Alberta Childhood Cancer Survivorship Research Program
Abstract
:Simple Summary
Abstract
1. Introduction
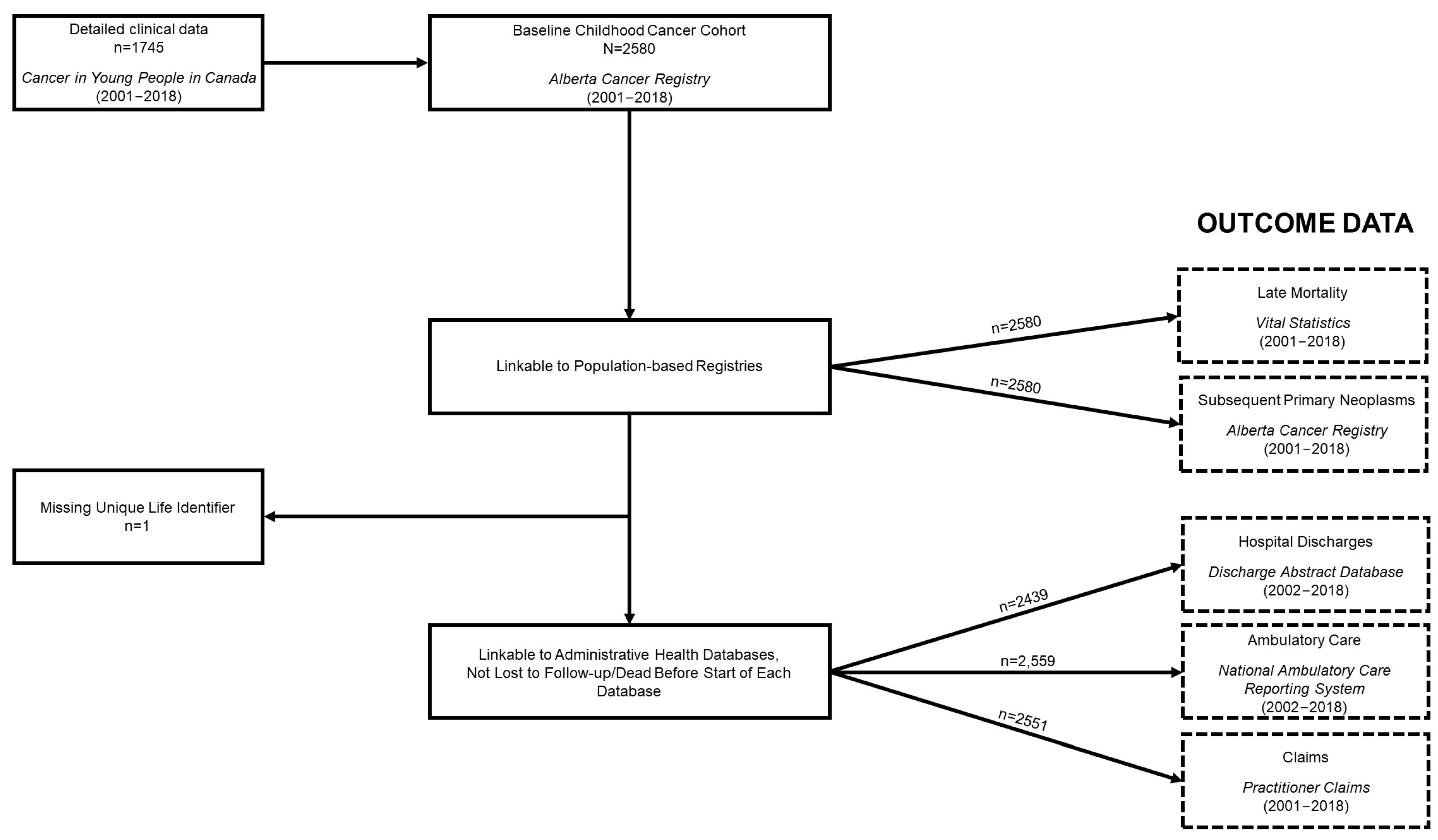
2. Materials and Methods
2.1. Study Setting
2.2. Study Population
2.3. Long-Term Survivor Clinic (LTSC)
2.4. Late-Effect Ascertainment
2.4.1. Alberta Cancer Registry (ACR)
2.4.2. Discharge Abstract Database (DAD)
2.4.3. National Ambulatory Care Reporting System (NACRS)
2.4.4. Practitioner Claims
2.4.5. Long-Term Survivor Questionnaire (LTSQ)
3. Results
3.1. Cohort Characteristics
3.2. Late Effects
4. Discussion
4.1. Strengths of the Research Program
4.2. Comparisons with the Literature
4.3. Limitations
4.4. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Noone, A.-M.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z. SEER cancer statistics review, 1975–2010. Bethesda, MD: National Cancer Institute. Mach. Learn. -Based Model. Diagn. 2013, 21, 12. [Google Scholar]
- Fidler, M.M.; Frobisher, C.; Guha, J.; Wong, K.; Kelly, J.; Winter, D.L.; Sugden, E.; Duncan, R.; Whelan, J.; Reulen, R.C.; et al. Long-term adverse outcomes in survivors of childhood bone sarcoma: The British Childhood Cancer Survivor Study. Br. J. Cancer 2015, 112, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Reulen, R.C.; Bright, C.J.; Henson, K.E.; Kelly, J.S.; Jenney, M.; Ng, A.; Whelan, J.; Winter, D.L.; Frobisher, C.; et al. Respiratory mortality of childhood, adolescent and young adult cancer survivors. Thorax 2018, 73, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Reulen, R.C.; Henson, K.; Kelly, J.; Cutter, D.; Levitt, G.A.; Frobisher, C.; Winter, D.L.; Hawkins, M.M.; British Childhood Cancer Survivor Study Steering, G. Population-Based Long-Term Cardiac-Specific Mortality Among 34 489 Five-Year Survivors of Childhood Cancer in Great Britain. Circulation 2017, 135, 951–963. [Google Scholar] [CrossRef]
- Fidler, M.M.; Reulen, R.C.; Winter, D.L.; Kelly, J.; Jenkinson, H.C.; Skinner, R.; Frobisher, C.; Hawkins, M.M. Long term cause specific mortality among 34,489 five year survivors of childhood cancer in Great Britain: Population based cohort study. BMJ 2016, 354, i4351. [Google Scholar] [CrossRef]
- Fidler, M.M.; Reulen, R.C.; Winter, D.L.; Allodji, R.S.; Bagnasco, F.; Bárdi, E.; Bautz, A.; Bright, C.J.; Byrne, J.; Feijen, E.A.M.; et al. Risk of Subsequent Bone Cancers Among 69 460 Five-Year Survivors of Childhood and Adolescent Cancer in Europe. J. Natl. Cancer Inst. 2018, 110, 183–194. [Google Scholar] [CrossRef]
- Bright, C.J.; Hawkins, M.M.; Winter, D.L.; Alessi, D.; Allodji, R.S.; Bagnasco, F.; Bárdi, E.; Bautz, A.; Byrne, J.; Feijen, E.A.M.; et al. Risk of Soft-Tissue Sarcoma Among 69,460 Five-Year Survivors of Childhood Cancer in Europe. J. Natl. Cancer Inst. 2018, 110, 649–660. [Google Scholar] [CrossRef]
