Predicting Overall Survival for Patients with Malignant Mesothelioma Following Radiotherapy via Interpretable Machine Learning
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Patient Characteristics
2.2. Radiation Treatment Planning and Delivery
2.3. Statistical Analysis
2.4. Patient Overall Survival (OS) and Survival Prediction
2.5. Machine Learning Methods for Predicting Overall Survival
2.6. Normal Tissue Complication Probability (NTCP)
3. Results
Achieved Dosimetric Endpoints and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gariazzo, C.; Gasparrini, A.; Marinaccio, A. Asbestos Consumption and Malignant Mesothelioma Mortality Trends in the Major User Countries. Ann. Glob. Health 2023, 89, 11. [Google Scholar] [CrossRef] [PubMed]
- Røe, O.D.; Stella, G.M. Malignant pleural mesothelioma: History, controversy and future of a manmade epidemic. Eur. Respir. Rev. 2015, 24, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Shavelle, R.; Vavra-Musser, K.; Lee, J.; Brooks, J. Life Expectancy in Pleural and Peritoneal Mesothelioma. Lung Cancer Int. 2017, 2017, 2782590. [Google Scholar] [CrossRef]
- Opitz, I.; Lardinois, D.; Arni, S.; Hillinger, S.; Vogt, P.; Odermatt, B.; Rousson, V.; Weder, W. Local recurrence model of malignant pleural mesothelioma for investigation of intrapleural treatment. Eur. J. Cardio-Thorac. Surg. 2007, 31, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Mott, F.E. Mesothelioma: A review. Ochsner J. 2012, 12, 70–79. [Google Scholar]
- National Comprehensive Cancer Network. Mesothelioma: Pleural (Version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/meso_pleural.pdf (accessed on 15 June 2023).
- Pan, X.; Levin-Epstein, R.; Huang, J.; Ruan, D.; King, C.R.; Kishan, A.U.; Steinberg, M.L.; Qi, X.S. Dosimetric predictors of patient-reported toxicity after prostate stereotactic body radiotherapy: Analysis of full range of the dose–volume histogram using ensemble machine learning. Radiother. Oncol. 2020, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhang, T.; Yang, Q.; Yang, D.; Rwigema, J.-C.; Qi, X.S. Survival prediction for oral tongue cancer patients via probabilistic genetic algorithm optimized neural network models. Br. J. Radiol. 2020, 93, 20190825. [Google Scholar] [CrossRef]
- Fu, J.; Zhong, X.; Li, N.; Van Dams, R.; Lewis, J.H.; Sung, K.; Raldow, A.C.; Jin, J.; Qi, X.S. Deep learning-based radiomic features for improving neoadjuvant chemoradiation response prediction in locally advanced rectal cancer. Phys. Med. Biol. 2020, 65, 075001. [Google Scholar] [CrossRef]
- Pan, X.; Liu, C.; Feng, T.; Qi, X.S. A multi-objective based radiomics feature selection method for response prediction following radiotherapy. Phys. Med. Biol. 2023, 68, 055018. [Google Scholar] [CrossRef]
- Courtiol, P.; Maussion, C.; Moarii, M.; Pronier, E.; Pilcer, S.; Sefta, M.; Manceron, P.; Toldo, S.; Zaslavskiy, M.; Le Stang, N.; et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat. Med. 2019, 25, 1519–1525. [Google Scholar] [CrossRef]
- Allione, A.; Viberti, C.; Cotellessa, I.; Catalano, C.; Casalone, E.; Cugliari, G.; Russo, A.; Guarrera, S.; Mirabelli, D.; Sacerdote, C.; et al. Blood cell DNA methylation biomarkers in preclinical malignant pleural mesothelioma: The EPIC prospective cohort. Int. J. Cancer 2022, 152, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Pölsterl, S.; Navab, N.; Katouzian, A. Fast training of support vector machines for survival analysis. In Joint European Conference on Machine Learning and Knowledge Discovery in Databases; Springer: Cham, Switzerland, 2015; pp. 243–259. [Google Scholar]
- Wu, Y.-L.; Li, W.-F.; Yang, K.-B.; Chen, L.; Shi, J.-R.; Chen, F.-P.; Huang, X.-D.; Lin, L.; Zhang, X.-M.; Li, J.; et al. Long-Term Evaluation and Normal Tissue Complication Probability (NTCP) Models for Predicting Radiation-Induced Optic Neuropathy after Intensity-Modulated Radiation Therapy (IMRT) for Nasopharyngeal Carcinoma: A Large Retrospective Study in China. J. Oncol. 2022, 2022, 3647462. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.2-11. Available online: https://CRAN.R-project.org/package=survival (accessed on 3 November 2022).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Kassambara, A.; VKosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0.4.9. 2021. Available online: https://CRAN.R-project.org/package=survminer (accessed on 3 November 2022).
