Novel Discovery of the Somatostatin Receptor (SSTR2) in Pleomorphic Adenomas via Immunohistochemical Analysis of Tumors of the Salivary Glands
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Z.; Li, L.; Wang, L.; Hu, Y.; Li, J. Salivary Gland Neoplasms in Oral and Maxillofacial Regions: A 23-Year Retrospective Study of 6982 Cases in an Eastern Chinese Population. Int. J. Oral Maxillofac. Surg. 2010, 39, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Thielker, J.; Grosheva, M.; Ihrler, S.; Wittig, A.; Guntinas-Lichius, O. Contemporary Management of Benign and Malignant Parotid Tumors. Front. Surg. 2018, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aro, K.; Valle, J.; Tarkkanen, J.; Mäkitie, A.; Atula, T. Repeatedly Recurring Pleomorphic Adenoma: A Therapeutic Challenge. Acta Otorhinolaryngol. Ital. 2019, 39, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Koochakzadeh, S.; Neskey, D.M.; Nguyen, S.A.; Lentsch, E.J. Carcinoma Ex Pleomorphic Adenoma: A Review of Incidence, Demographics, Risk Factors, and Survival. Am. J. Otolaryngol. 2019, 40, 102279. [Google Scholar] [CrossRef]
- Kiciński, K.; Mikaszewski, B.; Stankiewicz, C. Risk Factors for Recurrence of Pleomorphic Adenoma. Otolaryngol. Pol. 2016, 70, 1–7. [Google Scholar] [CrossRef]
- Christe, A.; Waldherr, C.; Hallett, R.; Zbaeren, P.; Thoeny, H. MR Imaging of Parotid Tumors: Typical Lesion Characteristics in MR Imaging Improve Discrimination between Benign and Malignant Disease. AJNR Am. J. Neuroradiol. 2011, 32, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Mantsopoulos, K.; Koch, M.; Iro, H. Extracapsular Dissection as Sole Therapy for Small Low-Grade Malignant Tumors of the Parotid Gland. Laryngoscope 2017, 127, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Mansour, N.; Bas, M.; Stock, K.F.; Strassen, U.; Hofauer, B.; Knopf, A. Multimodal Ultrasonographic Pathway of Parotid Gland Lesions. Ultraschall Med. 2017, 38, 166–173. [Google Scholar] [CrossRef]
- Altin, F.; Alimoglu, Y.; Acikalin, R.M.; Yasar, H. Is Fine Needle Aspiration Biopsy Reliable in the Diagnosis of Parotid Tumors? Comparison of Preoperative and Postoperative Results and the Factors Affecting Accuracy. Braz. J. Otorhinolaryngol. 2019, 85, 275–281. [Google Scholar] [CrossRef]
