lin, F. Application of immunohistochemistry in thyroid pathology. Arch. Pathol. Lab. Med. 2015, 139, 67–82. [Google Scholar] [CrossRef]
- Koperek, O.; Kornauth, C.; Capper, D.; Berghoff, A.S.; Asari, R.; Niederle, B.; von Deimling, A.; Birner, P.; Preusser, M. Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carincoma. Am. J. Surg. Pathol. 2012, 36, 844–850. [Google Scholar] [CrossRef]
- Hess, J.R.; Newbern, D.K.; Beebe, K.L.; Walsh, A.M.; Schafernak, K.T. High prevalence of gene fusions and copy number alterations in pediatric radiation therapy-induced papillary and follicular thyroid carcinomas. Thyroid 2022, 32, 411–420. [Google Scholar] [CrossRef]
- Chou, A.; Fraser, S.; Toon, C.W.; Clarkson, A.; Sioson, L.; Farzin, M.; Cussigh, C.; Aniss, A.; O’Neill, C.; Watson, N.; et al. A detailed clinicopathologic study of ALK-translocated papillary thyroid carcinoma. Am. J. Clin. Pathol. 2015, 39, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, T.Y.; Güzelbey, B.; Turan Güzel, N.; Kuzan, B.M.; Çakır, M.S.; Canbey, C. Analysis of intra-observer and inter-observer variability of pathologists for non-benign thyroid fine needle aspiration cytology according to Bethesda system categories. Diagn. Cytopathol. 2021, 49, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Srivastava, R.; Singh, N.; Arora, V.K.; Bhatia, A. Implementation of The Bethesda System for Reporting Cytopathology: Interobserver concordance and reclassification of previously inconclusive aspirates. Diagn. Cytopathol. 2014, 42, 944–949. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Khan, M.A.; Kazi, F.; Noreen, F.; Nawaz, S.; Sohail, I. The interobserver reproducibility of thyroid cytopathology using Bethesda Reporting System: Analysis of 200 cases. J. Pak. Med. Assoc. 2013, 63, 1252–1255. [Google Scholar]
- Anand, B.; Ramdas, A.; Ambroise, M.M.; Kumar, N.P. The Bethesda System for Reporting Cytopathology: A cytohistological study. J. Thyroid Res. 2020, 2020, 8095378. [Google Scholar] [CrossRef] [PubMed]
- Słowińska-Klencka, D.; Klencki, M.; Duda-Szymańska, J.; Szwalski, J.; Popowicz, B. Low reproducibility of equivocal categories of The Bethesda System for Reporting Thyroid Cytology makes the associated risk of malignancy specific to the diagnostic center. Endocrine 2021, 74, 355–364. [Google Scholar] [CrossRef]
- Bhasin, T.S.; Mannan, R.; Manjari, M.; Mehra, M.; Gill Sekhon, A.K.; Chandey, M.; Sharma, S.; Kaur, P. Reproducibility of ‘The Bethesda System for Reporting Thyroid Cytopathology’: A multicenter study with review of the literature. J. Clin. Diagn. Res. 2013, 7, 1051–1054. [Google Scholar] [CrossRef]
