Early Increase in Circulating PD-1+CD8+ T Cells Predicts Favorable Survival in Patients with Advanced Gastric Cancer Receiving Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Fluorescence-Activated Cell Soring Analysis
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Correlation between T Cells and Inflammatory Markers
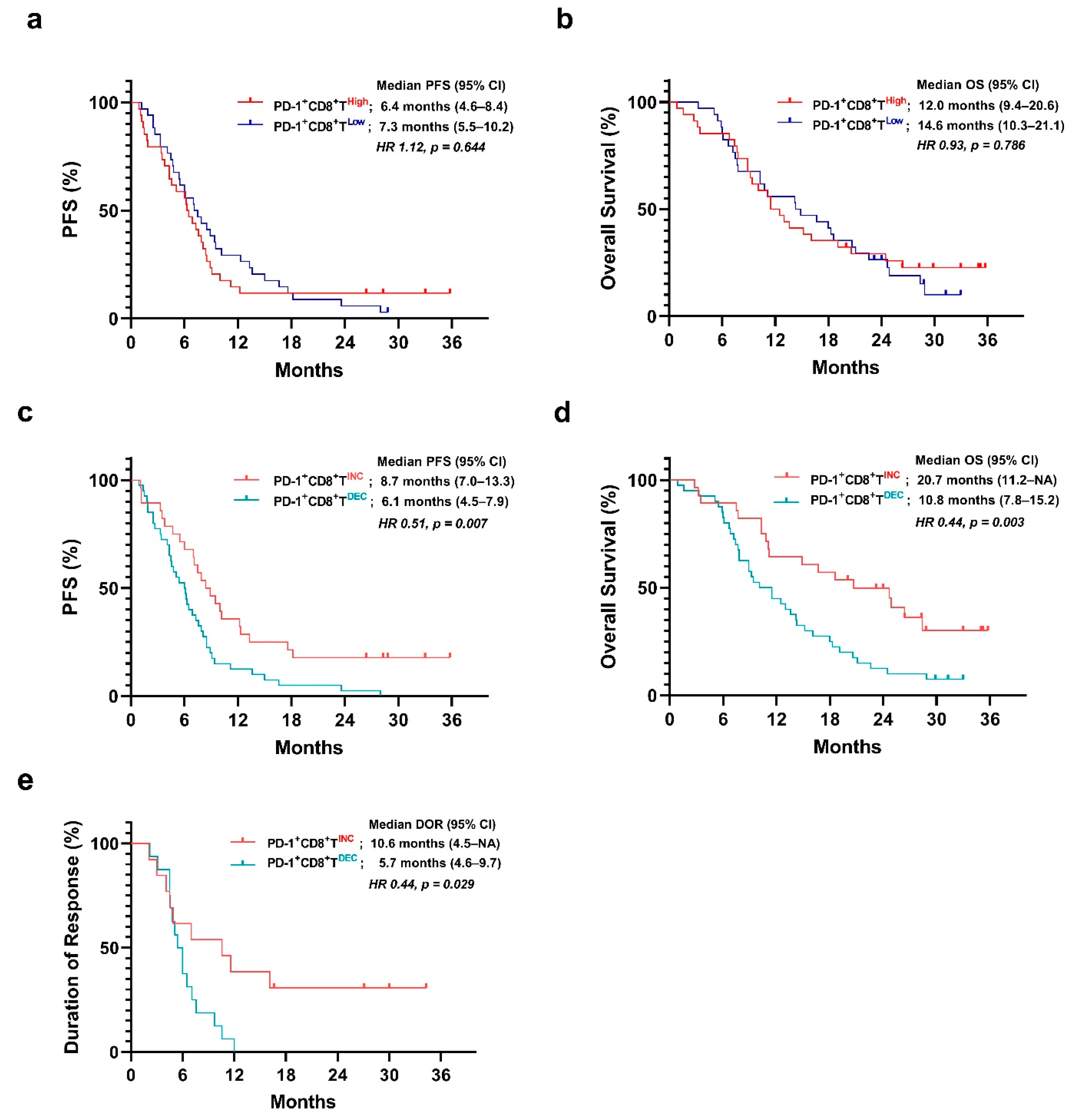
3.3. Baseline Frequency of PD-1+CD8+ T Cells Is Not Associated with Survival
3.4. Prognostic Value of the Early Change in the Frequency of PD-1+CD8+ T Cells
3.5. Change in CD69+CD8+ T Cells and Ki-67+CD8+ T Cells in Both Groups
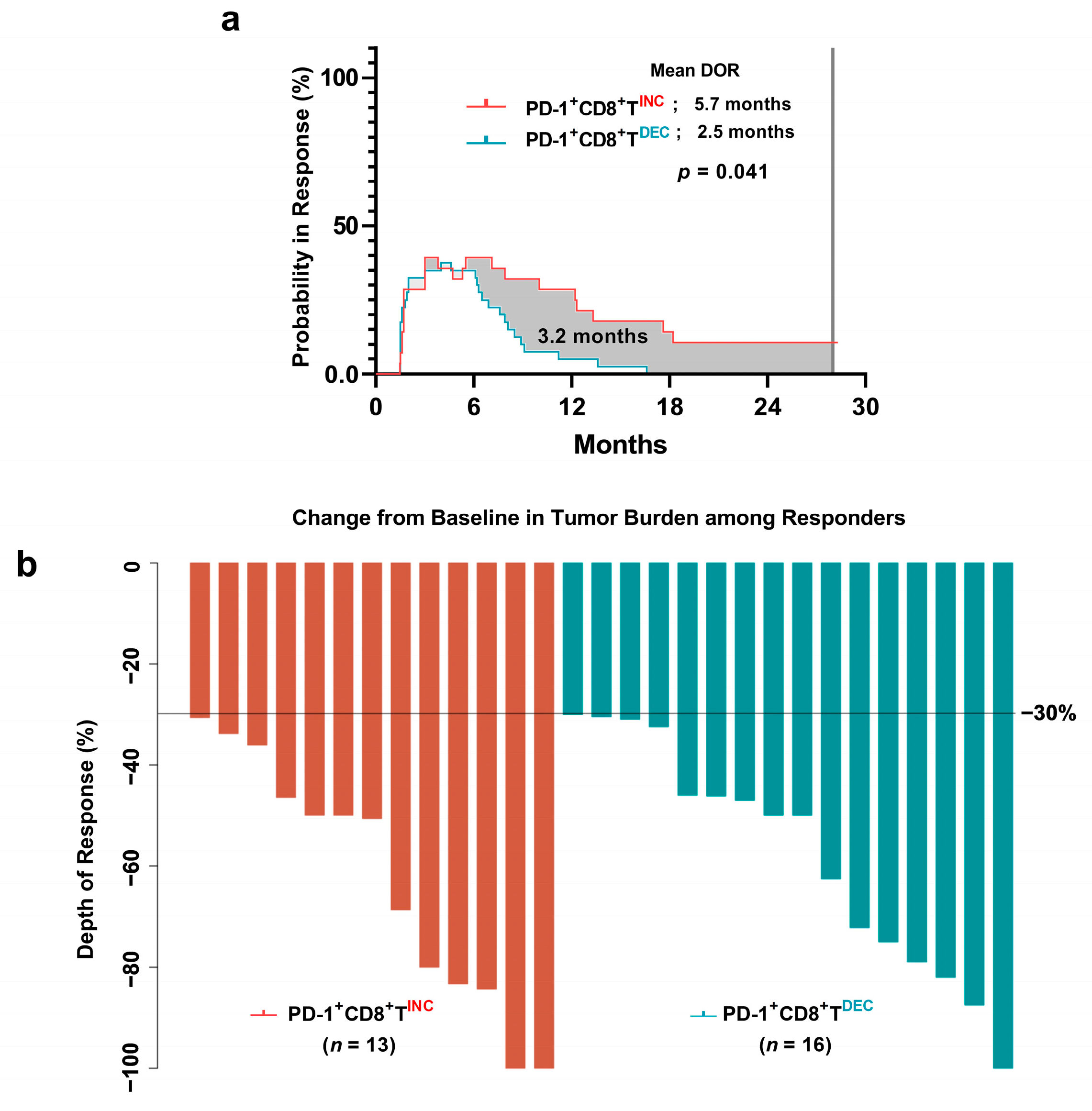
3.6. Duration of Response in PD-1+CD8+ T-Cell Groups
3.7. Difference in Depth of Response between the PD-1+CD8+ T-Cell Groups
3.8. Univariate and Multivariate Survival Analyses
3.9. Survival Analyses Stratified by Number of Metastatic Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Philip, M.; Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef]
- Yu, K.; Gu, Y.; Zhang, P.; Fang, H.; Cao, Y.; Wang, J.; Lin, C.; Liu, H.; Zhang, H.; He, H.; et al. Intratumoral PD-1(+)CD8(+) T cells associate poor clinical outcomes and adjuvant chemotherapeutic benefit in gastric cancer. Br. J. Cancer 2022, 127, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zhou, L.; Shen, H.; Shi, C.; Jia, S.; Ding, G.P.; Cao, L. Prognostic value of programmed cell death protein 1 expression on CD8+ T lymphocytes in pancreatic cancer. Sci. Rep. 2017, 7, 7848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Egelston, C.A.; Guo, W.; Tan, J.; Avalos, C.; Simons, D.L.; Lim, M.H.; Huang, Y.J.; Nelson, M.S.; Chowdhury, A.; Schmolze, D.B.; et al. Tumor-infiltrating exhausted CD8+ T cells dictate reduced survival in premenopausal estrogen receptor-positive breast cancer. JCI Insight 2022, 7, e153963. [Google Scholar] [CrossRef]
- Shen, Y.; Teng, Y.; Lv, Y.; Zhao, Y.; Qiu, Y.; Chen, W.; Wang, L.; Wang, Y.; Mao, F.; Cheng, P.; et al. PD-1 does not mark tumor-infiltrating CD8+ T cell dysfunction in human gastric cancer. J. Immunother. Cancer 2020, 8, e000422. [Google Scholar] [CrossRef]