- Rameeza, A.; Hemalata, M. Fine-Needle Aspiration Cytology of Salivary Gland Lesions. J. Oral Maxillofac. Pathol. 2022, 26, 52. [Google Scholar] [CrossRef]
- Silva, W.P.P.; Stramandinoli-Zanicotti, R.T.; Schussel, J.L.; Ramos, G.H.A.; Ioshi, S.O.; Sassi, L.M. Accuracy, Sensitivity and Specificity of Fine Needle Aspiration Biopsy for Salivary Gland Tumors: A Retrospective Study from 2006 to 2011. Asian Pac. J. Cancer Prev. 2016, 17, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Jethwa, A.R.; Khariwala, S.S.; Johnson, J.; Shin, J.J. Sensitivity, Specificity, and Posttest Probability of Parotid Fine-Needle Aspiration: A Systematic Review and Meta-Analysis. Otolaryngol. Neck Surg. Off. J. Am. Acad. Otolaryngol. Neck Surg. 2016, 154, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katabi, N.; Xu, B.; Jungbluth, A.A.; Zhang, L.; Shao, S.Y.; Lane, J.; Ghossein, R.; Antonescu, C.R. PLAG1 Immunohistochemistry Is a Sensitive Marker for Pleomorphic Adenoma: A Comparative Study with PLAG1 Genetic Abnormalities. Histopathology 2018, 72, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.; Fonseca, I.; Roque, L.; Pereira, T.; Ribeiro, C.; Bullerdiek, J.; Soares, J. PLAG1 Gene Alterations in Salivary Gland Pleomorphic Adenoma and Carcinoma Ex-Pleomorphic Adenoma: A Combined Study Using Chromosome Banding, in Situ Hybridization and Immunocytochemistry. Mod. Pathol. 2005, 18, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, A.A.; Altemani, A.; de Araujo, N.S.; Texeira, L.N.; de Araújo, V.C.; Soares, A.B. Estrogen Receptor, Progesterone Receptor, and HER-2 Expression in Recurrent Pleomorphic Adenoma. Clin. Pathol. 2019, 12, 2632010X19873384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, S.T.; Netea-Maier, R.T.; Aarntzen, E.J.H.G. 68Ga-DOTA-TOC Uptake in Pleomorphic Adenoma. Clin. Nucl. Med. 2018, 43, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, T.D.; Boy, C.; Bockisch, A.; Kotzerke, J.; Buchmann, I.; Ezziddin, S.; Scheidhauer, K.; Krause, B.J.; Schmidt, D.; Amthauer, H.; et al. Peptide receptor radionuclide therapy for patients with somatostatin receptor expressing tumours. German Guideline (S1). Nuklearmedizin 2015, 54, 1–11, quiz N2. [Google Scholar]