- Lokhandwala, P.M.; Abendroth, C.S.; Wang, M.; Mani, H.; Williams, N.C.; Walls, M.; Zander, D.S. Assessment of cytotechnologist-cytopathologist interpretive agreement using The Bethesda System for Reporting Thyroid Cytopathology. Diagn. Cytopathol. 2016, 44, 113–118. [Google Scholar] [CrossRef]
- Gerhard, R.; Teixeira, S.; Gaspar da Rocha, A.; Schmitt, F. Thyroid fine-needle aspiration cytology: Is there a place to virtual cytology? Diagn. Cytopathol. 2013, 41, 793–798. [Google Scholar] [CrossRef]
- Jing, X.; Knoepp, S.M.; Roh, M.H.; Hookim, K.; Placido, J.; Davenport, R.; Rasche, R.; Michael, C.W. Group consensus review minimizes the diagnosis of “follicular lesion of undetermined significance” and improves cytohistologic correlation. Diagn. Cytopathol. 2012, 40, 1037–1042. [Google Scholar] [CrossRef] [PubMed]





| Cases Overall (208) | Mean | Mean | Cases with Surgical Follow-Up (74) | ||||
|---|---|---|---|---|---|---|---|
| Distribution | Size (cm) | TI-RADS pts | Benign (41) | Malignant (33) | Total (74) | ROM | |
| Category | |||||||
| TI-RADS 1 | 8 (3.8%) | 1.6 | 0.0 | 2 (2.7%) | 0 (0%) | 2 (2.7%) | 0% |
| TI-RADS 2 | 13 (6.3%) | 1.9 | 1.9 | 4 (5.4%) | 1 (1.4%) 1 FTC | 5 (6.8%) | 20% |
| TI-RADS 3 | 56 (26.9%) | 1.8 | 3.0 | 10 (13.5%) | 7 (9.5%) 5 PTC, 2 FTC | 17 (23.0%) | 41.2% |
| TI-RADS 4 | 100 (48.1%) | 1.4 | 4.6 | 20 (27.0%) | 12 (16.2%) 10 PTC, 2 FTC | 32 (43.2%) | 37.5% |
| TI-RADS 5 | 31 (14.9%) | 1.7 | 7.8 | 5 (6.8%) | 13 (17.6%) 13 PTC | 18 (23.0%) | 72.2% |
| Composition | |||||||
| Cystic | 11 (5.3%) | 1.6 | 0.8 | 3 (4.1%) | 0 (0%) | 3 (4.1%) | 0% |
| Spongiform | 3 (1.4%) | 1.6 | 1.0 | 1 (1.4%) | 0 (0%) | 1 (1.4%) | 0% |
| Mixed | 25 (12.0%) | 2.2 | 3.5 | 9 (12.2%) | 1 (1.4%) 1 FTC | 10 (13.5%) | 10% |
| Solid | 169 (81.3%) | 1.5 | 4.7 | 28 (37.8%) | 32 (43.2%) 28 PTC, 4 FTC | 60 (81.1%) | 53.3% |
| Echogenicity | |||||||
| Anechoic | 9 (4.3%) | 1.8 | 0.3 | 3 (4.1%) | 0 (0%) | 3 (4.1%) | 0% |
| Hyperechoic/isoechoic | 84 (40.4%) | 2.0 | 3.9 | 18 (24.3%) | 16 (21.6%) 13 PTC, 3 FTC | 34 (45.9%) | 47.1% |
| Hypoechoic | 112 (53.8%) | 1.3 | 4.9 | 19 (25.7%) | 16 (21.6%) 14 PTC, 2 FTC | 35 (47.3%) | 45.7% |
| Very hypoechoic | 3 (1.4%) | 1.3 | 6.7 | 1 (1.4%) | 1 (1.4%) 1 PTC | 2 (2.7%) | 50% |
| Shape | |||||||
| Wider-than-tall | 18 (8.7%) | 2.0 | 7.3 | 36 (48.6%) | 28 (37.8%) 23 PTC, 5 FTC | 64 (81.1%) | 43.8% |
| Taller-than-wide | 190 (91.3%) | 1.6 | 4.0 | 5 (6.8%) | 5 (6.8%) 5 PTC | 10 (13.5%) | 50% |
| Margins | |||||||
| Smooth | 109 (52.4%) | 1.5 | 3.7 | 23 (31.1%) | 13 (17.6%) 10 PTC, 3 FTC | 36 (48.6%) | 36.1% |
| Ill-defined | 71 (34.1%) | 1.5 | 4.3 | 11 (14.9%) | 13 (17.6%) 12 PTC, 1 FTC | 24 (32.4%) | 54.2% |
| Lobulated/irregular | 28 (13.5%) | 2.1 | 6.5 | 7 (9.5%) | 7 (9.5%) 6 PTC, 1 FTC | 14 (18.9%) | 50% |
