Vaccination against Extracellular Vimentin for Treatment of Urothelial Cancer of the Bladder in Client-Owned Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
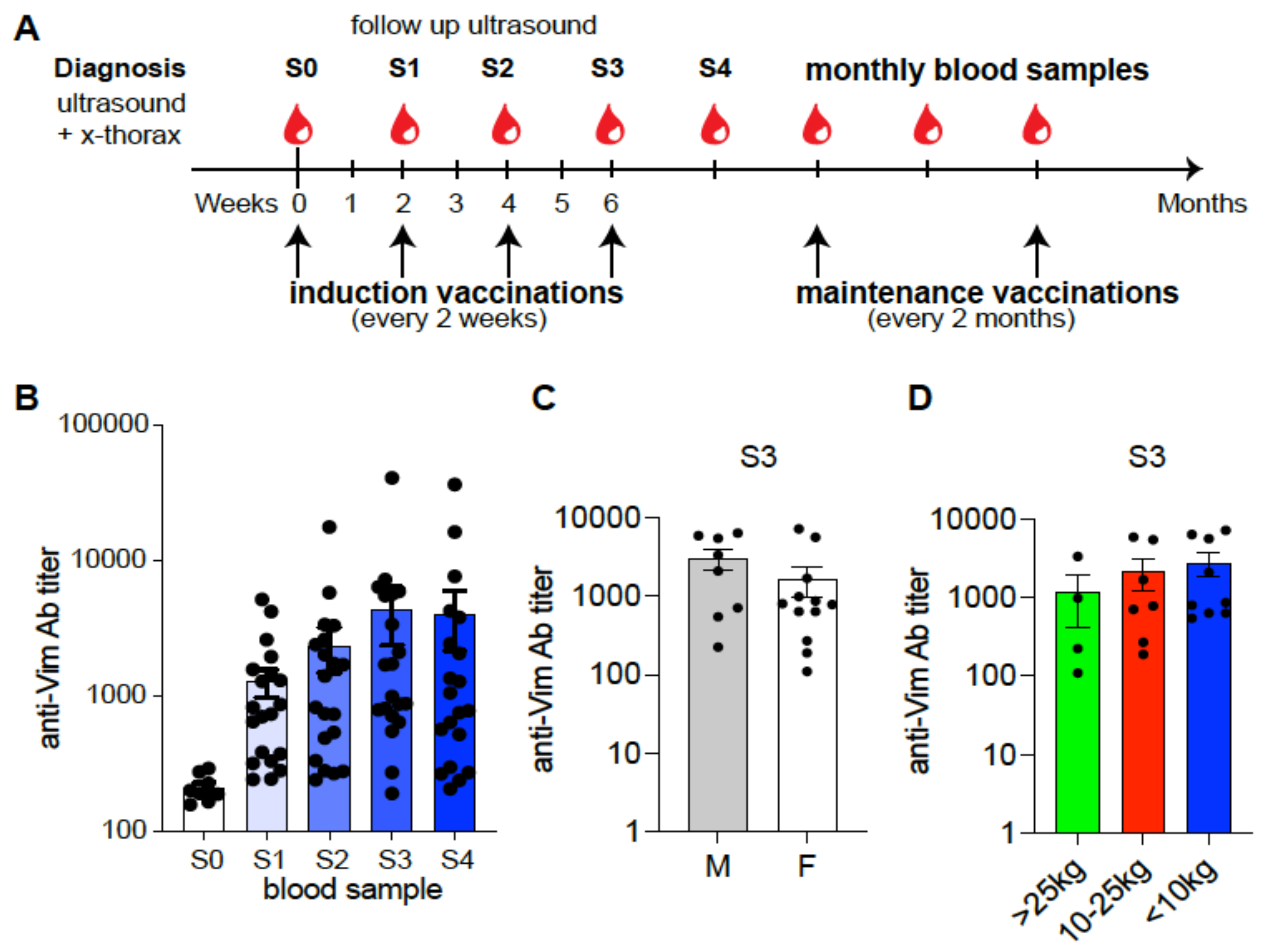
2.1. Study Design and Participants
Study Design
2.2. Immune Response Measurement
2.2.1. Anti-eVim Antibody ELISA
2.2.2. Immunofluorescence
2.3. Patient Monitoring and Evaluation
2.4. Statistical Analysis
3. Results
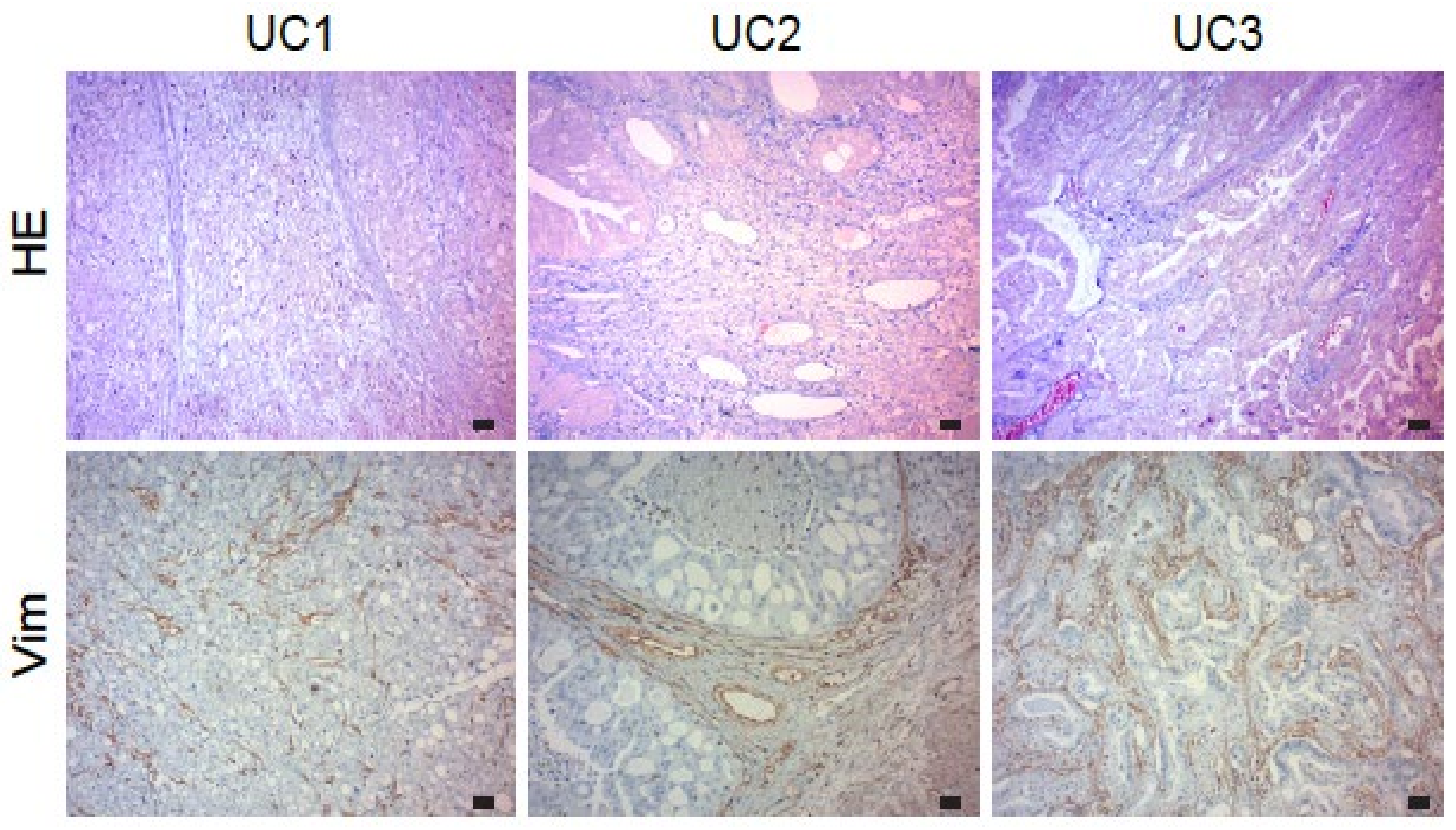
3.1. Expression of Vimentin in Urothelial Carcinoma
3.2. Study Population
3.3. Anti-eVim Antibody Response
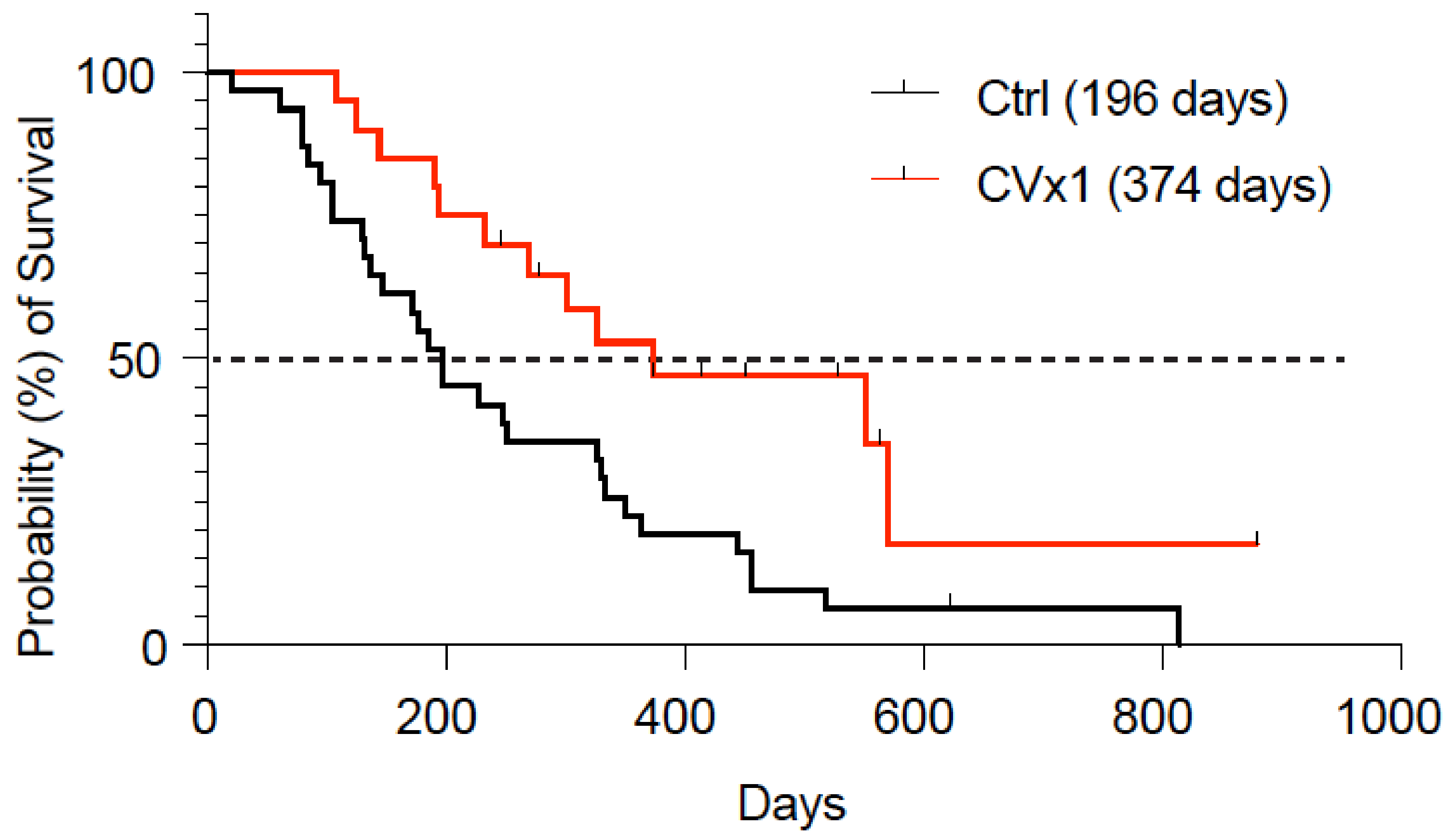
3.4. Clinical Response Data
3.5. Adverse Events
3.6. Reactivity of CVx1 Sera with Mouse and Human Vimentin
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Sievert, K.D.; Amend, B.; Nagele, U.; Schilling, D.; Bedke, J.; Horstmann, M.; Henenlotter, J.; Kruck, S.; Stenzl, A. Economic aspects of bladder cancer: What are the benefits and costs? World J. Urol. 2009, 27, 295–300. [Google Scholar] [CrossRef]
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef]
- Svatek, R.S.; Hollenbeck, B.K.; Holmäng, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Prim. 2017, 3, 17022. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Basouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Mukherjee, N.; Svatek, R.S.; Mansour, A.M. Role of immunotherapy in bacillus Calmette–Guérin-unresponsive non–muscle invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 103–108. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Roviello, G.; Catalano, M.; Santi, R.; Palmieri, V.E.; Vannini, G.; Galli, I.C.; Buttitta, E.; Villari, D.; Rossi, V.; Nesi, G. Immune checkpoint inhibitors in urothelial bladder cancer: State of the art and future perspectives. Cancers 2021, 13, 4411. [Google Scholar] [CrossRef]
- Bergman, P.J.; Camps-Palau, M.A.; McKnight, J.A.; Leibman, N.F.; Craft, D.M.; Leung, C.; Liao, J.; Riviere, I.; Sadelain, M.; Hohenhaus, A.E.; et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 2006, 24, 4582–4585. [Google Scholar] [CrossRef]