- Rai, U.; Thrimawithana, T.R.; Valery, C.; Young, S.A. Therapeutic Uses of Somatostatin and Its Analogues: Current View and Potential Applications. Pharmacol. Ther. 2015, 152, 98–110. [Google Scholar] [CrossRef]
- Mackie, E.J.; Trechsel, U.; Bruns, C. Somatostatin Receptors Are Restricted to a Subpopulation of Osteoblast-like Cells during Endochondral Bone Formation. Development 1990, 110, 1233–1239. [Google Scholar] [CrossRef]
- Lerner, U.H.; Persson, E.; Lundberg, P. Chapter 47—Kinins and neuro-osteogenic factors. In Principles of Bone Biology, 3rd ed.; Bilezikian, J.P., Raisz, L.G., Martin, T.J., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1025–1057. ISBN 978-0-12-373884-4. [Google Scholar]
- Satpathy, Y.; Spadigam, A.E.; Dhupar, A.; Syed, S. Epithelial and Stromal Patterns of Pleomorphic Adenoma of Minor Salivary Glands: A Histopathological and Histochemical Study. J. Oral Maxillofac. Pathol. 2014, 18, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Gubod, E.B.R.; Ramanathan, A.; Chong Mei Yee, S.; Tilakaratne, W.M. Bone Formation in Pleomorphic Adenoma: A Case Report. Cureus 2023, 14, e22868. [Google Scholar] [CrossRef] [PubMed]
- Kotzerke, J.; Buesser, D.; Naumann, A.; Runge, R.; Huebinger, L.; Kliewer, A.; Freudenberg, R.; Brogsitter, C. Epigenetic-Like Stimulation of Receptor Expression in SSTR2 Transfected HEK293 Cells as a New Therapeutic Strategy. Cancers 2022, 14, 2513. [Google Scholar] [CrossRef] [PubMed]
- Remes, S.M.; Leijon, H.L.; Vesterinen, T.J.; Arola, J.T.; Haglund, C.H. Immunohistochemical Expression of Somatostatin Receptor Subtypes in a Panel of Neuroendocrine Neoplasias. J. Histochem. Cytochem. 2019, 67, 735–743. [Google Scholar] [CrossRef]
- Tripathy, K.; Mishra, A.; Singh, A.K.; Panda, P.L.; Mahapatra, A.; Lenka, A. Immunocytochemistry Using Liquid-Based Cytology: A Tool in Hormone Receptor Analysis of Breast Cancer. J. Cytol. 2018, 35, 260–264. [Google Scholar] [CrossRef]
- Konukiewitz, B.; Schlitter, A.M.; Jesinghaus, M.; Pfister, D.; Steiger, K.; Segler, A.; Agaimy, A.; Sipos, B.; Zamboni, G.; Weichert, W.; et al. Somatostatin Receptor Expression Related to TP53 and RB1 Alterations in Pancreatic and Extrapancreatic Neuroendocrine Neoplasms with a Ki67-Index above 20%. Mod. Pathol. 