| Extrathyroidal extension | 0 (0%) | N/A | N/A | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Echogenic foci | |||||||
| None/lg comet-tail artifacts | 170 (81.7%) | 1.6 | 3.7 | 35 (47.3%) | 16 (21.6%) 11 PTC, 5 FTC | 51 (68.9%) | 31.4% |
| Macrocalcifications | 2 (1.0%) | 1.7 | 4.5 | 0 (0%) | 0 (0%) | 0 (0%) | N/A |
| Peripheral (rim) calcs | 4 (1.9%) | 1.4 | 6.3 | 1 (1.4%) | 0 (0%) | 1 (1.4%) | 0% |
| Punctate echogenic foci | 32 (15.4%) | 1.8 | 7.5 | 5 (6.8%) | 17 (23.0%) 17 PTC | 22 (29.7%) | 77.3% |
| Cases with surgical follow-up (74) | Combined TI-RADS and Bethesda score excluding Bethesda I (69) | ||||||
| TI-RADS points | ROM (Malignant/Total) | Benign (38) | Malignant (31) | Total (69) | ROM | ||
| 0 | 0% (0/2) | Combined score 3 | 1 (1.4%) | 0 (0%) | 1 (1.4%) | 0% | |
| 1 | N/A (0/0) | Combined score 4 | 3 (4.3%) | 0 (0%) | 3 (4.3%) | 0% | |
| 2 | 20% (1/5) 1 FTC | Combined score 5 | 6 (8.7%) | 1 (1.4%) 1 FTC | 7 (10.1%) | 14.3% | |
| 3 | 41.2% (7/17) 5 PTC, 2 FTC | Combined score 6 | 14 (20.3%) | 3 (4.3%) 2 PTC, 1 FTC | 17 (24.6%) | 17.6% | |
| 4 | 31.3% (5/16) 4 PTC, 1 FTC | Combined score 7 | 11 (15.9%) | 2 (2.9%) 2 FTC | 13 (18.8%) | 15.4% | |
| 5 | 0% (0/2) | Combined score 8 | 2 (2.9%) | 5 (7.2%) 4 PTC, 1 FTC | 7 (10.1%) | 71.4% | |
| 6 | 50% (7/14) 6 PTC, 1 FTC | Combined score 9 | 0 (%) | 4 (5.8%) 4 PTC | 4 (5.8%) | 100% | |
| 7 | 50%% (5/10) 5 PTC | Combined score 10 | 1 (1.4%) | 6 (8.7% 6 PTC | 7 (10.1%) | 85.7% | |
| 8 | 100% (2/2) 2 PTC | Combined score 11 | 0 (0%) | 10 (14.5%) 10 PTC | 10 (14.5%) | 100% | |
| 9 | 100% (3/3) 3 PTC | ||||||
| 10 | 100% (1/1) 1 PTC | ||||||
| 11 | 100% (1/1) 1 PTC | ||||||
| 12 | 100% (1/1) 1 PTC | ||||||
| Ref. | Study Period, Location, Readers, Agreement, AUC | Age | Follow-Up | TR1 ROM (M/T) | TR2 ROM (M/T) | TR3 ROM (M/T) | TR4 ROM (M/T) | TR5 ROM (M/T) |
|---|---|---|---|---|---|---|---|---|
| [45] | 1996–2017 Loyola University Medical Center (USA), 2 readers, intra-k = 0.69–0.77; p < 0.001, inter-k = 0.37; p < 0.002, AUC = 0.75 (95% CI, 0.64–0.86) | ≤18 y | FNA or surgical | 25% (1/4) | 0% (0/4) | 0% (0/6) | 8.3% (2/24) | 47.2% (17/36) |
| [46] | 8/2007–8/2017 Children’s Hospital of Eastern Ontario (Canada), 4 readers, pairwise agreement 50.9% (95% CI, 46.3–55.5%), AUC = 0.72 (95% CI, 0.61–0.82) | <18 y | FNA, surgical, or 2+ y clinical/US stability | 3.9% (0.5/12.75) | 6.5% (0.75/11.5) | 10% (1/10) | 21.2% (6/28.25) | 38% (4.75/12.5) |
| [47] | 1/2015–2018 Aydın Adnan Menderes (Turkey), 2 readers | <18 y | Surgical * | 0% (0/2) | N/A (0/0) | 0% (0/4) | 0% (0/2) | 100% (5/5) |
| Surgical or 1 y clinical/US stability | 0% (0/65) | 0% (0/2) | 0% (0/12) | 0% (0/21) | 100% (5/5) | |||