- Bow, S.; Guth, A. Cancer Immunotherapy. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Thamm, D.H., Liptak., J.M., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 231–250. [Google Scholar]
- Van Beijnum, J.R.; Huijbers, E.J.M.; van Loon, K.; Blanas, A.; Akbari, P.; Roos, A.; Wong, T.J.; Denisov, S.S.; Hackeng, T.M.; Jimenez, C.R.; et al. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat. Commun. 2022, 13, 2842. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Dings, R.P.; van der Linden, E.; Zwaans, B.M.M.; Ramaekers, F.C.S.; Mayo, K.H.; Griffioen, A.W. Gene expression of tumor angiogenesis dissected: Specific targeting of colon cancer angiogenic vasculature. Blood 2006, 108, 2339–2348. [Google Scholar] [CrossRef]
- Huijbers, E.J.M.; van Beijnum, J.R.; Lê, C.T.; Langman, S.; Nowak-Sliwinska, P.; Mayo, K.H.; Griffioen, A.W. An improved conjugate vaccine technology; induction of antibody responses to the tumor vasculature. Vaccine 2018, 36, 3054–3060. [Google Scholar] [CrossRef]
- Van Loon, K.; Huijbers, E.J.M.; de Haan, J.D.; Griffioen, A.W. Cancer Vaccination against Extracellular Vimentin Efficiently Adjuvanted with Montanide ISA 720/CpG. Cancers 2022, 14, 2593. [Google Scholar] [CrossRef]
- Patrick, D.J.; Fitzgerald, S.D.; Sesterhenn, I.A.; Davis, C.J.; Kiupel, M. Classification of Canine Urinary Bladder Urothelial Tumours Based on the World Health Organization/International Society of Urological Pathology Consensus Classification. J. Comp. Pathol. 2006, 135, 190–199. [Google Scholar] [CrossRef]
- Knapp, D.W.; Dhawan, D.; Ramos-Vara, J.A.; Ratliff, T.L.; Cresswell, G.M.; Utturkar, S.; Sommer, B.C.; Fulkerson, C.M.; Hahn, N.M. Naturally-Occurring Invasive Urothelial Carcinoma in Dogs, a Unique Model to Drive Advances in Managing Muscle Invasive Bladder Cancer in Humans. Front. Oncol. 2019, 9, 1493. [Google Scholar] [CrossRef]
- Dow, S. A Role for Dogs in Advancing Cancer Immunotherapy Research. Front. Immunol. 2020, 10, 2935. [Google Scholar] [CrossRef]