- Lang, M.; Binder, M.; Richter, J.; Schratz, P.; Pfisterer, F.; Coors, S.; Au, Q.; Casalicchio, G.; Kotthoff, L.; Bischl, B. mlr3: A modern object-oriented machine learning framework in R. J. Open Source Softw. 2019, 4, 1903. [Google Scholar] [CrossRef]
- Lang, M.; Au, Q.; Coors, S.; Schratz, P. mlr3learners: Recommended Learners for ‘mlr3’. R Package Version 0.5.5. 2022. Available online: https://CRAN.R-project.org/package=mlr3learners (accessed on 9 November 2022).
- Sonabend, R.; Schratz, P.; Fischer, S. mlr3extralearners: Extra Learners For mlr3; R Package Version 0.5.49. Available online: https://mlr3extralearners.mlr-org.com/ (accessed on 9 November 2022).
- Sonabend, R.; Király, F.J.; Bender, A.; Bischl, B.; Lang, M. mlr3proba: An R package for machine learning in survival analysis. Bioinformatics 2021, 37, 2789–2791. [Google Scholar] [CrossRef]
- Becker, M.; Schratz, P.; Lang, M.; Bischl, B. mlr3fselect: Feature Selection for ‘mlr3’. R Package Version 0.7.2. 2022. Available online: https://CRAN.R-project.org/package=mlr3fselect (accessed on 9 November 2022).
- Becker, M.; Lang, M.; Richter, J.; Bischl, B.; Schalk, D. mlr3tuning: Tuning for ‘mlr3’. R Package Version 0.15.0. 2022. Available online: https://CRAN.R-project.org/package=mlr3tuning (accessed on 9 November 2022).
- Lang, M.; Bischl, B.; Richter, J.; Sun, X.; Binder, M. Paradox: Define and Work with Parameter Spaces for Complex Algorithms. R Package Version 0.10.0. 2022. Available online: https://CRAN.R-project.org/package=paradox (accessed on 9 November 2022).
- Kuhn, M. Caret: Classification and Regression Training. R Package Version 6.0-93. 2022. Available online: https://CRAN.R-project.org/package=caret (accessed on 9 November 2022).
- Chang, J.H.; Gehrke, C.; Prabhakar, R.; Gill, S.; Wada, M.; Joon, D.L.; Khoo, V. RADBIOMOD: A simple program for utilising biological modelling in radiotherapy plan evaluation. Phys. Medica 2015, 32, 248–254. [Google Scholar] [CrossRef]
- Burman, C.; Kutcher, G.; Emami, B.; Goitein, M. Fitting of normal tissue tolerance data to an analytic function. Int. J. Radiat. Oncol. 1991, 21, 123–135. [Google Scholar] [CrossRef]