2017, 30, 587–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hothorn, T.; Zeileis, A. Partykit: A Modular Toolkit for Recursive Partytioning in R. J. Mach. Learn. Res. 2015, 16, 3905–3909. [Google Scholar]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Luers, J.C.; Guntinas-Lichius, O.; Klussmann, J.P.; Küsgen, C.; Beutner, D.; Grosheva, M. The Incidence of Warthin Tumours and Pleomorphic Adenomas in the Parotid Gland over a 25-Year Period. Clin. Otolaryngol. 2016, 41, 793–797. [Google Scholar] [CrossRef]
- Franzen, A.M.; Kaup Franzen, C.; Guenzel, T.; Lieder, A. Increased Incidence of Warthin Tumours of the Parotid Gland: A 42-Year Evaluation. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2593–2598. [Google Scholar] [CrossRef]
- Psychogios, G.; Vlastos, I.; Thölken, R.; Zenk, J. Warthin’s Tumour Seems to Be the Most Common Benign Neoplasm of the Parotid Gland in Germany. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2081–2084. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Laissue, J.A.; Gebbers, J.O. Somatostatin and Vasoactive Intestinal Peptide Receptors in Human Mesenchymal Tumors: In Vitro Identification. Cancer Res. 1996, 56, 1922–1931. [Google Scholar] [PubMed]
- To, V.S.; Chan, J.Y.; Tsang, R.K.; Wei, W.I. Review of Salivary Gland Neoplasms. ISRN Otolaryngol. 2012, 2012, 872982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zheng, J.; Lu, X.; Wang, Y.; Meng, F.; Zhao, J.; Guo, C.; Yu, L.; Zhu, Z.; Zhang, T. Radiomics-Based Comparison of MRI and CT for Differentiating Pleomorphic Adenomas and Warthin Tumors of the Parotid Gland: A Retrospective Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Oh, J.; Bukhari, H.; Ng, T.; Chau, N.; Tran, E. Primary Parotid Merkel Type Small Cell Neuroendocrine Carcinoma with Oligometastasis to the Brain and Adrenal Gland: Case Report and Review of Literature. Head Neck Pathol. 2021, 15, 311–318. [Google Scholar] [CrossRef]
- Said-Al-Naief, N.; Sciandra, K.; Gnepp, D.R. Moderately Differentiated Neuroendocrine Carcinoma (Atypical Carcinoid) of the Parotid Gland: Report of Three Cases with Contemporary Review of Salivary Neuroendocrine Carcinomas. Head Neck Pathol. 2013, 7, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin Receptor PET Ligands—The next Generation for Clinical Practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar]