| [48] | 1/2004–7/2017 Brigham and Women’s and Boston Children’s Hospitals (USA), 4 readers | ≤18 y | FNA or surgical; for ND FNAs, US size decrease after 1+ y or increased activity on NM scan | 5.9% (2/34) | 4.8% (4/83) | 6.4% (7/109) | 15.5% (18/116) | 74.2% (46/62) |
| [49] | Dr. Sami Ulus Children’s Hospital (Turkey), AUC = 0.89 (95% CI, 0.80–0.98) | ≤18 y | FNA or surgical | 0% (0/5) | 5.6% (2/36) | 42.9% (3/7) | 68.4% (13/19) | 100% (1/1) |
| [6] | 1/2015–3/2019 Nationwide Children’s Hospital (USA), 2 readers, inter-rater Spearman correlation and kappa statistic both 0.51; p < 0.00001 | ≤21 y | Surgical | 0% (0/1) | 0% (0/8) | 36.4% (8/22) | 66.7% (6/9) | 60% (3/5) |
| [50] | 1/2017–3/2021 University of Campania “L. Vanvitelli” (Italy), 2 readers (3rd if needed for consensus), inter-k = 0.7; p≤0.002 | ≤18 y | FNA or surgical | 0% (0/4) | 20% (1/5) | 30% (3/10) | 12.5% (2/16) | 100% (6/6) |
| [5] | 1/2000–4/2020 Asan Medical Center (South Korea), 3 readers, intra-class correlation coefficient for inter-reader agreement, 0.68 (95% CI, 0.63–0.73) | ≤18 y | FNA or surgical | 0% (0/11) | 15.9% (11/69) | 33.3% (14/42) | 59.6% (31/52) | 93.2% (96/103) |
| [51] | 2007–2018 University of Pittsburgh (USA), 2 readers, weighted Cohen’s inter-k = 0.576, SE = 0.066, p < 0.001, AUC = 0.758 | ≤18 y | Surgical (91 cases) or clinical/FNA (15 cases) | 0% (0/3) | 25% (3/12) | 36.4% (8/22) | 55.2% (16/29) | 80% (32/40) |
| [52] | 2000–2020 Regina Margherita Children’s Hospital (Italy), 2 readers, Cohen’s inter-k = 0.85 | <18 y | FNA (75)/surgical (40), or none | 0% (0/20) | 0% (0/9) | 4.1% (2/49) | 15.6% (17/109) | 53.8% (7/13) |
| 7/2015–5/2022 Phoenix Children’s Hospital (USA) (current study) | ≤18 y | Surgical | 0% (0/2) | 20% (1/5) | 41.2% (7/17) | 37.5% (12/32) | 72.2% (13/18) | |
| Total (429/1458) | 2.2% (3.5/161.75) | 9.3% (22.75/244.5) | 16.6% (49/295) | 27.0% (123/455.25) | 76.5% (230.75/301.5) |
| Ref. | Study Period and Location | FNA Cases Age | Cases with Follow-Up | % Bethesda I ROM * RON † | % Bethesda II ROM * RON † | % Bethesda III ROM * RON † | % Bethesda IV ROM * RON † | % Bethesda V ROM * RON † | % Bethesda VI ROM * RON † |
|---|---|---|---|---|---|---|---|---|---|
| [53] | 1/2007–7/2011 University of Pittsburgh Medical Center (USA) | 179 from 142 pts ≤21 y | 96 surgical | 11.7% 21/179 0% 0/8 * | 45.8% 82/179 6.7% 2/30 * | 24.0% 43/179 28% 7/25 * | 10.6% 19/179 57.8% 11/19 * | 3.4% 6/179 100% 6/6 * 100% 6/6 † | 4.5% 8/179 100% 8/8 * 100% 8/8 † |
| [54] | 1/2007–1/2012 Children’s Hospital of Pittsburgh (USA) | 76 ≤18 y | 37 surgical | 3.9% 3/76 N/A * N/A † | 53.9% 41/76 44.4% 4/9 * 44.4% 4/9 † | 15.8% 12/76 0% 0/8 * 0% 0/8 † | 7.9% 6/76 50% 3/6 * 83.3% 5/6 † | 9.2% 7/76 85.7% 6/7 * 100% 7/7 † | 9.2% 7/76 100% 7/7 * 100% 7/7 † |