- Kishan, A.U.; Cameron, R.B.; Wang, P.C.; Alexander, S.; Qi, S.X.; Low, D.A.; Kupelian, P.A.; Steinberg, M.L.; Lee, J.M.; Selch, M.T.; et al. Tomotherapy improves local control and changes failure patterns in locally advanced malignant pleural mesothelioma. Pract. Radiat. Oncol. 2015, 5, 366–373. [Google Scholar] [CrossRef]
- Taioli, E.; Wolf, A.S.; Camacho-Rivera, M.; Flores, R.M. Women with Malignant Pleural Mesothelioma Have a Threefold Better Survival Rate Than Men. Ann. Thorac. Surg. 2014, 98, 1020–1024. [Google Scholar] [CrossRef]
- Wolf, A.S.; Richards, W.G.; Tilleman, T.R.; Chirieac, L.; Hurwitz, S.; Bueno, R.; Sugarbaker, D.J. Characteristics of Malignant Pleural Mesothelioma in Women. Ann. Thorac. Surg. 2010, 90, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, R.R.; Yang, C.-F.J.; Speicher, P.J.; Gulack, B.C.; Hartwig, M.G.; D'Amico, T.A.; Harpole, D.H.; Berry, M.F. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J. Surg. Res. 2015, 196, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Le Stang, N.; Burke, L.; Blaizot, G.; Gibbs, A.R.; Lebailly, P.; Clin, B.; Girard, N.; Galateau-Sallé, F.; Networks, F.T.M.A.E. Differential Diagnosis of Epithelioid Malignant Mesothelioma with Lung and Breast Pleural Metastasis: A Systematic Review Compared with a Standardized Panel of Antibodies—A New Proposal That May Influence Pathologic Practice. Arch. Pathol. Lab. Med. 2019, 144, 446–456. [Google Scholar] [CrossRef]
- Galetta, D.; Catino, A.; Misino, A.; Logroscino, A.; Fico, M. Sarcomatoid Mesothelioma: Future Advances in Diagnosis, Biomolecular Assessment, and Therapeutic Options in a Poor-Outcome Disease. Tumori J. 2015, 102, 127–130. [Google Scholar] [CrossRef]
- Verma, V.; Ahern, C.A.; Berlind, C.G.; Lindsay, W.D.; Shabason, J.; Sharma, S.; Culligan, M.J.; Grover, S.; Friedberg, J.S.; Simone, C.B. Survival by Histologic Subtype of Malignant Pleural Mesothelioma and the Impact of Surgical Resection on Overall Survival. Clin. Lung Cancer 2018, 19, e901–e912. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, S.; Danel, C.; Scherpereel, A.; Mazières, J.; Lantuejoul, S.