MRD as Biomarker for Response to Donor Lymphocyte Infusion after Allogeneic Hematopoietic Cell Transplantation in Patients with AML
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatment
2.2. Cytogenetic and Molecular Analysis
2.3. Error-Corrected Sequencing of Patient-Specific Mutations
2.4. Bioinformatics and Statistical Analyses
3. Results
3.1. Patient and Treatment Characteristics
3.2. Impact of Mutations and Baseline Characteristics at Diagnosis on Outcome of Patients Receiving DLIs
3.3. Remission and MRD Status at the Time of First DLI and Its Associations
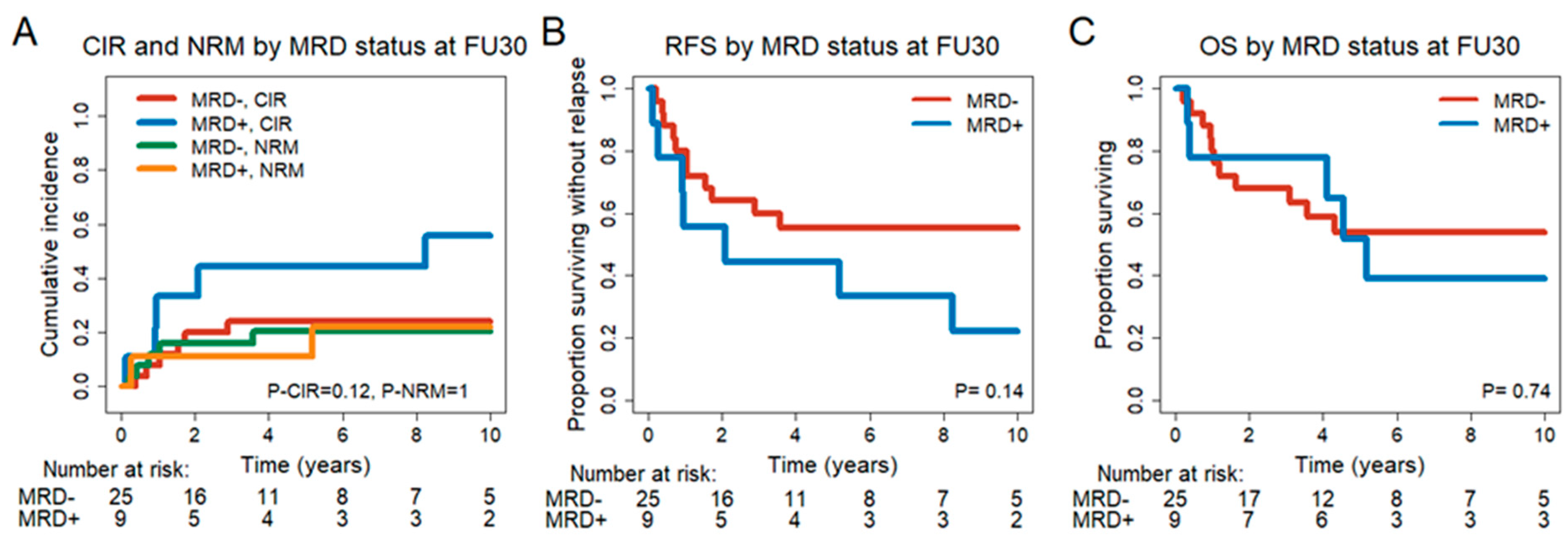
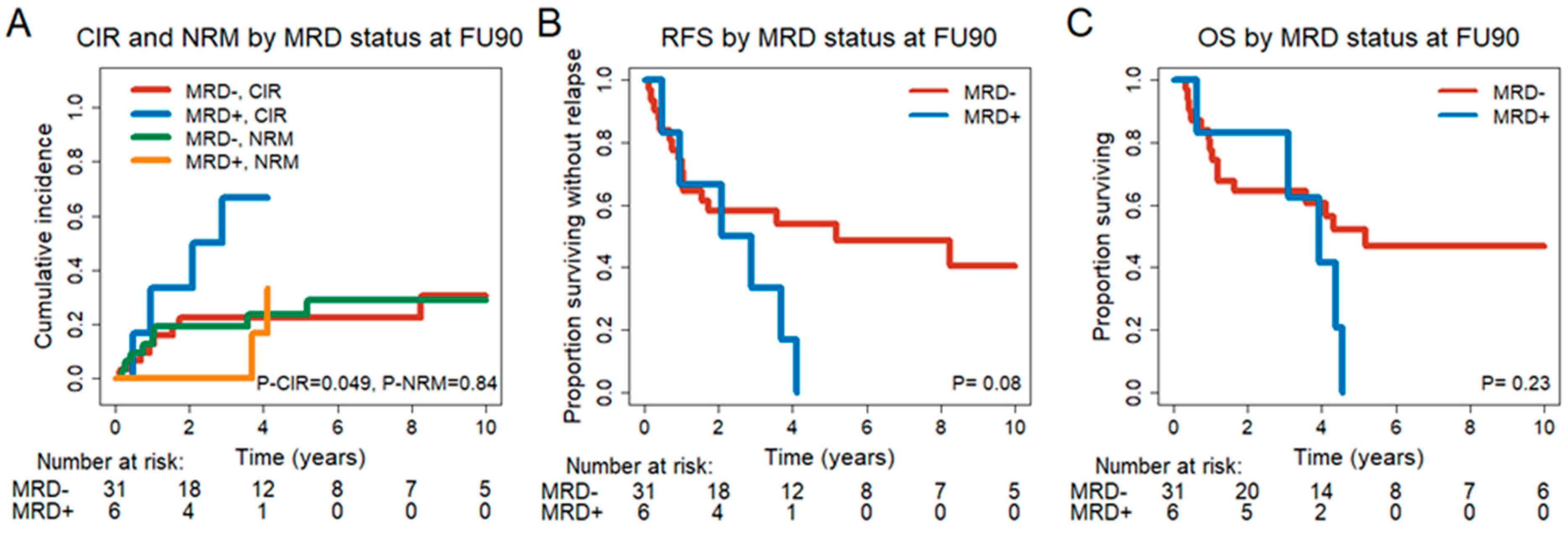
3.4. MRD Conversion Day 30 and Day 90 after DLIs
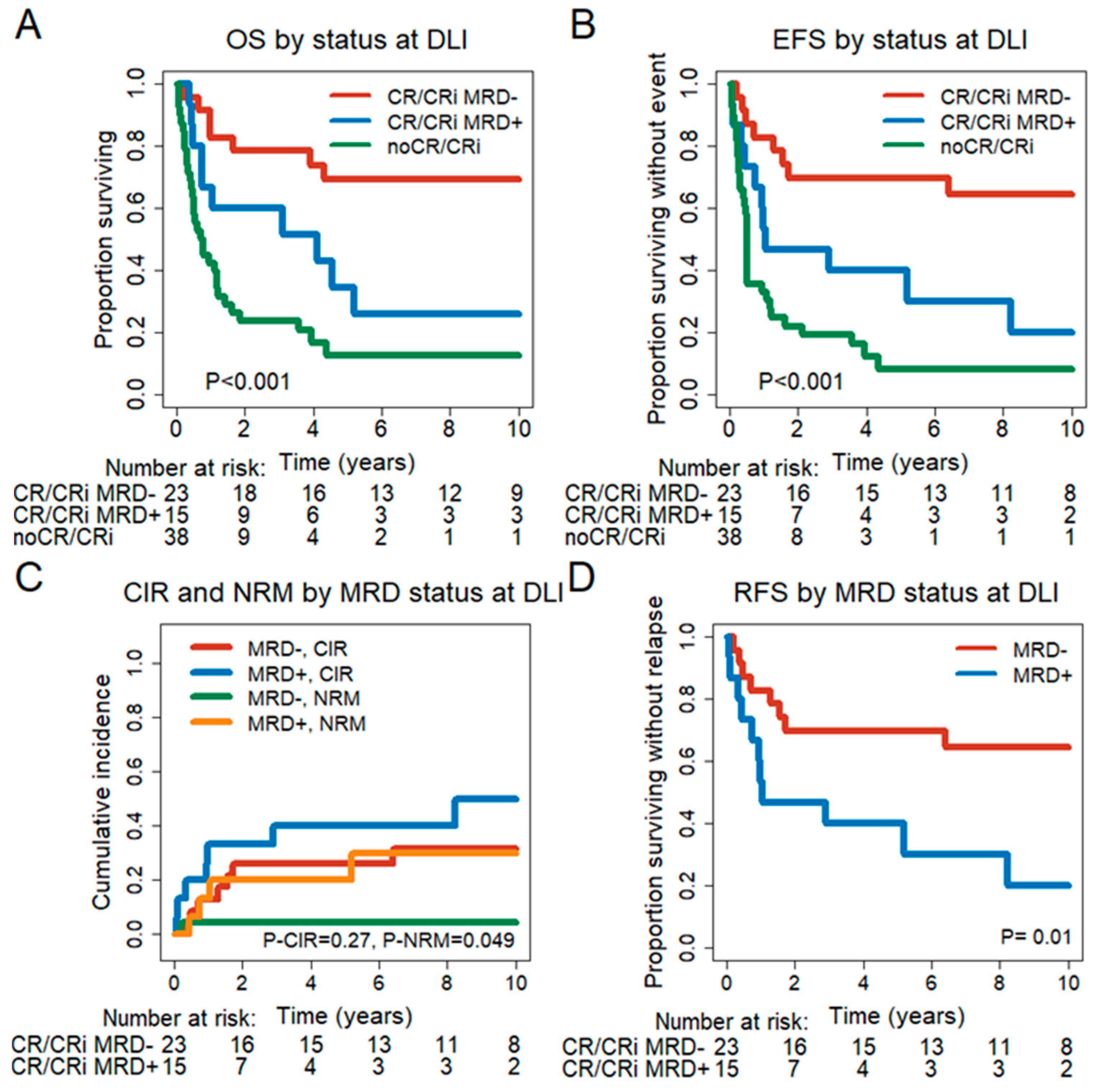
3.5. Prognostic Effect of Remission and MRD Status at the Time of First DLI
3.6. Prognostic Effect of MRD Status in CR/CRi Patients 30 and 90 Days after DLI
3.7. Impact of DLIs on MRD− Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sweeney, C.; Vyas, P. The Graft-Versus-Leukemia Effect in AML. Front. Oncol. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Leotta, S.; Condorelli, A.; Sciortino, R.; Milone, G.A.; Bellofiore, C.; Garibaldi, B.; Schininà, G.; Spadaro, A.; Cupri, A.; Milone, G. Prevention and Treatment of Acute Myeloid Leukemia Relapse after Hematopoietic Stem Cell Transplantation: The State of the Art and Future Perspectives. J. Clin. Med. 2022, 11, 253. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Nagler, A.; Niederwieser, D.; Castagna, L.; Tabrizi, R.; Stadler, M.; Kuball, J.; Cornelissen, J.; Vorlicek, J.; et al. Treatment, Risk Factors, and Outcome of Adults with Relapsed AML after Reduced Intensity Conditioning for Allogeneic Stem Cell Transplantation. Blood 2012, 119, 1599–1606. [Google Scholar] [CrossRef]
- Zilberberg, J.; Feinman, R.; Korngold, R. Strategies for the Identification of T Cell-Recognized Tumor Antigens in Hematological Malignancies for Improved Graft-versus-Tumor Responses after Allogeneic Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1000–1007. [Google Scholar] [CrossRef]
- Orti, G.; Barba, P.; Fox, L.; Salamero, O.; Bosch, F.; Valcarcel, D. Donor Lymphocyte Infusions in AML and MDS: Enhancing the Graft-versus-Leukemia Effect. Exp. Hematol. 2017, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Mittermuller, J.; Clemm, C.; Holler, E.; Ledderose, G.; Brehm, G.; Heim, M.; Wilmanns, W. Donor Leukocyte Transfusions for Treatment of Recurrent Chronic Myelogenous Leukemia in Marrow Transplant Patients. Blood 1990, 76, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Castagna, L.; Sarina, B.; Bramanti, S.; Perseghin, P.; Mariotti, J.; Morabito, L. Donor Lymphocyte Infusion after Allogeneic Stem Cell Transplantation. Transfus. Apher. Sci. 2016, 54, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.J. Graft-versus-Leukemia Effects of Transplantation and Donor Lymphocytes. Blood 2008, 112, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R. Advances in Understanding the Pathogenesis of Graft-versus-Host Disease. Br. J. Haematol. 