- Boria, P.A.; Glickman, N.W.; Schmidt, B.R.; Widmer, W.R.; Mutsaers, A.J.; Adams, L.G.; Snyder, P.W.; DiBernardi, L.; De Gortari, A.E.; Bonney, P.L.; et al. Carboplatin and piroxicam therapy in 31 dogs with transitional cell carcinoma of the urinary bladder. Vet. Comp. Oncol. 2005, 3, 73–80. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef]
- Honkisz, S.I.; Naughton, J.F.; Weng, H.Y.; Fourez, L.M.; Knapp, D.W. Evaluation of two-dimensional ultrasonography and computed tomography in the mapping and measuring of canine urinary bladder tumors. Vet. J. 2018, 232, 23–26. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Babiker, A.Y.; Alwanian, W.M.; Elsiddig, S.A.; Faragalla, H.E.; Aly, S.M. Association of cytokeratin and vimentin protein in the genesis of transitional cell carcinoma of urinary bladder patients. Dis. Markers 2015, 2015, 204759. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ruple-Czerniak, A.; Ramos-Vara, J.A.; Naughton, J.F.; Fulkerson, C.M.; Honkisz, S.I. A nonselective cyclooxygenase inhibitor enhances the activity of vinblastine in a naturally-occurring canine model of invasive urothelial carcinoma. Bladder Cancer 2016, 2, 241–250. [Google Scholar] [CrossRef]
- Knapp, D.W.; Glickman, N.W.; Widmer, W.R.; DeNicola, D.B.; Adams, L.G.; Kuczek, T.; Bonney, P.L.; DeGortari, A.E.; Han, C.; Glickman, L.T. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother. Pharmacol. 2000, 46, 221–226. [Google Scholar] [CrossRef]
- Allstadt, S.D.; Rodriguez, C.O.; Boostrom, B.; Rebhun, R.B.; Skorupski, K.A. Randomized Phase III Trial of Piroxicam in Combination with Mitoxantrone or Carboplatin for First-Line Treatment of Urogenital Tract Transitional Cell Carcinoma in Dogs. J. Vet. Intern. Med. 2015, 29, 261–267. [Google Scholar] [CrossRef]
- Knapp, D.W.; Richardson, R.C.; Chan, T.C.; Bottoms, G.D.; Widmer, W.R.; DeNicola, D.B.; Teclaw, R.; Bonney, P.L.; Kuczek, T. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J. Vet. Intern. Med. 1994, 8, 273–278. [Google Scholar] [CrossRef]