- Allodji, R.S.; Hawkins, M.M.; Bright, C.J.; Fidler-Benaoudia, M.M.; Winter, D.L.; Alessi, D.; Fresneau, B.; Journy, N.; Morsellino, V.; Bárdi, E.; et al. Risk of subsequent primary leukaemias among 69,460 five-year survivors of childhood cancer diagnosed from 1940 to 2008 in Europe: A cohort study within PanCareSurFup. Eur. J. Cancer 2019, 117, 71–83. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Frederiksen, L.E.; Mader, L.; Feychting, M.; Mogensen, H.; Madanat-Harjuoja, L.; Malila, N.; Tolkkinen, A.; Hasle, H.; Winther, J.F.; Erdmann, F. Surviving childhood cancer: A systematic review of studies on risk and determinants of adverse socioeconomic outcomes. Int. J. Cancer 2019, 144, 1796–1823. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Leisenring, W.; Krull, K.R.; Ness, K.K.; Friedman, D.L.; Armstrong, G.T.; Stovall, M.; Park, E.R.; Oeffinger, K.C.; Hudson, M.M.; et al. Unemployment among adult survivors of childhood cancer: A report from the childhood cancer survivor study. Med. Care 2010, 48, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Mader, L.; Michel, G.; Roser, K. Unemployment Following Childhood Cancer. Dtsch. Arztebl. Int. 2017, 114, 805–812. [Google Scholar] [CrossRef]
- Fidler, M.M.; Ziff, O.J.; Wang, S.; Cave, J.; Janardhanan, P.; Winter, D.L.; Kelly, J.; Mehta, S.; Jenkinson, H.; Frobisher, C.; et al. Aspects of mental health dysfunction among survivors of childhood cancer. Br. J. Cancer 2015, 113, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Reulen, R.C.; Bright, C.J.; Winter, D.L.; Fidler, M.M.; Wong, K.; Guha, J.; Kelly, J.S.; Frobisher, C.; Edgar, A.B.; Skinner, R.; et al. Pregnancy and Labor Complications in Female Survivors of Childhood Cancer: The British Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2017, 109, djx056. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.F.; Reulen, R.C.; Winter, D.L.; Guha, J.; Fidler, M.M.; Kelly, J.; Lancashire, E.R.; Pritchard-Jones, K.; Jenkinson, H.C.; Sugden, E.; et al. Risk of Adverse Health and Social Outcomes Up to 50 Years After Wilms Tumor: The British Childhood Cancer Survivor Study. J. Clin. Oncol. 2016, 34, 1772–1779. [Google Scholar] [CrossRef]
- Schulte, F.; Brinkman, T.M.; Li, C.; Fay-McClymont, T.; Srivastava, D.K.; Ness, K.K.; Howell, R.M.; Mueller, S.; Wells, E.; Strother, D.; et al. Social adjustment in adolescent survivors of pediatric central nervous system tumors: A report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 3596–3608. [Google Scholar] [CrossRef]
- Robison, L.L.; Mertens, A.C.; Boice, J.D.; Breslow, N.E.; Donaldson, S.S.; Green, D.M.; Li, F.P.; Meadows, A.T.; Mulvihill, J.J.; Neglia, J.P.; et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med. Pediatr. Oncol. 2002, 38, 229–239. [Google Scholar] [CrossRef]
- Hawkins, M.M.; Lancashire, E.R.; Winter, D.L.; Frobisher, C.; Reulen, R.C.; Taylor, A.J.; Stevens, M.C.; Jenney, M. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer 2008, 50, 1018–1025. [Google Scholar] [CrossRef]
- Statistics Canada. Census Profile, 2016 Census—Alberta [Province]. Available online: https://www12.statcan.gc.ca/census-recensement/2016/dppd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=48&Geo2=&Code2=&SearchText=Alberta&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=48&type=0 (accessed on 4 May 2023).