2019, 173, 190–205. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, J.H.; Kim, S.; Seol, M.; Lee, Y.S.; Lee, J.S.; Kim, W.K.; Chi, H.S.; Lee, K.H. Treatment of Relapsed Acute Myeloid Leukemia after Allogeneic Bone Marrow Transplantation with Chemotherapy Followed by G-CSF-Primed Donor Leukocyte Infusion: A High Incidence of Isolated Extramedullary Relapse. Leukemia 2004, 18, 1789–1797. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhäuser, M.; Finke, J.; Fassas, A.; Volin, L.; Gürman, G.; Maertens, J.; Bordigoni, P.; et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse after Allogeneic Stem-Cell Transplantation in Adults with Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison with Other Strategies by the EBMT Acute Leukem. J. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Sandmaier, B.M.; Inamoto, Y.; Bruno, B.; Hari, P.; Chauncey, T.; Martin, P.J.; Storb, R.; Maloney, D.G.; Storer, B.; et al. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies after Allogeneic Hematopoietic Cell Transplantation: Prognostic Relevance of the Initial CD3+ T Cell Dose. Biol. Blood Marrow Transplant. 2013, 19, 949–957. [Google Scholar] [CrossRef]
- Takami, A.; Yano, S.; Yokoyama, H.; Kuwatsuka, Y.; Yamaguchi, T.; Kanda, Y.; Morishima, Y.; Fukuda, T.; Miyazaki, Y.; Nakamae, H.; et al. Donor Lymphocyte Infusion for the Treatment of Relapsed Acute Myeloid Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective Analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell. Biol. Blood Marrow Transplant. 2014, 20, 1785–1790. [Google Scholar] [CrossRef]