- Egelston, C.A.; Avalos, C.; Tu, T.Y.; Simons, D.L.; Jimenez, G.; Jung, J.Y.; Melstrom, L.; Margolin, K.; Yim, J.H.; Kruper, L.; et al. Human breast tumor-infiltrating CD8(+) T cells retain polyfunctionality despite PD-1 expression. Nat. Commun. 2018, 9, 4297. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, P.; Ma, Y.; Yin, Z.; Xu, H.; Xiang, L.; Zhang, W.; Li, S.; Liang, X. Peripheral Polyfunctional PD1(+) CD8(+) T cells demonstrated strong immune protection in non-small cell lung cancer. Eur. J. Immunol. 2022, 52, 1369–1371. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Diana, A.; Wang, L.M.; D’Costa, Z.; Allen, P.; Azad, A.; Silva, M.A.; Soonawalla, Z.; Liu, S.; McKenna, W.G.; Muschel, R.J.; et al. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 40992–41004. [Google Scholar] [CrossRef] [Green Version]
- Yeong, J.; Lim, J.C.T.; Lee, B.; Li, H.; Ong, C.C.H.; Thike, A.A.; Yeap, W.H.; Yang, Y.; Lim, A.Y.H.; Tay, T.K.Y.; et al. Prognostic value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J. Immunother. Cancer 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Choo, J.; Kua, L.F.; Soe, M.Y.; Asuncion, B.R.; Tan, B.K.J.; Teo, C.B.; Tay, R.Y.K.; So, J.; Shabbir, A.; Guowei, K.; et al. Clinical relevance of PD-1 positive CD8 T-cells in gastric cancer. Gastric Cancer 2023, 26, 393–404. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Fishman, M.N.; Escudier, B.; McDermott, D.F.; Drake, C.G.; Kluger, H.; Stadler, W.M.; Perez-Gracia, J.L.; McNeel, D.G.; Curti, B.; et al. Immunomodulatory Activity of Nivolumab in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2016, 22, 5461–5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairfax, B.P.; Taylor, C.A.; Watson, R.A.; Nassiri, I.; Danielli, S.; Fang, H.; Mahe, E.A.; Cooper, R.; Woodcock, V.; Traill, Z.; et al. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat. Med. 2020, 26, 193–199. [Google Scholar] [CrossRef]
- Kwon, M.; An, M.; Klempner, S.J.; Lee, H.; Kim, K.M.; Sa, J.K.; Cho, H.J.; Hong, J.Y.; Lee, T.; Min, Y.W.; et al. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov. 2021, 11, 2168–2185. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Xie, F.; Luo, H.; Liu, Z.; Lv, W.; Shi, D.; Yu, D.; Gao, P.; Chen, C.; et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat. Commun. 2023, 14, 8. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, J.; Ku, B.M.; Koh, J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Cheon, J.; Min, Y.J.; Park, S.H.; et al. The First-week Proliferative Response of Peripheral Blood PD-1(+)CD8(+) T Cells Predicts the Response to Anti-PD-1 Therapy in Solid Tumors. Clin. Cancer Res. 2019, 25, 2144–2154. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Salas-Benito, D.; Perez-Gracia, J.L.; Ponz-Sarvise, M.; Rodriguez-Ruiz, M.E.; Martinez-Forero, I.; Castanon, E.; Lopez-Picazo, J.M.; Sanmamed, M.F.; Melero, I. Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov. 2021, 11, 1353–1367. [Google Scholar] [CrossRef]
- Fumet, J.D.; Limagne, E.; Thibaudin, M.; Ghiringhelli, F. Immunogenic Cell Death and Elimination of Immunosuppressive Cells: A Double-Edged Sword of Chemotherapy. Cancers 2020, 12, 2637. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.J.; Lake, R.A.; van der Most, R.G.; Dick, I.M.; Nowak, A.K. Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. Br. J. Cancer 2012, 107, 1107–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Sakakura, K.; Mito, I.; Ida, S.; Chikamatsu, K. Dynamic changes in immune cell profile in head and neck squamous cell carcinoma: Immunomodulatory effects of chemotherapy. Cancer Sci. 2016, 107, 1065–1071. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Li, G.; Ji, X.; Ma, L.; Wang, X.; Li, Y.; Fan, C. Impact of the immune cell population in peripheral blood on response and survival in patients receiving neoadjuvant chemotherapy for advanced gastric cancer. Tumor Biol. 2017, 39, 1010428317697571. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Shi, J.; Jia, Y.; Dou, Y.; Li, Z.; Dong, B.; Guo, T.; Cheng, X.; Li, X.; Du, H.; et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in gastric cancer as determined by multiplex immunofluorescence and T cell receptor repertoire analysis. J. Immunother. Cancer 2022, 10, e003984. [Google Scholar] [CrossRef]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Veiga-Fernandes, H.; Walter, U.; Bourgeois, C.; McLean, A.; Rocha, B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 2000, 1, 47–53. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.; Kim, J.; Park, S.J.; Lee, M.A.; Park, J.M.; Choi, M.G.; Kang, D.; Song, K.Y.; Lee, H.H.; Seo, H.S.; et al. Prognostic value of soluble PD-L1 and exosomal PD-L1 in advanced gastric cancer patients receiving systemic chemotherapy. Sci. Rep. 2023, 13, 6952. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lonardi, S.; Masi, G.