- Alberta Health Services. Cancer Centre Information. Available online: https://www.albertahealthservices.ca/cancer/Page16313.aspx (accessed on 14 November 2021).
- Alberta Health Services. Oncology—Inpatients. Available online: https://www.albertahealthservices.ca/findhealth/Service.aspx?id=1001073&serviceAtFacilityID=1041710 (accessed on 14 November 2021).
- Mitra, D.; Hutchings, K.; Shaw, A.; Barber, R.; Sung, L.; Bernstein, M.; Carret, A.S.; Barbaros, V.; McBride, M.; Parker, L.; et al. Status Report—The Cancer in Young People in Canada surveillance system. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 73–76. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International classification of childhood cancer. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; Klarnebach, S.; Clement, F. Alberta Health Data Asset Directory. 2018. Available online: https://albertarwe.ca/rwe-in-alberta/ (accessed on 15 November 2021).
- North American Association of Central Cancer Registries Certification. Certified Registries. Available online: https://www.naaccr.org/certified-registries/ (accessed on 15 November 2021).
- Reynolds, K.; Spavor, M.; Brandelli, Y.; Kwok, C.; Li, Y.; Disciglio, M.; Carlson, L.E.; Schulte, F.; Anderson, R.; Grundy, P.; et al. A comparison of two models of follow-up care for adult survivors of childhood cancer. J. Cancer Surviv. 2019, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IACR—Alberta Cancer Registry. Available online: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=887&Itemid=498 (accessed on 1 May 2022).
- Dickie, L.; Johnson, C.; Adams, S.; Negoita, S. Solid Tumor Rules; National Cancer Institute: Rockville, MD, USA, 2021.
- Ruhl, J.; Adamo, M.; Dickie, L.; Sun, L.; Johnson, C. Hematopoietic and Lymphoid Neoplasm Coding Manual; National Cancer Institute: Bethesda, MD, USA, 2021.
- ICD-9 to ICD-10 Coding with Reference to Causes of Death Grouping in Alberta Working Document, Version 1.0; Government of Alberta: Edmonton, AB, Canada, 2006.
- Overview of Administrative Health Datasets; Analytics and Performance Reporting Branch—Alberta Health Services: Calgary, AB, Canada, 2017.
- International Statistical Classification of Diseases and Related Health Problems; Canadian Institute for Health Information: Ottawa, ON, Canada, 2015.
- Canadian Classification of Health Interventions (CCI) Volume 4: Alphabetical Index; Canadian Institute for Health Information: Ottawa, ON, Canada, 2015.
- Canadian Institute of Health Information. ICD-10 Canada Volume 1. 2018. Available online: https://secure.cihi.ca/free_products/CodingStandards_v2018_EN.pdf (accessed on 1 May 2022).
- Canadian Institute of Health Information. ICD-9/CCP and ICD-9-CM. Available online: https://www.cihi.ca/en/submit-data-and-view-standards/icd-9ccp-and-icd-9-cm (accessed on 1 May 2022).