- Henry, C.J.; McCaw, D.L.; Turnquist, S.E.; Tyler, J.W.; Bravo, L.; Sheafor, S.; Straw, R.C.; Dernell, W.S.; Madewell, B.R.; Jorgensen, L.; et al. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin. Cancer Res. 2003, 9, 906–911. [Google Scholar]
- Robat, C.; Burton, J.; Thamm, D.; Vail, D. Retrospective evaluation of doxorubicin-piroxicam combination for the treatment of transitional cell carcinoma in dogs. J. Small Anim. Pract. 2013, 54, 67–74. [Google Scholar] [CrossRef]
- Marconato, L.; Zini, E.; Lindner, D.; Suslak-Brown, L.; Nelson, V.; Jeglum, A.K. Toxic effects and antitumor response of gemcitabine in combination with piroxicam treatment in dogs with transitional cell carcinoma of the urinary bladder. J. Am. Vet. Med. Assoc. 2011, 238, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.R.; Rêma, A.; Mesquita, J.R.; Taulescu, M.; Seixas, F.; Gärtner, F.; Amorim, I. Vimentin and Ki-67 immunolabeling in canine gastric carcinomas and their prognostic value. Vet. Pathol. 2022, 59, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, E.J.M.; Ringvall, M.; Femel, J.; Kalamajski, S.; Lukinius, A.; Åbrink, M.; Hellamn, L.; Olsson, A.K. Vaccination against the extra domain-B of fibronectin as a novel tumor therapy. FASEB J. 2010, 24, 4535–4544. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Damen, C.A.; Martinotti, S.; Blijham, G.H.; Groenewegen, G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: The role of angiogenic factors. Cancer Res. 1996, 56, 1111–1117. [Google Scholar]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; Griffioen, C.J.; Huinen, Z.R.; Sopesens, N.G.; Schulz, R.; Jenkins, S.V.; Dings, R.P.M.; Groenendijk, F.H.; Huijbers, E.J.M.; et al. Proinflammatory activity of VEGF-targeted treatment through reversal of tumor endothelial cell anergy. Angiogenesis 2022, 26, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, E.J.M.; Khan, K.A.; Kerbel, R.S.; Griffioen, A.W. Tumors resurrect an embryonic vascular program to escape immunity. Sci. Immunol. 2022, 7, eabm6388. [Google Scholar] [CrossRef]