- Heuser, M.; Heida, B.; Büttner, K.; Wienecke, C.P.; Teich, K.; Funke, C.; Brandes, M.; Klement, P.; Liebich, A.; Wichmann, M.; et al. Posttransplantation MRD Monitoring in Patients with AML by Next-Generation Sequencing Using DTA and Non-DTA Mutations. Blood Adv. 2021, 5, 2294–2304. [Google Scholar] [CrossRef]
- Thol, F.; Gabdoulline, R.; Liebich, A.; Klement, P.; Schiller, J.; Kandziora, C.; Hambach, L.; Stadler, M.; Koenecke, C.; Flintrop, M.; et al. Measurable Residual Disease Monitoring by Ngs before Allogeneic Hematopoietic Cell Transplantation in AML. Blood 2018, 132, 1703–1713. [Google Scholar] [CrossRef]
- Press, R.D.; Eickelberg, G.; Froman, A.; Yang, F.; Stentz, A.; Flatley, E.M.; Fan, G.; Lim, J.Y.; Meyers, G.; Maziarz, R.T.; et al. Next-Generation Sequencing-Defined Minimal Residual Disease before Stem Cell Transplantation Predicts Acute Myeloid Leukemia Relapse. Am. J. Hematol. 2019, 94, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.C.; et al. 2021 Update on MRD in Acute Myeloid Leukemia: A Consensus Document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2573–2767. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- McGowan-Jordan, J.; Simons, A.; Schmid, M. ISCN 2016: An International System for Human Cytogenomic Nomenclature. Reprint of: Cytogenetic and Genome Research 2016. Karger. 2016. Available online: https://cir.nii.ac.jp/crid/1130282272168007424 (accessed on 26 July 2023).