- Cella, D.; Weinfurt, K.; Revicki, D.; Pilkonis, P.; DeWalt, D.; DeVellis, R.; Cook, K.; Buysse, D.; Amtmann, D.; Yount, S.; et al. Patient-Reported Outcomes Measurement Information System Pediatric Profile-25 v2.0 (PROMIS Pediatric Profile-25 v2.0). Available online: https://eprovide.mapi-trust.org/instruments/patient-reported-outcomes-measurement-information-system-pediatric-profile-25-v2.0 (accessed on 1 May 2022).
- Winther, J.F.; Kenborg, L.; Byrne, J.; Hjorth, L.; Kaatsch, P.; Kremer, L.C.; Kuehni, C.E.; Auquier, P.; Michel, G.; De Vathaire, F.; et al. Childhood cancer survivor cohorts in Europe. Acta Oncol. 2015, 54, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Bejarano-Quisoboni, D.; Pelletier-Fleury, N.; Allodji, R.S.; Lacour, B.; GrosClaude, P.; Pacquement, H.; Doz, F.; Berchery, D.; Pluchart, C.; Bondiau, P.-Y.; et al. Health care expenditures among long-term survivors of pediatric solid tumors: Results from the French Childhood Cancer Survivor Study (FCCSS) and the French network of cancer registries (FRANCIM). PLoS ONE 2022, 17, e0267317. [Google Scholar] [CrossRef] [PubMed]
- Chi ldhood Cancer Survivor Study Progress Report; St. Jude: Memphis, TN, USA, 2020.
- McBride, M.L.; Rogers, P.C.; Sheps, S.B.; Glickman, V.; Broemeling, A.M.; Goddard, K.; Hu, J.; Lorenzi, M.; Peacock, S.; Pritchard, S.; et al. Childhood, adolescent, and young adult cancer survivors research program of British Columbia: Objectives, study design, and cohort characteristics. Pediatr. Blood Cancer 2010, 55, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Pole, J.D.; Gu, L.Y.; Kirsh, V.; Greenberg, M.L.; Nathan, P.C. Subsequent Malignant Neoplasms in a Population-Based Cohort of Pediatric Cancer Patients: A Focus on the First 5 Years. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1585–1592. [Google Scholar] [CrossRef]
- Marjerrison, S.; Pole, J.D.; Sung, L. Inferior survival among Aboriginal children with cancer in Ontario. Cancer 2014, 120, 2751–2759. [Google Scholar] [CrossRef]
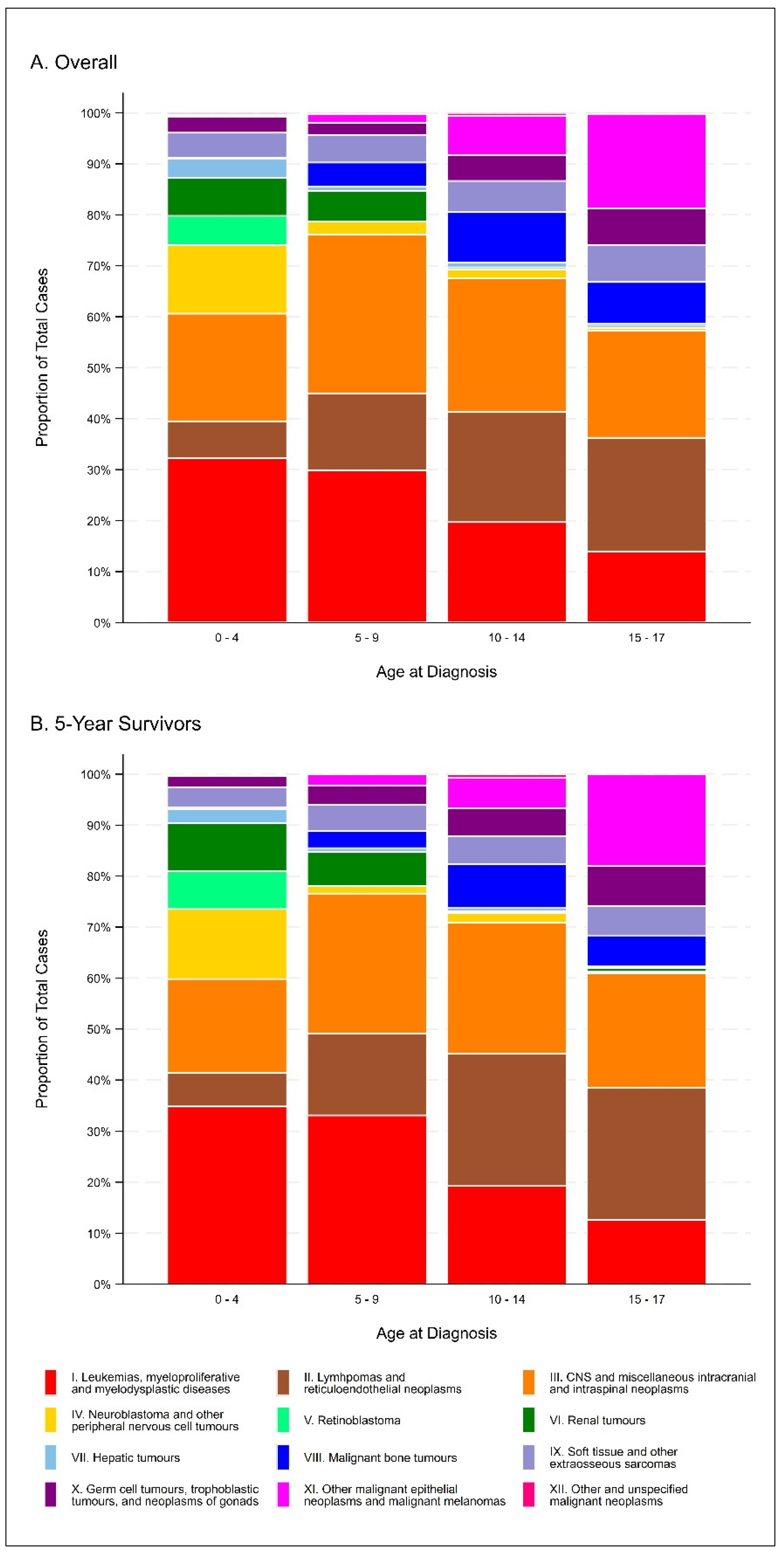
| Characteristic | Overall Number (%) | 5-Year Survivors Number (%) |
|---|---|---|
| Total | 2581 (100.0) | 1385 (100.0) |
| Sex | ||
| Male | 1354 (52.5) | 740 (53.4) |
| Female | 1227 (47.5) | 645 (46.6) |
| Time Since Diagnosis (years) 1 | ||
| 0–<5 | 1196 (46.3) | 0 (0.0) |
| 5–<10 | 627 (24.3) | 627 (45.3) |
| 10–<14 | 522 (20.2) | 522 (37.7) |
| 15 | 236 (9.1) | 236 (17.0) |
| Median (IQR) | 5.6 (1.9–10.9) | 10.3 (7.5–13.7) |
| ICCC-3 Diagnosis Category | ||
| Leukemias, myeloproliferative diseases, and myelodysplastic diseases | 654 (25.3) | 364 (26.3) |
| Lymphomas and reticuloendothelial neoplasms | 385 (14.9) | 233 (16.8) |