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Gounant, V.; Antoine, M.; et al. Shorter Survival in Malignant Pleural Mesothelioma Patients with High PD-L1 Expression Associated with Sarcomatoid or Biphasic Histology Subtype: A Series of 214 Cases From the Bio-MAPS Cohort. Clin. Lung Cancer 2019, 20, e564–e575. [Google Scholar] [CrossRef]
- Murphy, D.J.; Gill, R.R. Overview of treatment related complications in malignant pleural mesothelioma. Ann. Transl. Med. 2017, 5, 235. [Google Scholar] [CrossRef]
- Allen, A.M.; Czerminska, M.; Jänne, P.A.; Sugarbaker, D.J.; Bueno, R.; Harris, J.R.; Court, L.; Baldini, E.H. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int. J. Radiat. Oncol. 2006, 65, 640–645. [Google Scholar] [CrossRef]
- Nabavi, N.; Wei, J.; Lin, D.; Collins, C.C.; Gout, P.W.; Wang, Y. Pre-clinical models for malignant mesothelioma research: From chemical-induced to patient-derived cancer xenografts. Front. Genet. 2018, 9, 232. [Google Scholar] [CrossRef]
- Testa, J.R.; Berns, A. Preclinical Models of Malignant Mesothelioma. Front. Oncol. 2020, 10, 101. [Google Scholar] [CrossRef]
- Feinstein, J.; Kittaneh, M. Biomarkers Progress and Therapeutic Implications in Malignant Mesothelioma. In Mesothelioma; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/73196 (accessed on 9 November 2022).
- Zhang, Y.; Wong, G.; Mann, G.; Muller, S.; Yang, J.Y.H. SurvBenchmark: Comprehensive benchmarking study of survival analysis methods using both omics data and clinical data. GigaScience 2022, 11, giac071. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; Galjart, B.; Van Vugt, J.L.A.; Bagante, F.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; et al. Performance of prognostic scores and staging systems in predicting long-term survival outcomes after surgery for intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2017, 116, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Escanilla, N.S.; Hellerstein, L.; Kleiman, R.; Kuang, Z.; Shull, J.D.; Page, D. Recursive Feature Elimination by Sensitivity Testing. Proc. Int. Conf. Mach. Learn. Appl. 2018, 2018, 40–47. [Google Scholar]