- Heuser, M.; Gabdoulline, R.; Löffeld, P.; Dobbernack, V.; Kreimeyer, H.; Pankratz, M.; Flintrop, M.; Liebich, A.; Klesse, S.; Panagiota, V.; et al. Individual Outcome Prediction for Myelodysplastic Syndrome (MDS) and Secondary Acute Myeloid Leukemia from MDS after Allogeneic Hematopoietic Cell Transplantation. Ann. Hematol. 2017, 96, 1361–1372. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Gray, B.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Papaemmanuil, E.; Martincorena, I.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Heuser, M.; Thol, F.; Bolli, N.; Ganly, P.; et al. Precision Oncology for Acute Myeloid Leukemia Using a Knowledge Bank Approach. Nat. Genet. 2017, 49, 332–340. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia with Genomic Evidence of Residual Disease. J. Clin. Oncol. 2020, 38, 1273. [Google Scholar] [CrossRef]
- Wong, Z.C.; Dillon, L.W.; Hourigan, C.S. Measurable Residual Disease in Patients Undergoing Allogeneic Transplant for Acute Myeloid Leukemia. Best Pr. Res. Clin. Haematol. 2023, 36, 101468. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Moon, J.H.; Ahn, J.S.; Kim, Y.K.; Lee, S.S.; Ahn, S.Y.; Jung, S.H.; Yang, D.H.; Lee, J.J.; Choi, S.H.; et al. Next-Generation Sequencing–Based Posttransplant Monitoring of Acute Myeloid Leukemia Identifies Patients at High Risk of Relapse. Blood 2018, 132, 1604–1613. [Google Scholar] [CrossRef]
- Shah, M.V.; Jorgensen, J.L.; Saliba, R.M.; Wang, S.A.; Alousi, A.M.; Andersson, B.S.; Bashir, Q.; Ciurea, S.O.; Kebriaei, P.; Marin, D.; et al. Early Post-Transplant Minimal Residual Disease Assessment Improves Risk Stratification in Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 1514–1520. [Google Scholar] [CrossRef]
- Zhao, X.S.; Yan, C.H.; Liu, D.H.; Xu, L.P.; Liu, Y.R.; Liu, K.Y.; Qin, Y.Z.; Wang, Y.; Huang, X.J. Combined Use of WT1 and Flow Cytometry Monitoring Can Promote Sensitivity of Predicting Relapse after Allogeneic HSCT without Affecting Specificity. Ann. Hematol. 2013, 92, 1111–1119. [Google Scholar] [CrossRef]
- Dey, B.R.; McAfee, S.; Colby, C.; Sackstein, R.; Saidman, S.; Tarbell, N.; Sachs, D.H.; Sykes, M.; Spitzer, T.R. Impact of Prophylactic Donor Leukocyte Infusions on Mixed Chimerism, Graft-versus-Host Disease, and Antitumor Response in Patients with Advanced Hematologic Malignancies Treated with Nonmyeloablative Conditioning and Allogeneic Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2003, 9, 320–329. [Google Scholar] [CrossRef]
- Lutz, C.; Massenkeil, G.; Nagy, M.; Neuburger, S.; Tamm, I.; Rosen, O.; Dörken, B.; Arnold, R. A Pilot Study of Prophylactic Donor Lymphocyte Infusions to Prevent Relapse in Adult Acute Lymphoblastic Leukemias after Allogeneic Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2008, 41, 805–812. [Google Scholar] [CrossRef]