| CNS and miscellaneous intracranial and intraspinal neoplasms | 624 (24.2) | 314 (22.7) |
| Neuroblastoma and other peripheral nervous cell tumors | 161 (6.2) | 81 (5.9) |
| Retinoblastoma | 57 (2.2) | 38 (2.7) |
| Renal tumors | 109 (4.2) | 69 (5.0) |
| Hepatic tumors | 48 (1.9) | 19 (1.4) |
| Malignant bone tumors | 124 (4.8) | 56 (4.0) |
| Soft tissue and other extraosseous sarcomas | 148 (5.7) | 68 (4.9) |
| Germ cell tumors, trophoblastic tumors, and neoplasms of gonads | 109 (4.2) | 61 (4.4) |
| Other malignant epithelial neoplasms and malignant melanomas | 153 (5.9) | 78 (5.6) |
| Other and unspecified malignant neoplasms | 9 (0.4) | 4 (0.3) |
| Year of Diagnosis | ||
| 2001–2005 | 620 (24.0) | 502 (36.3) |
| 2006–2010 | 676 (26.2) | 549 (39.6) |
| 2011–2015 | 786 (30.5) | 334 (24.1) |
| 2016–2018 | 499 (19.3) | 0 (0.0) |
| Age at Diagnosis (years) | ||
| 0–4 | 1000 (38.7) | 510 (36.8) |
| 5–9 | 503 (19.5) | 269 (19.4) |
| 10–14 | 554 (21.5) | 312 (22.5) |
| 15–17 | 524 (20.3) | 294 (21.2) |
| Median (IQR) | 7.6 (2.9–14.1) | 8.1 (3.2–14.4) |
| Attained Age (years) 1 | ||
| 0–4 | 318 (12.3) | 0 (0.0) |
| 5–9 | 451 (17.5) | 134 (9.7) |
| 10–14 | 510 (19.8) | 279 (20.1) |
| 15–19 | 582 (22.6) | 302 (21.8) |
| 20–24 | 404 (15.7) | 354 (25.6) |
| 25–29 | 226 (8.8) | 226 (16.3) |
| 30–34 | 89 (3.5) | 89 (6.4) |
| 35 | 1 (<0.1) | 1 (0.1) |
| Median (IQR) | 15.1 (8.7–20.8) | 19.7 (14.0–24.5) |
| CYP-C Treatment | ||
| No treatment | 70 (2.7) | 19 (1.4) |
| CT only | 588 (22.8) | 317 (22.9) |
| RT only | 10 (0.4) | 2 (0.1) |
| Surgery only | 215 (8.3) | 127 (9.2) |
| CT + RT | 154 (6.0) | 81 (5.9) |
| CT + Surgery | 347 (13.4) | 178 (12.9) |
| RT + Surgery | 71 (2.8) | 39 (2.8) |
| CT + RT + Surgery | 290 (11.2) | 140 (10.1) |
| Patients with missing treatment information | 836 (32.4) | 482 (34.8) |
| Zone of Diagnosis | ||
| South | 164 (6.4) | 89 (6.4) |
| Calgary | 915 (35.5) | 471 (34.0) |
| Central | 343 (13.3) | 202 (14.6) |
| Edmonton | 815 (31.6) | 440 (31.8) |
| North | 343 (13.3) | 182 (13.1) |
| Alberta, zone unknown | 1 (<0.1) | 1 (0.1) |
| Chemotherapy Agent | Number of Survivors | Dose Available (%) | Median (mg/m2) (IQR) |
|---|---|---|---|
| Bleomycin Blenoxane Bleo | 96 | 95 (99.0) | 60.6 (46.0–69.1) |
| Busulphan Busulfan (Myleran) | 4 | 4 (100.0) | 346.3 (295.3–429.1) |
| Carboplatin CBDCA Paraplatin Carboplatinum | 209 | 164 (78.5) | 1480.5 (983.0–2250.2) |
| Carmustine (BCNU) Bis-Chloroethyl-Nitrosourea BiCNU | 2 | 2 (100.0) | 309.9 (58.4–561.4) |
| Cisplatin CDDP Platinol Cisplatinum Cis-diamminedicloro-platinum II P | 232 | 232 (100.0) | 368.4 (230.4–454.3) |
| Cyclophosphamide Cytoxan CTX Procytox | 837 | 831 (99.3) | 3002.6 (1124.9–5293.9) |