- Borboudakis, G.; Tsamardinos, I. Forward-backward selection with early dropping. J. Mach. Learn. Res. 2019, 20, 276–314. [Google Scholar]
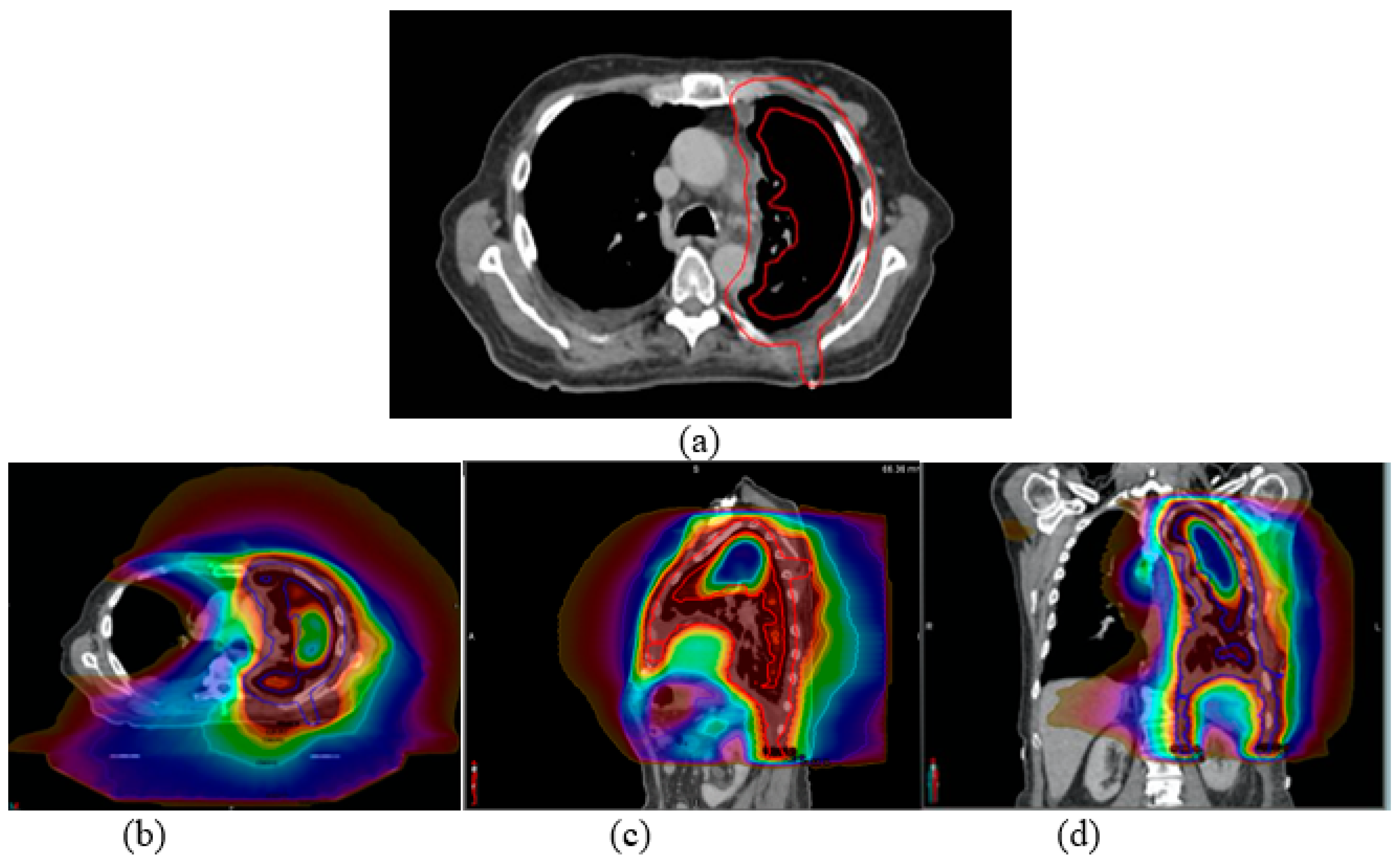
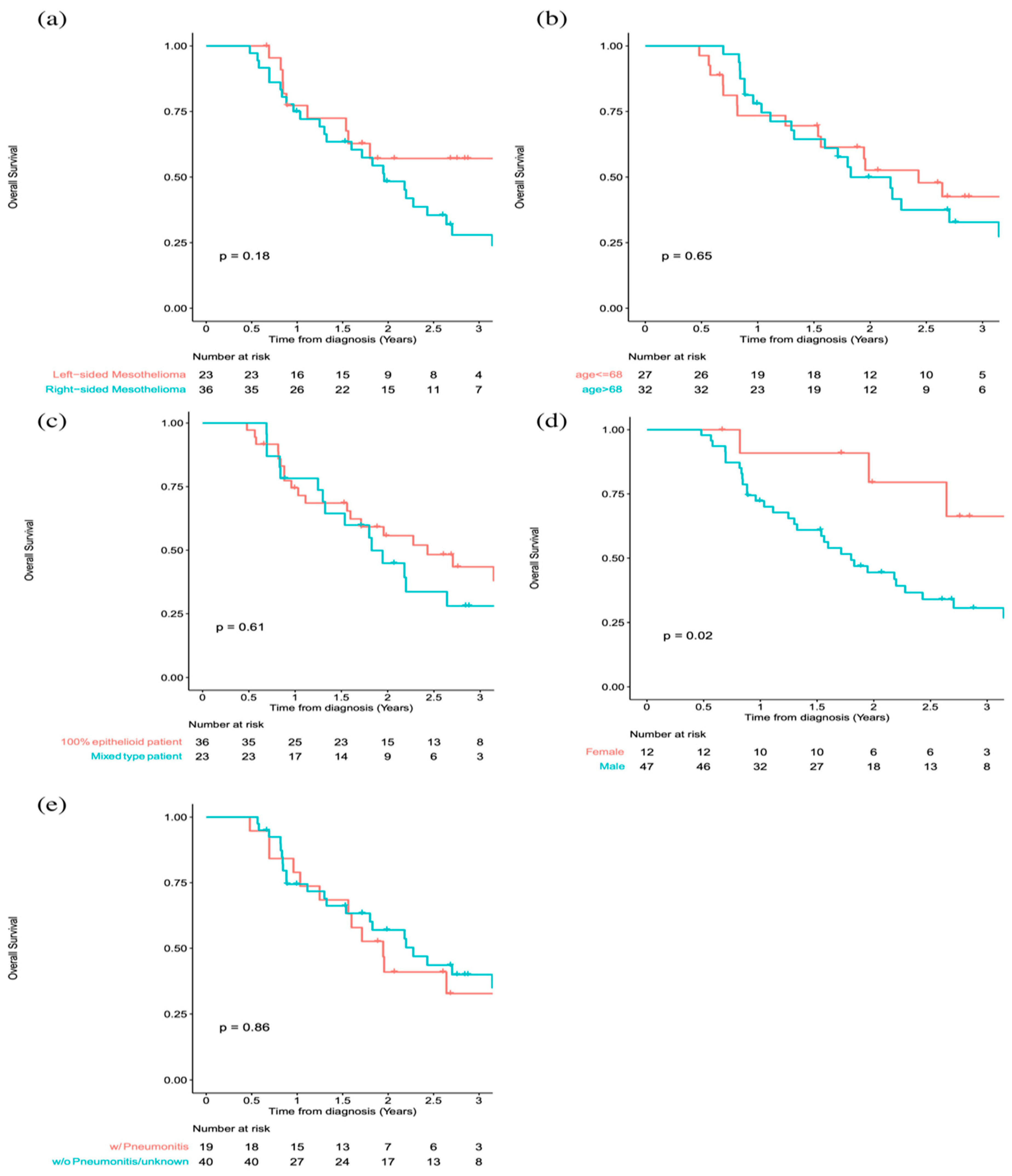
| Variables | Left-Sided Mesothelioma (n = 23) | Right-Sided Mesothelioma (n = 37) |
|---|---|---|
| Mean ± SD * | Mean ± SD * | |
| Age (year) | 65.96 ± 10.4 | 69.25 ± 7.87 |
| Gender | ||
| Male | 16 (69.6%) | 32 (86.5%) |
| Female | 7 (30.4%) | 5 (13.5%) |
| Staging | ||
| T1 | 1 | 1 |
| T2 | 6 | 5 |
| T3 | 12 | 26 |
| T4 | 4 | 5 |
| N0 | 10 | 13 |
| N1 | 5 | 6 |
| N2 | 8 | 18 |
| M0 | 22 | 37 |
| M1 | 1 | 0 |
| Histologic subtype | ||
| Epithelioid | 13 | 22 |
| Sarcomatoid | 0 | 0 |
| Mixed histology | 10 | 15 |
| Surgery type | ||
| Pleurectomy and decortication | 23 | 36 ** |
| LSM * (n = 23) | RSM * (n = 37) | LSM vs. RSM | |||
|---|---|---|---|---|---|
| Dosimetric Variables | Median | IQR ** | Median | IQR ** | p-Value |
| Target | |||||
| PTV volume (cc) | 2107 | 750 | 2193 | 574 | 0.82 |
| V100% (%) | 94.3 | 1.0 | 94.2 | 2.0 | 0.42 |
| Lung | |||||
| Ipsi lung mean dose (Gy) | 38.9 | 4.5 | 37.1 | 3.5 | 0.02 |
| Ipsi lung V20Gy (%) | 92.0 | 6.9 | 86.1 | 9.3 | 0.001 |
| Ipsilateral lung volume (cc) | 989.5 | 322 | 1154.2 | 498.4 | 0.06 |
| Ipsilateral lung-PTV volume (cc) | 593.4 | 273.8 | 730.5 | 315.3 | 0.01 |
| Ipsi lung-PTV mean dose (Gy) | 34.0 | 4.5 | 31.8 | 4.4 | 0.002 |
| Ipsi lung-PTV V20Gy (%) | 86.7 | 10.6 | 77.2 | 15.3 | <0.001 |
| Contralateral lung volume (cc) | 1727 | 515 | 1692.3 | 377.5 | 0.68 |
| Contra lung V5Gy (%) | 49.5 | 13.0 | 48.2 | 23.5 | 0.72 |
| Contra lung mean dose (Gy) | 6.4 | 1.5 | 6.8 | 1.7 | 0.58 |
| Contra lung V20Gy (%) | 1.6 | 3.4 | 1.6 | 3.4 | 0.63 |
| Total lung volume (cc) | 2756 | 658 | 2851 | 667 | 0.39 |
| Total lung Mean Dose (Gy) | 18.3 | 2.9 | 19.2 | 1.7 | 0.13 |
| Total lung V20Gy (%) | 35.0 | 7.2 | 36.4 | 4.3 | 0.13 |
| Total lung-PTV mean dose (Gy) | 13.2 | 1.8 | 14.1 | 2.6 | 0.03 |
| Total lung-PTV V20Gy (%) | 22.3 | 7.2 | 25.4 | 4.9 | 0.07 |