- Fulkerson, C.E.; Knapp, D.W. Tumors of the urinary system. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 645–656. [Google Scholar]
- Szigetvari, N.M.; Dhawan, D.; Ramos-Vara, J.A.; Leamon, C.P.; Klein, P.J.; Audrey Ruple, A.; Heng, H.G.; Pugh, M.R.; Rao, S.; Vlahov, I.R.; et al. Phase I/II clinical trial of the targeted chemotherapeutic drug, folate-tubulysin, in dogs with naturally-occurring invasive urothelial carcinoma. Oncotarget 2018, 9, 37042–37053. [Google Scholar] [CrossRef]
- Lynn Gustafson, T.; Biller, B. Use of Toceranib Phosphate in the Treatment of Canine Bladder Tumors: 37 Cases. J. Am. Anim. Hosp. Assoc. 2019, 55, 243–248. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occuring model for cancer biology and drug development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef]
- Iwasaki, R.; Shimosato, Y.; Yoshikawa, R.; Goto, S.; Yoshida, K.; Murakami, M.; Kawabe, M.; Sakai, H.; Mori, T. Survival analysis in dogs with urinary transitional cell carcinoma that underwent whole-body computed tomography at diagnosis. Vet. Comp. Oncol. 2019, 17, 385–393. [Google Scholar] [CrossRef]
- Burgess, K.E.; DeRegis, C.J. Urologic Oncology. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Deville, S.; Dupuis, L.; Bertrand, F.; Aucouturier, J. Adjuvant formulation for veterinary vaccines: MontanideTM Gel safety profile. Procedia Vaccinol. 2009, 1, 140–147. [Google Scholar] [CrossRef]
- NIH US National Library of Medicine. CpG 7909/Montanide ISA 720 with or without Cyclophosphamide in Combination Either with NY-ESO-1-derived Peptides or the NY-ESO-1 Protein for NY-ESO-1-expressing Tumors (NCT00819806). 2009. Available online: https://clinicaltrials.gov/ct2/show/NCT00819806 (accessed on 2 November 2022).

| Dogs (n) | Gender, n (%) | Breed, n (%) | Weight, Median | Age, Average | Prior Tx * | Stage, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | Single | Mixed | kg (range) | year (range) | n (%) | T2N0M0 | T2N1M0 | T2N0M1 | T2N2M1 | T3N0M0 | T3N1M1 | ||