| Cytarabine (IT ONLY) Ara-C Cytosar Cytosine arabinoside | 558 | 441 (79.0) | 95.6 (75.4–188.3) |
| Cytarabine (ONLY IV ≥ 500 mg/m2 per dose) Ara-C Cytosar Cytosine arabinoside | 146 | 145 (99.3) | 15,009.4 (10,349.3–24,129.0) |
| Daunomycin Daunorubicin Cerubidine DNR | 300 | 299 (99.7) | 102.1 (96.5–166.2) |
| Doxorubicin Adriamycin ADR | 786 | 780 (99.2) | 109.9 (74.7–217.8) |
| Doxorubicin-Pegylated Liposomal (DOXIL) PLD | 4 | 4 (100.0) | 111.9 (78.0–436.7) |
| Etoposide (VP16) VePesid ETOP | 564 | 559 (99.1) | 1335.1 (598.9–1875.0) |
| Etoposide Phosphate | 3 | 2 (66.7) | 2107.5 (1200.0–3015.0) |
| Hydrocortisone (IT ONLY) | 113 | 89 (78.8) | 80.3 (40.8–162.6) |
| Idarubicin Idamycin 4-Demethoxydaunorubicin | 28 | 28 (100.0) | 11.0 (09.9–20.9) |
| Ifosfamide Isophosphamide IFOS Ifex Holoxan | 217 | 215 (99.1) | 20,354.5 (6052.1–46,236.1) |
| Lomustine (CCNU) CeeNU Chloroethyl-Cyclohexyl-Nitrosurea | 20 | 18 (90.0) | 445.8 (299.3–568.5) |
| Melphalan L-PAM Alkeran L-Sarcolysin | 5 | 5 (100.0) | 177.5 (100.4–192.5) |
| Methotrexate (IT ONLY) MTX Amethopterin | 558 | 446 (79.9) | 243.6 (113.1–300.0) |
| Methotrexate (IV ≥ 500 mg/m2 ONLY) MTX Amethopterin | 355 | 352 (99.2) | 14,491.6 (6319.5–20,000.0) |
| Mitoxantrone Novantrone DHAD Dihydrochloride | 57 | 57 (100.0) | 46.6 (34.4–48.5) |
| Oxaliplatin Eloxatin | 4 | 4 (100.0) | 178.6 (53.5–286.2) |
| Procarbazine PCB Natulan Matulane | 7 | 6 (85.7) | 733.0 (62.3–1600.0) |
| Teniposide (Vumon) VM-26 | 21 | 21 (100.0) | 404.2 (383.7–529.0) |
| Thiotepa TESPA Triethylene Thiophosphoramide | 12 | 11 (91.7) | 502.2 (16.8–1271.8) |
| Radiotherapy Site | Patients | Patients with Dosage (%) | Median (cGy) (IQR) |
| All Sites Combined | 525 | 513 (97.7) | 2550 (1800–4776) |
| Abdomen | 86 | 86 (100.0) | 1490 (1080–2400) |
| Central Nervous System 1 | 266 | 265 (99.6) | 3600 (1800–5400) |
| Chest | 6 | 6 (100.0) | 2325 (1500–4500) |
| Face 2 | 16 | 16 (100.0) | 3870 (2000–4320) |
| Limb | 43 | 42 (97.7) | 4500 (2200–5000) |
| Liver | 3 | 3 (100.0) | 600 (450–1200) |
| Lung | 32 | 32 (100.0) | 1500 (1200–2130) |
| Lymph Nodes 3 | 56 | 56 (100.0) | 2100.0 (2100–3075) |
| Nasopharynx | 5 | 5 (100.0) | 4150 (3600–4500) |
| Neck | 56 | 56 (100.0) | 2100 (2100–3375) |
| Pelvis | 35 | 35 (100.0) | 2100 (1050–3600) |
| Skull | 18 | 17 (94.4) | 2400 (1500–4500) |
| Spleen | 11 | 11 (100.0) | 2100 (2100–2100) |
| Testis | 3 | 3 (100.0) | 3600 (1200–8659) |
| Thorax 4 | 40 | 40 (100.0) | 2100 (2100–2550) |
| Other | 85 | 82 (96.5) | 2603 (2000–5000) |
| Missing 5 | 17 | 3 (17.6) | 2160 (1440–5400) |
| Characteristic | Overall Number (%) | 5-Year Survivors Number (%) |
|---|---|---|
| Total | 836 (100.0) | 482 (100.0) |
| Sex | ||
| Male | 399 (47.7) | 238 (49.4) |