- Bentestuen, M.; Gossili, F.; Almasi, C.E.; Zacho, H.D. Prevalence and Significance of Incidental Findings on 68 Ga-DOTA-Conjugated Somatostatin Receptor-Targeting Peptide PET/CT: A Systematic Review of the Literature. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2022, 22, 44. [Google Scholar] [CrossRef]
- Kunikowska, J.; Królicki, L.; Pawlak, D.; Zerizer, I.; Mikołajczak, R. Semiquantitative Analysis and Characterization of Physiological Biodistribution of (68)Ga-DOTA-TATE PET/CT. Clin. Nucl. Med. 2012, 37, 1052–1057. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and Tumoral Uptake of (68)Ga-DOTATATE: Standardized Uptake Values and Challenges in Interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef]
- Punjabi, L.S.; Daryl Seow, M.K.; Ahmed, S.S. Lymphoepithelial Carcinoma of the Salivary Gland-A Great Cytologic Mimicker in the Head and Neck Region, and the First Report of SSTR2 Expression on Cytologic Material. Diagn. Cytopathol. 2022, 50, 525–528. [Google Scholar] [CrossRef]
- Viswanathan, K.; Sadow, P.M. Somatostatin Receptor 2 (SSTR2) Is Highly Sensitive and Specific for Epstein-Barr Virus-Associated Nasopharyngeal Carcinoma. Hum. Pathol. 2021, 117, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Meirovitz, A.; Shouchane-Blum, K.; Maly, A.; Bersudski, E.; Hirshoren, N.; Abrams, R.; Popovtzer, A.; Orevi, M.; Weinberger, J. The Potential of Somatostatin Receptor 2 as a Novel Therapeutic Target in Salivary Gland Malignant Tumors. J. Cancer Res. Clin. Oncol. 2021, 147, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Glas, A.S.; Vermey, A.; Hollema, H.; Robinson, P.H.; Roodenburg, J.L.; Nap, R.E.; Plukker, J.T. Surgical Treatment of Recurrent Pleomorphic Adenoma of the Parotid Gland: A Clinical Analysis of 52 Patients. Head Neck 2001, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Kawata, R.; Higashino, M.; Nishikawa, S.; Inui, T.; Terada, T.; Haginomori, S.I.; Kurisu, Y.; Hirose, Y. Recurrent Benign Pleomorphic Adenoma of the Parotid Gland: Facial Nerve Identification and Risk Factors for Facial Nerve Paralysis at Re-Operation. Auris Nasus Larynx 2019, 46, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.R.; Li, T.; Ter-Minassian, M.; Yang, J.; Chan, J.A.; Brais, L.K.; Masugi, Y.; Thiaglingam, A.; Brooks, N.; Nishihara, R.; et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016, 45, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20, 3049. [Google Scholar] [CrossRef] [Green Version]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R.; Group, P.S. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.T.; Wolin, E.M.; Ruszniewski, P.; Investigators, C. Lanreotide Autogel/Depot in Advanced Enteropancreatic Neuroendocrine Tumours: Final Results of the CLARINET Open-Label Extension Study. Endocrine 2021, 71, 502–513. [Google Scholar] [CrossRef]



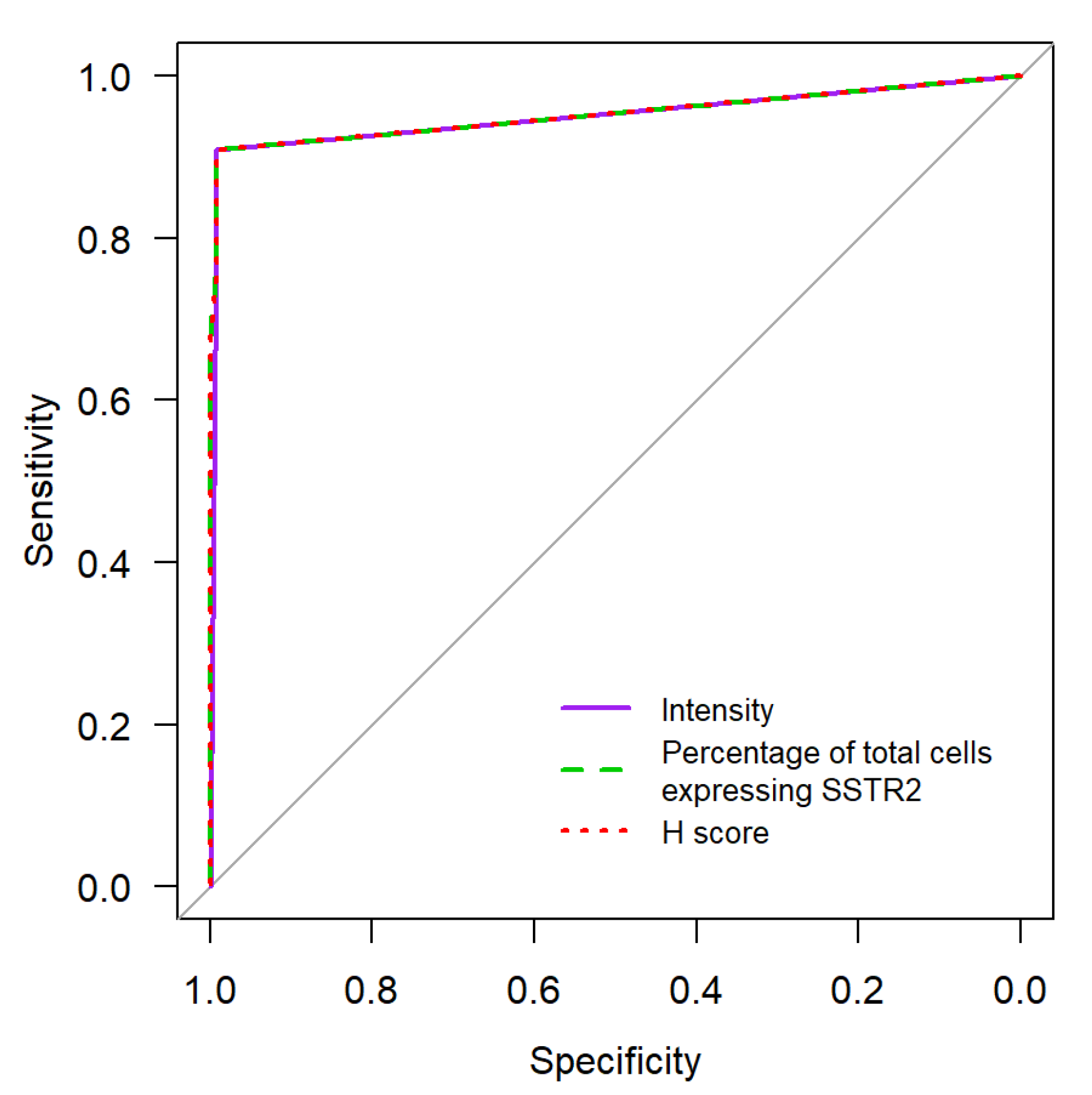
| Staining | Score | Evaluation |
|---|---|---|
| No staining was observed; faint membrane staining was observed in ≤10% of tumor cells | 0 | None |
| Incomplete, barely visible staining in >10% of tumor cells | 1 | Mild |
| Incomplete and/or weak circumferential staining in >10% of tumor cells, or complete, intense staining in ≤10% of tumor cells | 2 | Moderate |
| Complete, intense staining in >10% of tumor cells | 3 | Strong |
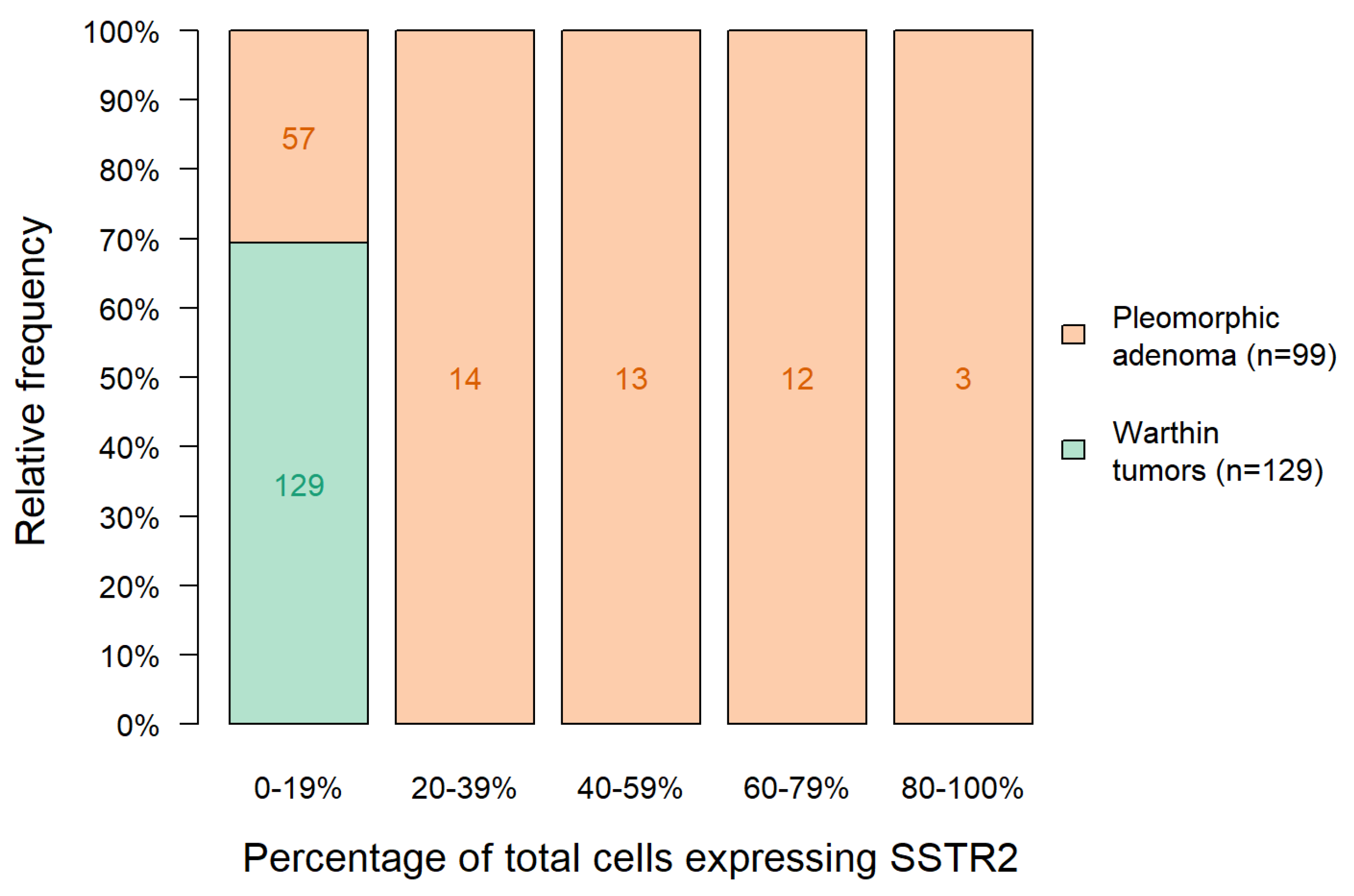
| Percentage of Cells Expressing SSTR2 | 0–19% | 20–39% | 40–59% | 60–79% | 80–100% | Total |
|---|---|---|---|---|---|---|
| Warthin tumor | 129 | 0 | 0 | 0 | 0 | 129 |
| Pleomorphic adenoma (PA) | 57 | 14 | 13 | 12 | 3 | 99 |
| Basal cell adenoma | 9 | 0 | 0 | 0 | 0 | 9 |
| Oncocytoma | 7 | 0 | 0 | 0 | 0 | 7 |
| Primary squamous cell cancer | 3 | 0 | 0 | 0 | 0 | 3 |
| MALT | 2 | 0 | 0 | 0 | 0 | 2 |
| Mucoepidermoid carcinoma | 2 | 0 | 0 | 0 | 0 | 2 |
| Myoepithelioma | 2 | 0 | 0 | 0 | 0 | 2 |
| Secretory carcinoma | 1 | 0 | 0 | 0 | 0 | 1 |
| Neuroendocrine Carcinoma | 0 | 0 | 0 | 0 | 1 | 1 |
| Lymphoma | 1 | 0 | 0 | 0 | 0 | 1 |
| Carcinoma ex pleomorphic adenoma (CXPA) | 1 | 0 | 0 | 0 | 0 | 1 |
| Adenoid cystic carcinoma | 1 | 0 | 0 | 0 | 0 | 1 |
| Reticulary myoepithelioma | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 216 | 14 | 13 | 12 | 4 | 259 |
| Percentage of Total Cells Expressing SSTR2 | ||
|---|---|---|
| Positivity in % | All Other Tumors (n = 160) | Pleomorphic Adenoma (n = 99) |
| 0–20 | 159 | 57 |
| 20–40 | 0 | 14 |
| 40–60 | 0 | 13 |
| 60–80 | 0 | 12 |
| 80–100 | 1 * | 3 |
| Intensity of SSTR2 Expression (Grading: 0–3) * | |||||