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Spadi, R.; Zaniboni, A.; et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: Results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann. Oncol. 2015, 26, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Latouche, A.; Vaflard, P.; Ricci, F.; Loirat, D.; Hescot, S.; Sablin, M.P.; Rouzier, R.; Kamal, M.; Morel, C.; et al. Comparative Analysis of Durable Responses on Immune Checkpoint Inhibitors Versus Other Systemic Therapies: A Pooled Analysis of Phase III Trials. JCO Precis. Oncol. 2019, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tian, L.; McCaw, Z.R.; Luo, X.; Talukder, E.; Rothenberg, M.; Xie, W.; Choueiri, T.K.; Kim, D.H.; Wei, L.J. Analysis of Response Data for Assessing Treatment Effects in Comparative Clinical Studies. Ann. Intern. Med. 2020, 173, 368–374. [Google Scholar] [CrossRef]
- Huang, B.; Tian, L.; Talukder, E.; Rothenberg, M.; Kim, D.H.; Wei, L.J. Evaluating Treatment Effect Based on Duration of Response for a Comparative Oncology Study. JAMA Oncol. 2018, 4, 874–876. [Google Scholar] [CrossRef]
- Park, Y.; Seo, A.N.; Koh, J.; Nam, S.K.; Kwak, Y.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Lee, H.S. Expression of the immune checkpoint receptors PD-1, LAG3, and TIM3 in the immune context of stage II and III gastric cancer by using single and chromogenic multiplex immunohistochemistry. Oncoimmunology 2021, 10, 1954761. [Google Scholar] [CrossRef]
- Saito, H.; Shimizu, S.; Kono, Y.; Murakami, Y.; Shishido, Y.; Miyatani, K.; Matsunaga, T.; Fukumoto, Y.; Ashida, K.; Fujiwara, Y. PD-1 Expression on Circulating CD8(+) T-Cells as a Prognostic Marker for Patients with Gastric Cancer. Anticancer Res. 2019, 39, 443–448. [Google Scholar] [CrossRef]
- Yong, W.P.; Rha, S.Y.; Tan, I.B.; Choo, S.P.; Syn, N.L.; Koh, V.; Tan, S.H.; Asuncion, B.R.; Sundar, R.; So, J.B.; et al. Real-Time Tumor Gene Expression Profiling to Direct Gastric Cancer Chemotherapy: Proof-of-Concept “3G” Trial. Clin. Cancer Res. 2018, 24, 5272–5281. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Wang, M.; Wu, C.; Zhou, H.; Chen, C.; Diede, S. Comparison of Duration of Response vs Conventional Response Rates and Progression-Free Survival as Efficacy End Points in Simulated Immuno-oncology Clinical Trials. JAMA Netw. Open 2021, 4, e218175. [Google Scholar] [CrossRef]