| Female | 437 (52.3) | 244 (50.6) |
| Time Since Diagnosis (years) 1 | ||
| 0–<5 | 354 (42.3) | 0 (0.0) |
| 5–<10 | 243 (29.1) | 243 (50.4) |
| 10–<14 | 174 (20.8) | 174 (36.1) |
| 15 | 65 (7.8) | 65 (13.5) |
| Median (IQR) | 6.0 (2.2–10.8) | 9.9 (7.1–13.2) |
| ICCC-3 Diagnosis Category | ||
| Leukemias, myeloproliferative diseases, and myelodysplastic diseases | 108 (12.9) | 53 (11.0) |
| Lymphomas and reticuloendothelial neoplasms | 144 (17.2) | 99 (20.5) |
| CNS and miscellaneous intracranial and intraspinal neoplasms | 247 (29.6) | 147 (30.5) |
| Neuroblastoma and other peripheral nervous cell tumors | 16 (1.9) | 7 (1.5) |
| Retinoblastoma | 40 (4.8) | 31 (6.4) |
| Renal tumors | 6 (0.7) | 5 (1.0) |
| Hepatic tumors | 3 (0.4) | 1 (0.2) |
| Malignant bone tumors | 45 (5.4) | 19 (3.9) |
| Soft tissue and other extraosseous sarcomas | 39 (4.7) | 21 (4.4) |
| Germ cell tumors, trophoblastic tumors, and neoplasms of gonads | 55 (6.6) | 31 (6.4) |
| Other malignant epithelial neoplasms and malignant melanomas | 131 (15.7) | 67 (13.9) |
| Other and unspecified malignant neoplasms | 2 (0.2) | 1 (0.2) |
| Year of Diagnosis | ||
| 2001–2005 | 215 (25.7) | 165 (34.2) |
| 2006–2010 | 218 (26.1) | 179 (37.1) |
| 2011–2015 | 276 (33.0) | 138 (28.6) |
| 2016–2018 | 127 (15.2) | 0 (0.0) |
| Age at Diagnosis (years) | ||
| 0–4 | 161 (19.3) | 80 (16.6) |
| 5–9 | 65 (7.8) | 32 (6.6) |
| 10–14 | 128 (15.3) | 78 (16.2) |
| 15–17 | 482 (57.7) | 292 (60.6) |
| Median (IQR) | 15.4 (8.5–16.8) | 15.6 (11.0–17.0) |
| Attained Age (years) 1 | ||
| 0–4 | 63 (7.5) | 0 (0.0) |
| 5–9 | 73 (8.7) | 31 (6.4) |
| 10–14 | 75 (9.0) | 37 (7.7) |
| 15–19 | 211 (25.2) | 48 (10.0) |
| 20–24 | 201 (24.0) | 153 (31.7) |
| 25–29 | 138 (16.5) | 138 (28.6) |
| 30–34 | 74 (8.9) | 74 (15.4) |
| 35 | 1 (0.1) | 1 (0.2) |
| Median (IQR) | 19.9 (14.8–25.2) | 24.2 (20.4–28.2) |
| Zone of Diagnosis | ||
| South | 64 (7.7) | 38 (7.9) |
| Calgary | 285 (34.1) | 151 (31.3) |
| Central | 118 (14.1) | 77 (16.0) |
| Edmonton | 250 (29.9) | 152 (31.5) |
| North | 118 (14.1) | 63 (13.1) |
| Alberta, zone unknown | 1 (0.1) | 1 (0.2) |
| Database and Outcome | Overall (%) | 5-Year Survivors (%) |
|---|---|---|
| Alberta Cancer Registry | ||
| Subsequent primary neoplasms observed | 94 | 55 |
| Number of survivors | 83 (3.2) | 48 (3.5) |
| Vital Statistics | ||
| Alive | 2173 (84.2) | 1347 (97.3) |
| Deceased | 408 (15.8) | 38 (2.7) |
| Discharge Abstract Database | ||
| Number of inpatient events | 16,669 | 995 |
| Number of survivors | 2254 (87.3) | 331 (23.9) |
| National Ambulatory Care Reporting System | ||
| Number of ambulatory/outpatient events | 445,150 | 60,906 |
| Number of survivors | 2533 (98.1) | 1296 (93.6) |
| Practitioner Claims | ||