| [55] | 1/2000–12/2013 Ann & Robert H. Lurie Children’s Hospital of Chicago (USA) | 187 from 180 pts 177 ≤ 18 y 3 > 18 y | 81 surgical | 5.9% 11/187 N/A * N/A † | 61.0% 114/187 10.5% 3/29 * 20.7% 6/29 † | 13.9% 26/187 18.8% 3/16 * 43.8% 7/16 † | 9.6% 18/187 27.7% 5/18 * 72.2% 13/18 † | 3.2% 6/187 100% 6/6 * 100% 6/6 † | 6.4% 12/187 100% 12/12 * 100% 12/12 † |
| [56] | 1/1998–7/2013 Royal North Shore Children’s Hospital, Children’s Westmead Hospital (Australia) | 66 from 56 pts <18 y | 31 surgical | 10.6% 7/66 0% 0/3 * 0% 0/3 † | 57.6% 38/66 0% 0/9 * 22.2% 2/9 † | 16.7% 11/66 22.2% 2/9 * 44.4% 4/9 † | 6.1% 4/66 100% 4/4 * 100% 4/4 † | 4.5% 3/66 100% 3/3 * 100% 3/3 † | 4.5% 3/66 100% 3/3 * 100% 3/3 † |
| [57] | 1/1998–11/2010 North Shore-Long Island Jewish Health System (USA) | 282 from 282 pts <20 y | 78 surgical | 20.9% 59/282 10% 1/10 * | 48.2% 136/282 0% 0/17 * | 2.1% 6/282 50% 2/4 * | 14.2% 40/282 47.4% 9/19 * | 2.1% 6/282 100% 4/4 * 100% 4/4 † | 12.4% 35/282 100% 13/13 * 100% 24/24 † |
| [58] | 1995–2014 Indiana University Health, 2005–2014 University of California, Davis Medical Center (USA) | 186 from 154 pts ≤18 y | 61 surgical + 57 ≥ 2 y clinical | 14.5% 27/186 0% 0/19 * 0% 0/19 † | 61.3% 114/186 1.5% 1/68 * 8.8% 6/68 † | 11.3% 21/186 26.3% 5/19 * 52.6% 10/19 † | 4.3% 8/186 57.1% 4/7 * 100% 7/7 † | 1.6% 3/186 100% 3/3 * 100% 3/3 † | 7.0% 13/186 100% 13/13 * 100% 13/13 † |
| [59] | 8/2010–7/2014 Ganesh Shankar Vidyarthi Memorial Medical College, Bharat Scan and Research Institute (India) | 218 <18 y | 44 surgical | 5.5% 12/218 0% 0/2 * 0% 0/2 † | 69.3% 151/218 0% 0/12 * 0% 0/12 † | 10.6% 23/218 8.3% 1/12 * 75% 9/12 † | 8.3% 18/218 10% 1/10 * 80% 8/10 † | 2.3% 5/218 100% 2/2 * 100% 2/2 † | 4.1% 9/218 100% 6/6 * 100% 6/6 † |
| [60] | 1992–2015 The Hospital for Sick Children (Canada) | 207 from 178 pts <18 y | 65 surgical | 26.1% 54/207 0% 0/12 * 41.7% 5/12 † | 52.2% 108/207 15.8% 3/19 * 52.6% 10/19 † | 8.2% 17/207 66.7% 6/9 * 77.8% 7/9 † | 0% 0/207 N/A * N/A † | 4.8% 10/207 71.4% 5/7 * 71.4% 5/7 † | 8.7% 18/207 100% 18/18 * 100% 18/18 † |
| [61] | 9/2008–12/2015 Connecticut Children’s Medical Center (USA) | 46 from 46 pts <18 y | 46 surgical | 2.2% 1/46 0% 0/1 * | 32.6% 15/46 0% 0/15 * | 39.1% 18/46 5.6% 1/18 * | 8.7% 4/46 25% 1/4 * | 2.2% 1/46 100% 1/1 * 100% 1/1 † | 15.2% 7/46 100% 7/7 * 100% 7/7 † |
| [62] | 1/2001–12/2016 Agostino Gemelli Hospital of Catholic University, Loyola University (Italy, USA) | 95 <19 y | 95 surgical | 5.3% 5/95 0% 0/5 * 60% 3/5 † | 22.1% 21/95 4.8% 1/21 * 61.9% 13/21 † | 9.5% 9/95 11/1% 1/9 * 88.8% 8/9 † | 26.3% 25/95 20% 5/25 * 96% 24/25 † | 7.4% 7/95 100% 7/7 * 100% 7/7 † | 29.5% 28/95 100% 28/28 * 100% 28/28 † |