- Duan, R.; Li, X.; Zeng, D.; Chen, X.; Shen, B.; Zhu, D.; Zhu, L.; Yu, Y.; Wang, D. Tumor Microenvironment Status Predicts the Efficacy of Postoperative Chemotherapy or Radiochemotherapy in Resected Gastric Cancer. Front. Immunol. 2020, 11, 609337. [Google Scholar] [CrossRef] [PubMed]

| Increased PD-1+CD8+ T-Cell Group (n = 28) | Decreased PD-1+CD8+ T-Cell Group (n = 40) | ||||
|---|---|---|---|---|---|
| % | % | p Value * | |||
| Age (years) | 0.234 | ||||
| <65 | 12 | (42.9%) | 23 | (57.5%) | |
| ≥65 | 16 | (57.1%) | 17 | (42.5%) | |
| Sex | 0.743 | ||||
| Male | 20 | (71.4%) | 30 | (75.0%) | |
| Female | 8 | (28.6%) | 10 | (25.0%) | |
| ECOG PS | 0.298 | ||||
| 0–1 | 25 | (89.3%) | 39 | (97.5%) | |
| 2 | 3 | (10.7%) | 1 | (2.5%) | |
| Differentiation | 0.074 | ||||
| Well to moderate | 12 | (42.9%) | 9 | (22.5%) | |
| Poor | 16 | (57.1%) | 31 | (77.5%) | |
| HER2 | 0.999 | ||||
| Positive | 2 | (7.1%) | 4 | (10.0%) | |
| Negative | 26 | (92.9%) | 36 | (90.0%) | |
| Disease status | 0.708 | ||||
| Locally advanced | 4 | (14.3%) | 4 | (10.0%) | |
| Metastatic | 24 | (85.7%) | 36 | (90.0%) | |
| Peritoneal seeding | 0.684 | ||||
| Yes | 14 | (50.0%) | 22 | (55.0%) | |
| No | 14 | (50.0%) | 18 | (45.0%) | |
| No. of metastatic sites | 0.071 | ||||
| ≥2 | 6 | (21.4%) | 17 | (42.5%) | |
| <2 | 22 | (78.6%) | 23 | (57.5%) | |
| CEA (ng/mL) | 0.034 | ||||
| >5 | 14 | (50.0%) | 10 | (25.0%) | |
| ≤5 | 14 | (50.0%) | 30 | (75.0%) | |
| CA 19-9 (U/mL) | 0.444 | ||||
| >37 | 8 | (28.6%) | 15 | (37.5%) | |
| ≤37 | 20 | (71.4%) | 25 | (62.5%) | |
| Tissue PD-L1 | 0.182 | ||||
| CPS ≥ 10 | 11 | (39.3%) | 19 | (47.5%) | |
| CPS < 10 | 13 | (46.4%) | 20 | (50.0%) | |
| Undetermined | 4 | (14.3%) | 1 | (2.5%) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | (95%CI) | p Value | HR | (95%CI) | p Value | |
| Overall Survival | ||||||
| Age (≥65) | 1.25 | 0.73–2.12 | 0.419 | |||
| Sex (male) | 1.11 | 0.60–2.03 | 0.746 | |||
| ECOG PS (2) | 1.26 | 0.75–2.11 | 0.376 | |||
| Differentiation (poorly) | 1.23 | 0.92–1.65 | 0.157 | |||
| HER2 (positive) | 1.77 | 0.69–4.51 | 0.232 | |||
| Disease status (metastatic) | 1.37 | 0.73–2.55 | 0.328 | |||
| No. of metastatic sites (≥2) | 3.21 | 1.83–5.63 | <0.001 | 2.37 | 1.26–4.44 | 0.007 |
| CEA (>5 ng/mL) | 0.87 | 0.49–1.53 | 0.622 | |||