| Estimated NTCP mean (%) | 9.7 | 2.4 | 9.1 | 1.6 | 0.20 |
| Kidney | |||||
| Ipsi kidney Mean dose (Gy) | 7.9 | 3.1 | 7.1 | 4.2 | 0.67 |
| Ipsi kidney D2/3 (Gy) | 3.4 | 1.3 | 4.0 | 3.4 | 0.31 |
| Contra kidney Mean dose (Gy) | 2.7 | 1.9 | 3.7 | 1.8 | 0.02 |
| Contra kidney D2/3 | 1.4 | 1.1 | 2.2 | 2.1 | 0.11 |
| Esophagus | |||||
| Mean dose (Gy) | 22.2 | 10.0 | 25.9 | 9.6 | 0.20 |
| Max dose (Gy) | 50.0 | 2.1 | 51.1 | 2.6 | 0.03 |
| Estimated NTCP mean (%) | 2.4 | 3.1 | 3.1 | 3.3 | 0.37 |
| Heart | |||||
| Mean dose (Gy) | 25.6 | 4.4 | 19.5 | 5.8 | <0.001 |
| V30Gy (%) | 30.8 | 11.9 | 19.5 | 14.1 | <0.001 |
| Spinal Cord | |||||
| Max dose (Gy) | 38.0 | 8.5 | 39.1 | 6.3 | 0.16 |
| Estimated NTCP mean (%) | 0.01 | 0.04 | 0.03 | 0.05 | 0.11 |
| Liver | |||||
| Mean dose (Gy) | 10.8 | 3.9 | 23.4 | 5.2 | <0.001 |
| V30Gy (%) | 0.8 | 4.2 | 28.8 | 14.7 | <0.001 |
| Stomach | |||||
| Mean dose (Gy) | 19.2 | 6.4 | 10.8 | 5.1 | <0.001 |
| V45Gy (%) | 7.4 | 7.7 | 4.4 | 3.0 | 0.06 |
| Estimated NTCP mean (%) | 6.9 | 5.3 | 0.3 | 0.4 | <0.001 |
| HR | Lower CI | Upper CI | p-Value | |
|---|---|---|---|---|
| Cox PH regression with dosimetric variables on the right side | ||||
| PTV_V100 | 0.77 | 0.42 | 1.42 | 0.4 |
| PTV_Max | 1.1 | 0.91 | 1.34 | 0.33 |
| PTV_Min | 1.28 | 1.11 | 1.48 | <0.001 |
| Total_Lung_PTV_Mean | 7.48 | 1.77 | 31.53 | <0.01 |
| Total_Lung_PTV_V20 | 0.53 | 0.33 | 0.85 | <0.01 |
| Ipsi_Lung_PTV_Mean | 0.85 | 0.63 | 1.14 | 0.28 |
| Contra_Lung_Volume | 1 | 1 | 1 | <0.01 |
| Contra_Lung_V5 | 0.86 | 0.78 | 0.95 | <0.01 |
| Contra_Lung_V20 | 1.71 | 1.17 | 2.5 | <0.01 |
| Esophagus_Max | 0.75 | 0.57 | 0.98 | 0.04 |
| Esophagus_Mean | 1.14 | 1.01 | 1.29 | 0.03 |
| Heart_Volume | 1.01 | 1 | 1.01 | <0.01 |
| Heart_V5 | 1.88 | 0.96 | 3.68 | 0.06 |
| (No stable fit with dosimetric variables on the left side was attained.) Cox PH regression with clinical variables | ||||
| gender_male | 4.23 | 1.22 | 14.63 | 0.02 |
| T Stage | 0.66 | 0.41 | 1.07 | 0.09 |
| N Stage | 1.60 | 1.07 | 2.38 | 0.02 |
| age | 1.02 | 0.98 | 1.06 | 0.38 |