- Tan, Y.; Du, K.; Luo, Y.; Shi, J.; Cao, L.; Zheng, Y.; Zheng, G.; Zhao, Y.; Ye, X.; Cai, Z.; et al. Superiority of Preemptive Donor Lymphocyte Infusion Based on Minimal Residual Disease in Acute Leukemia Patients after Allogeneic Hematopoietic Stem Cell Transplantation. Transfusion 2014, 54, 1493–1500. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Potter, V.T.; Barber, L.D.; Kulasekararaj, A.G.; Lim, Z.Y.; Pearce, R.M.; de Lavallade, H.; Kenyon, M.; Ireland, R.M.; Marsh, J.C.W.; et al. Outcome of Donor Lymphocyte Infusion after T Cell-Depleted Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2013, 19, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Antin, J.H. Donor Leukocyte Infusions in Myeloid Malignancies: New Strategies. Best Pract. Res. Clin. Haematol. 2006, 19, 737–755. [Google Scholar] [CrossRef]
- Montoya, M.; Schiavoni, G.; Mattei, F.; Gresser, I.; Belardelli, F.; Borrow, P.; Tough, D.F. Type I Interferons Produced by Dendritic Cells Promote Their Phenotypic and Functional Activation. Blood 2002, 99, 3263–3271. [Google Scholar] [CrossRef]
- Rettig, A.R.; Ihorst, G.; Bertz, H.; Lübbert, M.; Marks, R.; Waterhouse, M.; Wäsch, R.; Zeiser, R.; Duyster, J.; Finke, J. Donor Lymphocyte Infusions after First Allogeneic Hematopoietic Stem-Cell Transplantation in Adults with Acute Myeloid Leukemia: A Single-Center Landmark Analysis. Ann. Hematol. 2021, 100, 2339–2350. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M.; Stadler, M.; Finke, J.; Hurst, E.; Baron, F.; Ringden, O.; et al. Prophylactic Donor Lymphocyte Infusion after Allogeneic Stem Cell Transplantation in Acute Leukaemia—a Matched Pair Analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2019, 184, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Brecht, A.; Stadler, M.; Finke, J.; Baron, F.; Collin, M.; Bug, G.; et al. Long-Term Results and GvHD after Prophylactic and Preemptive Donor Lymphocyte Infusion after Allogeneic Stem Cell Transplantation for Acute Leukemia. Bone Marrow Transplant. 2022, 57, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Kerbage, F.; Sakr, R.; Lapierre, V.; Alexandrova, K.; Coman, T.; Leroux, S.; Lucas, N.; Pilorge, S.; Solary, E.; Bourhis, J.H.; et al. Donor Lymphocyte Infusions After Allogeneic Transplantation: A Single-Center Experience. Clin. Lymphoma Myeloma Leuk. 2020, 20, 209–211. [Google Scholar] [CrossRef]
- Yan, C.H.; Liu, D.H.; Liu, K.Y.; Xu, L.P.; Liu, Y.R.; Chen, H.; Han, W.; Wang, Y.; Qin, Y.Z.; Huang, X.J. Risk Stratification-Directed Donor Lymphocyte Infusion Could Reduce Relapse of Standard-Risk Acute Leukemia Patients after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2012, 119, 3256–3262. [Google Scholar] [CrossRef] [PubMed]
- Eefting, M.; von dem Borne, P.A.; de Wreede, L.C.; Halkes, C.J.M.; Kersting, S.; Marijt, E.W.A.; Veelken, H.; Frederik Falkenburg, J.H. Intentional Donor Lymphocyte-Induced Limited Acute Graft-versus-Host Disease Is Essential for Long-Term Survival of Relapsed Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation. Haematologica 2014, 99, 751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solomon, S.R.; Sizemore, C.A.; Zhang, X.; Brown, S.; Holland, H.K.; Morris, L.E.; Bashey, A. Preemptive DLI without Withdrawal of Immunosuppression to Promote Complete Donor T-Cell Chimerism Results in Favorable Outcomes for High-Risk Older Recipients of Alemtuzumab-Containing Reduced-Intensity Unrelated Donor Allogeneic Transplant: A Prospective. Bone Marrow Transplant. 