| CA 19-9 (>37 U/mL) | 1.32 | 0.76–2.31 | 0.320 | |||
| NLR (≥ median) | 2.12 | 1.23–3.64 | 0.006 | 2.37 | 0.81–2.70 | 0.206 |
| PLR (≥ median) | 1.31 | 0.77–2.22 | 0.326 | |||
| Increase in PD-1+CD8+ T-cell frequency | 0.43 | 0.24–0.77 | 0.004 | 0.51 | 0.29–0.92 | 0.025 |
| Progression-free Survival | ||||||
| Age (≥65) | 1.15 | 0.70–1.89 | 0.576 | |||
| Sex (male) | 1.08 | 0.61–1.89 | 0.789 | |||
| ECOG PS (2) | 1.06 | 0.64–1.77 | 0.824 | |||
| Differentiation (poorly) | 1.13 | 0.87–1.49 | 0.359 | |||
| HER2 (positive) | 1.66 | 0.71–3.92 | 0.244 | |||
| Disease status (metastatic) | 1.53 | 0.84–2.75 | 0.161 | |||
| No. of metastatic sites (≥2) | 3.33 | 1.89–5.88 | <0.001 | 2.55 | 1.34–4.86 | 0.004 |
| CEA (>5 ng/mL) | 1.15 | 0.68–1.94 | 0.597 | |||
| CA 19-9 (>37 U/mL) | 1.63 | 0.97–2.75 | 0.065 | 1.16 | 0.66–2.02 | 0.609 |
| NLR (≥median) | 2.28 | 1.36–3.84 | 0.002 | 1.67 | 0.93–2.98 | 0.085 |
| PLR (≥median) | 1.3 | 0.79–2.14 | 0.305 | |||
| Increase in PD-1+CD8+ T-cell frequency | 0.49 | 0.29–0.83 | 0.008 | 0.57 | 0.33–0.98 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.; Kim, J.; Park, S.J.; Kim, H.; Lee, M.A.; Kim, O.; Park, J.; Kang, N.; Kim, I.-H. Early Increase in Circulating PD-1+CD8+ T Cells Predicts Favorable Survival in Patients with Advanced Gastric Cancer Receiving Chemotherapy. Cancers 2023, 15, 3955. https://doi.org/10.3390/cancers15153955
Shin K, Kim J, Park SJ, Kim H, Lee MA, Kim O, Park J, Kang N, Kim I-H. Early Increase in Circulating PD-1+CD8+ T Cells Predicts Favorable Survival in Patients with Advanced Gastric Cancer Receiving Chemotherapy. Cancers. 2023; 15(15):3955. https://doi.org/10.3390/cancers15153955
Chicago/Turabian StyleShin, Kabsoo, Joori Kim, Se Jun Park, Hyunho Kim, Myung Ah Lee, Okran Kim, Juyeon Park, Nahyeon Kang, and In-Ho Kim. 2023. "Early Increase in Circulating PD-1+CD8+ T Cells Predicts Favorable Survival in Patients with Advanced Gastric Cancer Receiving Chemotherapy" Cancers 15, no. 15: 3955. https://doi.org/10.3390/cancers15153955
APA StyleShin, K., Kim, J., Park, S. J., Kim, H., Lee, M. A., Kim, O., Park, J., Kang, N., & Kim, I.-H. (2023). Early Increase in Circulating PD-1+CD8+ T Cells Predicts Favorable Survival in Patients with Advanced Gastric Cancer Receiving Chemotherapy. Cancers, 15(15), 3955. https://doi.org/10.3390/cancers15153955