| Study | 20 | 8 (40%) | 12 (60%) | 17 (85%) | 3 (15%) | 18.8 (4.4–72) | 11 (7–14) | 5 (25%) | 15 (75%) | 2 (10%) | 2 (10%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Ctrl | 31 | 12 (39%) | 19 (61%) | 25 (81%) | 6 (19%) | 14.4 (3.6–47) | 11 (6–15) | 6 (19%) | 24 (75%) | 2 (6%) | 2 (6%) | 0 (0%) | 1 (3%) | 2 (6%) |
| Dogs | N1, N2/M1 | CR | PR | SD | PD | PFI | OS | Refs. | |
|---|---|---|---|---|---|---|---|---|---|
| Total/Evaluable or Tumor Response | Any Metastasis, % of Total | ||||||||
| Drugs | (#) | (%) | (%) | (%) | (%) | (%) | days | days | |
| Current study | |||||||||
| CVx1/meloxicam | 25/20 | 5/5/10 | 10 | 10 | 75 | 5 | 257 | 374 | |
| Randomized trials | |||||||||
| Vinblastine/piroxicam | 27/26 | 0/4/4 | 0 | 58 | 33 | 8 | 199 | 299 | [26] |
| Cisplatin/piroxicam | 14/14 | 28/14/43 | 14 | 57 | 28 | 0 | 124 | 246 | [27] |
| Mitoxantrone/piroxicam | 26/NA | 8/NA/8 | 0 | 8 | 69 | 23 | 106 | 247 | [28] |
| Carboplatin/piroxicam | 24/NA | 29/NA/29 | 0 | 13 | 54 | 33 | 73 | 263 | [28] |
| Single-arm trials | |||||||||
| Piroxicam | 34/34 | 9/15/24 | 2 | 4 | 18 | 10 | NA | 181 | [29] |
| Mitoxantrone/piroxicam | 55/48 | NA/NA/11 | 2 | 33 | 46 | 19 | 194 | 291 | [30] |
| Carboplatin/piroxicam | 31/29 | 13/13/19 | 0 | 38 | 45 | 17 | NA | 161 | [21] |
| Doxorubicin/piroxicam | 34/23 | NA/NA/NA | 0 | 9 | 60 | 30 | 103 | 168 | [31] |
| Gemcitabine/piroxicam | 38/37 | 11/3/11 | 5 | 22 | 51 | 22 | NA | 230 | [32] |
| Dogs *, n = 20 (%) | Vaccinations, n = 172 (%) | ||||||
|---|---|---|---|---|---|---|---|
| Initial, n =79 | Maintenance, n = 66 | ||||||
| Grade 1 | Grade 2 | Grade 3–5 | Grade 1 | Grade 2 | Grade 1 | Grade 2 | |
| Administration site conditions | |||||||
| Injection site reactions | 9 (45%) | 7 (35%) | 0 (0%) | 27 (34%) | 8 (10%) | 10 (15%) | 5 (8%) |
| Lameness local extremity | 5 (25%) | 1 (5%) | 0 (0%) | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Constitutional clinical signs | |||||||