|---|---|---|---|---|---|
| Grading | None (0) | Mild (1) | Moderate (2) | Strong (3) | Total |
| Warthin tumor (Cystadenolymphoma) | 128 | 0 | 0 | 1 | 129 |
| Pleomorphic adenoma (PA) | 9 | 0 | 8 | 82 | 99 |
| Basal cell adenoma | 5 | 1 | 0 | 3 | 9 |
| Oncocytoma | 7 | 0 | 0 | 0 | 7 |
| Primary squamous cell cancer | 1 | 0 | 1 | 1 | 3 |
| MALT | 1 | 0 | 1 | 0 | 2 |
| Mucoepidermoid carcinoma | 1 | 0 | 1 | 0 | 2 |
| Myoepithelioma | 2 | 0 | 0 | 0 | 2 |
| Secretory carcinoma | 1 | 0 | 0 | 0 | 1 |
| Neuroendocrine Carcinoma | 0 | 0 | 0 | 1 | 1 |
| Lymphoma | 1 | 0 | 0 | 0 | 1 |
| Carcinoma ex pleomorphic adenoma (CXPA) | 0 | 0 | 0 | 1 | 1 |
| Adenoid cystic carcinoma | 0 | 1 | 0 | 0 | 1 |
| Reticulary myoepithelioma | 1 | 0 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, F.; Hofauer, B.; Wirth, M.; Wollenberg, B.; Stögbauer, F.; Notohamiprodjo, S.; Haller, B.; Reschke, R.; Knopf, A.; Strassen, U. Novel Discovery of the Somatostatin Receptor (SSTR2) in Pleomorphic Adenomas via Immunohistochemical Analysis of Tumors of the Salivary Glands. Cancers 2023, 15, 3917. https://doi.org/10.3390/cancers15153917
Johnson F, Hofauer B, Wirth M, Wollenberg B, Stögbauer F, Notohamiprodjo S, Haller B, Reschke R, Knopf A, Strassen U. Novel Discovery of the Somatostatin Receptor (SSTR2) in Pleomorphic Adenomas via Immunohistochemical Analysis of Tumors of the Salivary Glands. Cancers. 2023; 15(15):3917. https://doi.org/10.3390/cancers15153917
Chicago/Turabian StyleJohnson, Felix, Benedikt Hofauer, Markus Wirth, Barbara Wollenberg, Fabian Stögbauer, Susan Notohamiprodjo, Bernhard Haller, Robin Reschke, Andreas Knopf, and Ulrich Strassen. 2023. "Novel Discovery of the Somatostatin Receptor (SSTR2) in Pleomorphic Adenomas via Immunohistochemical Analysis of Tumors of the Salivary Glands" Cancers 15, no. 15: 3917. https://doi.org/10.3390/cancers15153917
APA StyleJohnson, F., Hofauer, B., Wirth, M., Wollenberg, B., Stögbauer, F., Notohamiprodjo, S., Haller, B., Reschke, R., Knopf, A., & Strassen, U. (2023). Novel Discovery of the Somatostatin Receptor (SSTR2) in Pleomorphic Adenomas via Immunohistochemical Analysis of Tumors of the Salivary Glands. Cancers, 15(15), 3917. https://doi.org/10.3390/cancers15153917