| Number of claims records | 396,074 | 88,885 |
| Number of survivors | 2549 (98.8) | 1317 (95.1) |
| Cohort | Alberta Childhood Cancer Survivorship Research Program | Childhood, Adolescent, and Young Adult Cancer Survivors Research Program | North American Childhood Cancer Survivor Study | Adult Life after Childhood Cancer in Scandinavia | British Childhood Cancer Survivor Study | Dutch Childhood Oncology Group LATER | Swiss Childhood Cancer Survivor Study | French Childhood Cancer Survivor Study |
| Country | Canada | Canada | Canada United States | Denmark Finland Iceland Norway Sweden | England Scotland Wales | The Netherlands | Switzerland | France |
| Identification of survivors | Registry-based | Registry-based | Institution-based | Registry-based | Registry-based | Registry-based | Registry-based | Registry-based |
| Number of survivors | 2580 | 3841 | 38,036 | 33,160 | 34,490 | 6168 | 4405 | 7670 |
| Age at cancer diagnosis | 0–17 | 0–24 | 0–20 | 0–19 | 0–14 | 0–17 | 0–20 | 0–19 |
| Diagnosis years | 2001–2018 | 1970–1995 | 1970–1999 | 1943–2008 | 1940–2006 | 1963–2002 | 1976–2014 | 1945–2000 |
| Cancer types | All | All | Leukemia, tumor of the central nervous system, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft-tissue sarcoma, and bone tumor | All | All | All | All | All except leukemia |
| Entry for follow-up | Diagnosis | 5 years post-diagnosis | 5 years post-diagnosis | 1-year post-diagnosis | 5 years post-diagnosis | 5 years post-diagnosis | 5 years post-diagnosis | 5 years post-diagnosis |
| Detailed treatment information | Yes (collection ongoing) | No | Yes | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, A.; Schulte, F.; Guilcher, G.M.T.; Truong, T.H.; Reynolds, K.; Spavor, M.; Logie, N.; Lee, J.; Fidler-Benaoudia, M.M. Alberta Childhood Cancer Survivorship Research Program. Cancers 2023, 15, 3932. https://doi.org/10.3390/cancers15153932
Harper A, Schulte F, Guilcher GMT, Truong TH, Reynolds K, Spavor M, Logie N, Lee J, Fidler-Benaoudia MM. Alberta Childhood Cancer Survivorship Research Program. Cancers. 2023; 15(15):3932. https://doi.org/10.3390/cancers15153932
Chicago/Turabian StyleHarper, Andrew, Fiona Schulte, Gregory M. T. Guilcher, Tony H. Truong, Kathleen Reynolds, Maria Spavor, Natalie Logie, Joon Lee, and Miranda M. Fidler-Benaoudia. 2023. "Alberta Childhood Cancer Survivorship Research Program" Cancers 15, no. 15: 3932. https://doi.org/10.3390/cancers15153932
APA StyleHarper, A., Schulte, F., Guilcher, G. M. T., Truong, T. H., Reynolds, K., Spavor, M., Logie, N., Lee, J., & Fidler-Benaoudia, M. M. (2023). Alberta Childhood Cancer Survivorship Research Program. Cancers, 15(15), 3932. https://doi.org/10.3390/cancers15153932