| Lethargy | 3 (15%) | 5 (25%) | 0 (0%) | 7 (9%) | 6 (6%) | 0 (0%) | 1 (2%) |
| Anorexia | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Nausea/vomiting | 5 (25%) | 0 (0%) | 0 (0%) | 4 (5%) | 0 (0%) | 3 (5%) | 0 (0%) |
| Fever | 2 (10%) | 0 (0%) | 0 (0%) | 4 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diarrhea | 2 (10%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Any * | 11 (55%) | 8 (40%) | 0 (0%) | 12 (60%) | 5 (25%) | 5 (25%) | 4 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engbersen, D.J.M.; van Beijnum, J.R.; Roos, A.; van Beelen, M.; de Haan, J.D.; Grinwis, G.C.M.; Schalken, J.A.; Witjes, J.A.; Griffioen, A.W.; Huijbers, E.J.M. Vaccination against Extracellular Vimentin for Treatment of Urothelial Cancer of the Bladder in Client-Owned Dogs. Cancers 2023, 15, 3958. https://doi.org/10.3390/cancers15153958
Engbersen DJM, van Beijnum JR, Roos A, van Beelen M, de Haan JD, Grinwis GCM, Schalken JA, Witjes JA, Griffioen AW, Huijbers EJM. Vaccination against Extracellular Vimentin for Treatment of Urothelial Cancer of the Bladder in Client-Owned Dogs. Cancers. 2023; 15(15):3958. https://doi.org/10.3390/cancers15153958
Chicago/Turabian StyleEngbersen, Diederik J. M., Judy R. van Beijnum, Arno Roos, Marit van Beelen, Jan David de Haan, Guy C. M. Grinwis, Jack A. Schalken, J. Alfred Witjes, Arjan W. Griffioen, and Elisabeth J. M. Huijbers. 2023. "Vaccination against Extracellular Vimentin for Treatment of Urothelial Cancer of the Bladder in Client-Owned Dogs" Cancers 15, no. 15: 3958. https://doi.org/10.3390/cancers15153958
APA StyleEngbersen, D. J. M., van Beijnum, J. R., Roos, A., van Beelen, M., de Haan, J. D., Grinwis, G. C. M., Schalken, J. A., Witjes, J. A., Griffioen, A. W., & Huijbers, E. J. M. (2023). Vaccination against Extracellular Vimentin for Treatment of Urothelial Cancer of the Bladder in Client-Owned Dogs. Cancers, 15(15), 3958. https://doi.org/10.3390/cancers15153958