2014, 49, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Mizuno, S.; Yano, S.; Takami, A.; Ishii, H.; Ikegame, K.; Najima, Y.; Kako, S.; Ashida, T.; Shiratori, S.; et al. Donor Lymphocyte Infusion after Haploidentical Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia. Ann. Hematol. 2022, 101, 643–653. [Google Scholar] [CrossRef]

| Characteristic | All (n = 76) | CR/CRi MRD− (n = 23) | CR/CRi MRD+ (n = 15) | no CR/CRi at DLI (n = 38) | p | p (MRD− vs. MRD+) |
|---|---|---|---|---|---|---|
| Age at diagnosis | 0.19 | 0.73 | ||||
| Median (years) | 52.9 | 50.7 | 52.8 | 55.9 | ||
| Range (years) | 17.4–67.5 | 18.3–67.5 | 30–66.9 | 17.4–66 | ||
| Patient sex | 0.049 | 0.013 | ||||
| Male—no. (%) | 42 (55) | 9 (39) | 12 (80) | 21 (55) | ||
| Female—no. (%) | 34 (45) | 14 (61) | 3 (20) | 17 (45) | ||
| Diagnosis | 0.69 | 0.46 | ||||
| De novo AML—no. (%) | 44 (58) | 15 (65) | 8 (53) | 21 (55) | ||
| sAML/tAML/MDS/AML—no. (%) | 32 (42) | 8 (35) | 7 (47) | 17 (45) | ||
| Extramedullary manifestation at diagnosis | 0.74 | 0.82 | ||||
| Yes—no. (%) | 8 (11) | 2 (9) | 1 (7) | 5 (13) | ||
| No—no. (%) | 68 (89) | 21 (91) | 14 (93) | 33 (87) | ||
| FAB subtype | 0.51 | 0.34 | ||||
| M0 + M1 + M2—no. (%) | 29 (38) | 11 (48) | 5 (33.3) | 13 (34) | ||
| M4 + M5 + M6 + M7—no. (%) | 22 (29) | 5 (22) | 5 (33.3) | 12 (32) | ||
| Not classifiable—no. (%) | 25 (33) | 7 (30) | 5 (33.3) | 13 (34) | ||
| FLT3-ITD status at diagnosis | 0.4 | 0.23 | ||||
| Mutated—no. (%) | 16 (21) | 7 (30) | 2 (13) | 7 (18) | ||
| Wild type—no. (%) | 59 (78) | 16 (70) | 13 (87) | 30 (79) | ||
| Missing—no. (%) | 1 (1) | 0 (0) | 0 (0) | 1 (3) | ||
| FLT3-TKD status at diagnosis | 0.2 | 0.07 | ||||
| Mutated—no. (%) | 4 (5) | 0 (0) | 2 (13) | 2 (5) | ||
| Wild type—no. (%) | 72 (95) | 23 (100) | 13 (87) | 36 (95) | ||
| Complex karyotype | 0.96 | 0.98 | ||||
| Yes—no. (%) | 11 (15) | 3 (13) | 2 (13) | 6 (16) | ||
| No—no. (%) | 64 (84) | 19 (83) | 13 (87) | 32 (84) | ||
| Missing—no. (%) | 1 (1) | 1 (4) | 0 (0) | 0 (0) | ||
| Monosomal karyotype | 0.66 | 0.61 | ||||
| Yes—no. (%) | 10 (13) | 3 (13) | 3 (20) | 4 (11) | ||
| No—no. (%) | 65 (86) | 19 (83) | 12 (80) | 34 (89) | ||
| Missing—no. (%) | 1 (1) | 1 (4) | 0 (0) | 0 (0) | ||
| 2022 ELN risk group | 0.77 | 0.85 | ||||
| Favorable + Intermediate—no. (%) | 31 (41) | 10 (43) | 7 (47) | 14 (37) | ||
| Adverse—no. (%) | 45 (59) | 13 (57) | 8 (53) | 24 (63) | ||
| MRC Grimwade | 0.73 | 0.48 | ||||
| Favorable + Intermediate—no. (%) | 56 (74) | 17 (74) | 10 (67) | 29 (76) | ||
| Adverse—no. (%) | 19 (25) | 5 (22) | 5 (33) | 9 (24) | ||
| Missing—no. (%) | 1 (1) | 1 (4) | 0 (0) | 0 (0) | ||
| ECOG performance status at diagnosis | 0.67 | 0.75 | ||||
| ECOG 0—no. (%) | 70 (92) | 22 (96) | 14 (93) | 34 (89) | ||