| [63] | 2001–2018 Vanderbilt University Medical Center (USA) | 302 from 253 pts ≤21 y | 104 surgical | 8.3% 25/302 0% 0/5 * 0% 0/5 † | 71.2% 215/302 7.5% 4/53 * 20.8% 11/53 † | 8.6% 26/302 20% 3/15 * 53.3% 8/15 † | 3.3% 10/302 25% 2/8 * 75% 6/8% † | 1.7% 5/302 100% 5/5 * 100% 5/5 † | 7.0% 21/302 100% 18/18 * 100% 18/18 † |
| [64] | 6/2003–5/2016 Istanbul University (Turkey) | 103 from 80 pts ≤19 y | 44 surgical | 8.7% 9/103 100% 1/1 * 100% 1/1 † | 49.5% 51/103 55.6% 5/9 * | 11.7% 12/103 100% 3/3 * 100% 3/3 † | 7.8% 8/103 71.4% 5/7 † | 6.8% 7/103 85.7% 6/7 * | 15.5% 16/103 100% 16/16 100% 16/16 † |
| [65] | 1/1998–11/2016 Boston Children’s Hospital and Brigham and Women’s Hospital (USA) | 430 from 334 pts <19 y | 190 surgical | 12.3% 53/430 30% 6/20 * | 64.0% 275/430 2.6% 2/76 * | 7.4% 32/430 53.8% 14/26 * | 3.3% 14/430 71.4% 10/14 * | 6.0% 26/430 76% 19/25 * | 7.0% 30/430 100% 29/29 * 100% 29/29 † |
| [66] | 1/2003–12/2013 Rhode Island Hospital (USA)–study only included Bethesda II FNAs | 46 from 43 pts <19 y | 14 surgical | N/A 14.3% 2/14 * 71.4% 10/14 † | |||||
| [67] | 1/2005–5/2017 Severance Children’s Hospital (South Korea) | 141 <18 y | 111 surgical | 6.4% 9/141 100% 2/2 * 100% 2/2 † | 22.0% 31/141 12.5% 1/8 * | 8.5% 12/141 75% 9/12 * | 1.4% 2/141 50% ½ * | 14.2% 20/141 100% 20/20 * 100% 20/20 † | 47.5% 67/141 100% 67/67 * 100% 67/67 † |
| [68] | 1/2011–9/2017 University of Michigan-Michigan Medicine (USA) | 201 from 148 pts ≤20 y | 100 surgical | 7.0% 14/201 14.2% 1/7 * 14.2% 1/7 † | 51.2% 103/201 0% 0/31 * 12.9% 4/31 † | 14.9% 30/201 31.3% 5/16 * 56.3% 9/16 † | 5.0% 10/201 11.1% 1/9 * 100% 9/9 † | 4.5% 9/201 100% 6/6 * 100% 6/6 † | 17.4% 35/201 100% 31/31 * 100% 31/31 † |
| [69] | 2008–2018 Cincinnati Children’s Hospital (USA) | 143 from 128 pts ≤22 y | 74 surgical | 18.9% 27/143 23.1% 3/13 * | 53.8% 77/143 11.1% 3/27 * | 15.4% 22/143 44.4% 8/18 * | 5.6% 8/143 28.6% 2/7 * | 3.5% 5/143 100% 5/5 * 100% 5/5 † | 2.8% 4/143 100% 4/4 * 100% 4/4 † |
| [70] | 12/2002–11/2018 Rady Children’s Hospital in San Diego (USA) | 203 from 171 pts ≤18 y | 92 surgical | 14.3% 29/203 33.3% 4/12 * 41.7% 5/12 † | 52.2% 106/203 26.3% 5/19 * 52.6% 10/19 † | 10.8% 22/203 31.3% 5/16 * 56.3% 9/16 † | 6.9% 14/203 38.5% 5/13 * 46.2% 6/13 † | 3.0% 6/203 83.3% 5/6 * 83.3% 5/6 † | 12.8% 26/203 100% 26/26 * 100% 26/26 † |
| [71] | 2011–2019 7 institutions in 5 Asian countries: Japan (2), Korea (2), Thailand, Philippines, Vietnam | 1217 ≤18 y | 300 surgical (Philippines and Vietnam excluded) | 15.9% 194/1217 30% 3/10 * 30% 3/10 † | 58.3% 709/1217 8.8% 8/91 * 33.0% 30/91 † | 2.6% 32/1217 66.7% 4/6 * 66.7% 4/6 † | 5.5% 67/1217 36.4% 16/44 * 95.5% 42/44 † | 2.3% 28/1217 100% 11/11 * 100% 11/11 † | 15.3% 186/1217 99.3% 137/138 * 99.3% 137/138 † |