| Cox PH regression with dosimetric and clinical variables | ||||
| Histology in Number (Epithelioid: 0; Mixed: 1) | 1.25 | 0.49 | 3.22 | 0.64 |
| gender_male | 4.54 | 0.78 | 26.37 | 0.09 |
| T Stage | 0.31 | 0.14 | 0.70 | <0.01 |
| N Stage | 2.46 | 1.38 | 4.36 | <0.01 |
| age | 1.01 | 0.97 | 1.06 | 0.56 |
| Pneumonitis | 0.93 | 0.35 | 2.49 | 0.89 |
| PTV_Side..L | 5.32 | 0.87 | 32.57 | 0.07 |
| PTV_Max | 0.87 | 0.71 | 1.08 | 0.21 |
| PTV_Mean | 1.67 | 1.01 | 2.76 | <0.05 |
| PTV_Min | 1.12 | 1.01 | 1.24 | 0.04 |
| Total_Lung_PTV_Volume | 1.00 | 1.00 | 1.00 | 0.51 |
| Ipsi_Lung_PTV_Mean | 0.93 | 0.78 | 1.11 | 0.42 |
| Esophagus_Volume | 1.01 | 0.97 | 1.06 | 0.59 |
| Esophagus_Max | 0.98 | 0.87 | 1.12 | 0.79 |
| Esophagus_Mean | 1.14 | 1.03 | 1.27 | 0.01 |
| Heart_Volume | 1.00 | 1.00 | 1.00 | 0.91 |
| Heart_V30 | 0.89 | 0.82 | 0.97 | <0.01 |
| Spinal_Cord_Volume | 1.02 | 0.99 | 1.04 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, V.R.; Chu, F.-I.; Yu, V.; Lee, A.; Low, D.; Moghanaki, D.; Lee, P.; Qi, X.S. Predicting Overall Survival for Patients with Malignant Mesothelioma Following Radiotherapy via Interpretable Machine Learning. Cancers 2023, 15, 3916. https://doi.org/10.3390/cancers15153916
Wang Z, Li VR, Chu F-I, Yu V, Lee A, Low D, Moghanaki D, Lee P, Qi XS. Predicting Overall Survival for Patients with Malignant Mesothelioma Following Radiotherapy via Interpretable Machine Learning. Cancers. 2023; 15(15):3916. https://doi.org/10.3390/cancers15153916
Chicago/Turabian StyleWang, Zitian, Vincent R. Li, Fang-I Chu, Victoria Yu, Alan Lee, Daniel Low, Drew Moghanaki, Percy Lee, and X. Sharon Qi. 2023. "Predicting Overall Survival for Patients with Malignant Mesothelioma Following Radiotherapy via Interpretable Machine Learning" Cancers 15, no. 15: 3916. https://doi.org/10.3390/cancers15153916
APA StyleWang, Z., Li, V. R., Chu, F.-I., Yu, V., Lee, A., Low, D., Moghanaki, D., Lee, P., & Qi, X. S. (2023). Predicting Overall Survival for Patients with Malignant Mesothelioma Following Radiotherapy via Interpretable Machine Learning. Cancers, 15(15), 3916. https://doi.org/10.3390/cancers15153916