| [6] | 1/2015–3/2019 Nationwide Children’s Hospital (USA) | 138 from 115 pts ≤21 y | 9.4% 13/138 | 79.0% 109/138 | 4.4% 6/138 | 1.5% 2/138 | 1.5% 2/138 | 4.4% 6/138 | |
| [72] | 1/2008–12/2018 Children’s Hospital of Philadelphia (USA) | 575 from 324 pts <18 y | 340 surgical | 4.3% 25/575 0% 0/6 * | 66.4% 382/575 1.8% 3/169 * | 7.8% 45/575 16.7% 7/42 * | 5.7% 33/575 54.5% 18/33 * | 2.3% 13/575 100% 13/13 * 100% 13/13 † | 13.4% 77/575 100% 77/77 * 100% 77/77 † |
| [73] | 2010–2021 Medical University of Lublin (Poland) | 67 ≤18 y | 37 surgical | 4.5% 3/67 N/A * | 70.1% 47/67 12.5% 2/16 * | 13.4% 9/67 44.4% 4/9 * | 4.5% 3/67 33.3% 1/3 * | 6.0% 4/67 100% 4/4 * 100% 4/4 † | 1.5% 1/67 100% 1/1 * 100% 1/1 † |
| [74] | 2005–2020 Hospital Universitari Vall d’Hebron (Spain) | 31 from 24 pts <18 y | 19 surgical | 25.8% 8/31 0% 0/3 * 33.3% 1/3 † | 41.9% 13/31 14.3% 1/7 * 42.9% 3/7 † | 12.9% 4/31 0% 0/3* 0% 0/3† | 6.5% 2/31 100% 2/2 * 100% 2/2 † | 0% 0/31 N/A * N/A † | 12.9% 4/31 100% 4/4 * 100% 4/4 † |
| [75] | 2000–2018 4 institutions: Portugal (1), Turkey (3) | 405 from 405 pts ≤21 y | 153 surgical | 10.9% 44/105 30% 3/10 * | 50.4% 204/405 15.2% 5/33 * | 9.9% 40/405 22.2% 4/18 * | 8.9% 36/105 44.4% 12/27 * | 5.9% 24/105 72.7% 16/22 * | 14.1% 57/405 86.0% 37/43 * |
| [76] | 2019–2021 Children’s Hospital of Philadelphia (USA) | 151 ≤19 y | 2.6% 4/151 | 25.8% 39/151 | 23.2% 35/151 | 9.3% 14/151 | 4.0% 6/151 | 35.1% 53/151 | |
| [77] | 1/2003–12/2019 Vanderbilt University Medical Center (USA) | 44 ≤21 y | 44 surgical | 0% 0/44 N/A * | 27.3% 12/44 33.3% 4/12 * | 15.9% 7/44 42.9% 3/7 * | 9.1% 4/44 25% 1/4 | 9.1% 4/44 100% 4/4 * 100% 4/4 † | 38.6% 17/44 100% 17/17 * 100% 17/17 † |
| [78] | 1/2010–10/2020 Children’s Hospital of Los Angeles (USA) | 112 ≤18 y | 112 surgical | 4.5% 5/112 20% 1/5 * | 9.8% 11/112 0% 0/11 * | 26.8% 30/112 16.7% 5/30 * | 11.6% 13/112 30.8% 4/13 * | 15.2% 17/112 94.1% 16/17 * | 32.1% 36/112 100% 36/36 * 100% 36/36 † |
| [79] | 1/2017–5/2021 University of Alabama at Birmingham (USA) | 49 ≤19 y | 44 surgical + 5 clinical | 4.1% 2/49 0% 0/2 * | 51.0% 25/49 4% 1/25 * | 14.3% 7/49 57.1% 4/7 * | 8.2% 4/49 50% 2/4 * | 6.1% 3/49 100% 3/3 * 100% 3/3 † | 16.3% 8/49 100% 8/8 * 100% 8/8 † |
| 7/2015–5/2022 Phoenix Children’s Hospital (USA)(current study) | 208 ≤18 y | 74 surgical | 7.7% 16/208 40% 2/5 * 40% 2/5 † | 56.7% 118/208 4.8% 1/21 * 19.0% 4/21 † | 21.6% 45/208 27.3% 6/22 * 59.1% 13/22 † | 2.4% 5/208 100% 5/5 * 100% 5/5 † | 1.4% 3/208 100% 2/2 * 100% 2/2 † | 10.1% 21/208 94.7% 18/19 * 94.7% 18/19 † | |
| Total | 5911 | 2486 surgical + 62 clinical | Freq. 11.4% 676/5911 ROM 16.8% 27/161 * RON 26.7% 23/86 † | Freq. 56.0% 3308/5911 ROM 7.2% 61/851 * RON 27.5% 111/403 † | Freq. 9.6% 567/5911 ROM 29.6% 112/379 * RON 55.8% 91/163 † | Freq. 6.4% 377/5911 ROM 42.3% 130/307 * RON 86.8% 131/151 † | Freq. 3.9% 230/5911 ROM 90.8% 178/196 RON 97.6% 122/125 † | Freq. 12.7% 752/5911 ROM 98.8% 652/660 * RON 99.7% 611/613 † |
| Level/Category/Score | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| TI-RADS 2 | 47.3 | 97.0 | 7.3 | 45.7 | 75.0 |
| TI-RADS 3 | 55.4 | 97.0 | 22.0 | 50.0 | 90.0 |
| TI-RADS 4 | 56.8 | 72.7 | 43.9 | 51.1 | 66.7 |
| TI-RADS 5 | 64.9 | 36.4 | 87.8 | 70.6 | 63.2 |
| Bethesda II | 60.8 | 95.5 | 10.0 | 60.9 | 60.0 |
| Bethesda III | 78.4 | 86.4 | 66.7 | 79.2 | 76.9 |
| Bethesda IV | 73.0 | 56.8 | 96.7 | 95.2 | 60.4 |
| Bethesda V | 66.2 | 45.5 | 96.7 | 95.2 | 54.7 |
| Bethesda VI | 63.5 | 40.9 | 96.7 | 94.7 | 52.7 |
| Combined 4 | 47.8 | 100.0 | 5.3 | 46.3 | 100.0 |
| Combined 5 | 55.1 | 100.0 | 18.4 | 50.0 | 100.0 |
| Combined 6 | 59.4 | 96.8 | 29.0 | 52.6 | 91.7 |
| Combined 7 | 73.9 | 87.1 | 63.2 | 65.9 | 85.7 |
| Combined 8 | 87.0 | 80.7 | 92.1 | 89.3 | 85.4 |
| Combined 9 | 82.6 | 64.5 | 97.4 | 95.2 | 77.1 |
| Combined 10 | 75.4 | 48.4 | 97.4 | 93.8 | 69.8 |
| Combined 11 | 68.1 | 29.0 | 100.0 | 100.0 | 63.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hess, J.R.; Van Tassel, D.C.; Runyan, C.E.; Morrison, Z.; Walsh, A.M.; Schafernak, K.T. Performance of ACR TI-RADS and the Bethesda System in Predicting Risk of Malignancy in Thyroid Nodules at a Large Children’s Hospital and a Comprehensive Review of the Pediatric Literature. Cancers 2023, 15, 3975. https://doi.org/10.3390/cancers15153975
Hess JR, Van Tassel DC, Runyan CE, Morrison Z, Walsh AM, Schafernak KT. Performance of ACR TI-RADS and the Bethesda System in Predicting Risk of Malignancy in Thyroid Nodules at a Large Children’s Hospital and a Comprehensive Review of the Pediatric Literature. Cancers. 2023; 15(15):3975. https://doi.org/10.3390/cancers15153975
Chicago/Turabian StyleHess, Jennifer R., Dane C. Van Tassel, Charles E. Runyan, Zachary Morrison, Alexandra M. Walsh, and Kristian T. Schafernak. 2023. "Performance of ACR TI-RADS and the Bethesda System in Predicting Risk of Malignancy in Thyroid Nodules at a Large Children’s Hospital and a Comprehensive Review of the Pediatric Literature" Cancers 15, no. 15: 3975. https://doi.org/10.3390/cancers15153975
APA StyleHess, J. R., Van Tassel, D. C., Runyan, C. E., Morrison, Z., Walsh, A. M., & Schafernak, K. T. (2023). Performance of ACR TI-RADS and the Bethesda System in Predicting Risk of Malignancy in Thyroid Nodules at a Large Children’s Hospital and a Comprehensive Review of the Pediatric Literature. Cancers, 15(15), 3975. https://doi.org/10.3390/cancers15153975