| ECOG 1—no. (%) | 6(8) | 1 (4) | 1 (7) | 4 (11) | ||
| HCT–CI at diagnosis | 0.86 | 0.65 | ||||
| 0–2—no. (%) | 67 (88) | 21 (91) | 13 (87) | 33 (87) | ||
| >2—no. (%) | 9 (12) | 2 (99 | 2 (13) | 5 (13) | ||
| WBC count at diagnosis | 0.29 | 0.4 | ||||
| Median—(×109/L) | 8 | 23.9 | 2.5 | 8.7 | ||
| Range—(×109/L) | 0.9–115.2 | 1.2–115.2 | 1.4–106.8 | 0.9–70.6 | ||
| Missing—no. (%) | 26 (34) | 6 (26) | 3 (20) | 17 (45) | ||
| Hemoglobin at diagnosis | 0.78 | 0.43 | ||||
| Median—g/dL | 9.8 | 9.8 | 9.6 | 10.7 | ||
| Range—g/dL | 5–15 | 5–14.4 | 7.9–12.3 | 5.2–15 | ||
| Missing—no. (%) | 26 (34) | 6 (26) | 3 (20) | 17 (45) | ||
| Platelet count at diagnosis | 0.77 | 0.93 | ||||
| Median—(×109/L) | 61 | 53.5 | 50.5 | 73 | ||
| Range—(×109/L) | 7–1104 | 7–212 | 23–1104 | 14–469 | ||
| Missing—no. (%) | 27 (36) | 7 (30) | 3 (20) | 17 (45) | ||
| Blasts in PB at diagnosis | 0.15 | 0.45 | ||||
| Median—% | 19.4 | 67 | 19.5 | 11 | ||
| Range—% | 0–93 | 0–93 | 0–82 | 0–86 | ||
| Missing—no. (%) | 29 (38) | 8 (35) | 3 (20) | 18 (47) | ||
| Blasts in BM at diagnosis | 0.92 | 0.9 | ||||
| Median—% | 48.4 | 40 | 46.7 | 50 | ||
| Range—% | 5–95 | 20–95 | 20–95 | 5–90 | ||
| Missing—no. (%) | 44 (58) | 15 (65) | 6 (40) | 23 (61) |
| Univariate Analysis Results | Multivariate Analysis Results | ||||||
|---|---|---|---|---|---|---|---|
| Endpoint, Cohort | Variables in the Model | HR | 95% CI | p | HR | 95% CI | p |
| EFS, n = 76 Before DLI | Extramedullary manifestation at diagnosis, yes vs. no | 2.48 | 1.11–5.57 | 0.028 | 2.41 | 1.06–5.49 | 0.036 |
| MRC Grimwade, adverse vs. favorable + intermediate | 2.06 | 1.12–3.77 | 0.020 | 1.88 | 1.02–3.47 | 0.044 | |
| No response or CR/CRi MRD+ vs. CR/CRi MRD− | 4.67 | 2.16–10.7 | <0.001 | 5.05 | 2.22–11.48 | <0.001 | |
| OS, n = 76 Before DLI | Extramedullary manifestation at diagnosis, yes vs. no | 2.89 | 1.28–6.56 | 0.011 | 3.15 | 1.35–7.36 | 0.008 |
| Complex karyotype, yes vs. no | 2.35 | 1.27–4.36 | 0.007 | 2.34 | 1.25–4.35 | 0.007 | |
| No response or CR/CRi MRD+ vs. CR/CRi MRD− | 4.67 | 2.16–10.07 | <0.001 | 5.33 | 2.23–12.76 | <0.001 | |
| CIR, n = 34 CR/CRi FU30 | Complex karyotype, yes vs. no | 3.43 | 0.87–13.56 | 0.078 | 6.72 | 1.35–33.41 | 0.02 |
| FU30 MRD+ vs. MRD− | 2.64 | 0.86–8.13 | 0.09 | 4.6 | 1.21–17.5 | 0.025 | |
| NRM, n = 34 CR/CRi FU30 | Pre DLI median age, ≤54 vs. >54 | 3.78 | 0.76–18.91 | 0.105 | 7.33 | 1.05–51.17 | 0.045 |
| Extramedullary manifestation at diagnosis, yes vs. no | 5.21 | 0.89–30.41 | 0.066 | 13.02 | 3.32–51.13 | <0.001 | |
| RFS, n = 34 CR/CRi FU30 | Complex karyotype, yes vs. no | 3.47 | 1.08–11.16 | 0.037 | 4.45 | 1.25–15.8 | 0.021 |
| CMV status alloHCT1 | 2.57 | 0.84–7.87 | 0.098 | 3.27 | 1.0–10.67 | 0.049 | |
| FU30 MRD+ vs. MRD | 2 | 0.77–5.17 | 0.154 | 3.91 | 1.31–11.65 | 0.015 | |
| OS, n = 34 CR/CRi FU30 | Extramedullary manifestation at diagnosis, yes vs. no | 5.1 | 1.39–18.67 | 0.014 | 22.69 | 3.77–136.46 | 0.001 |
| MRC Grimwade, adverse vs. favorable + intermediate | 2.87 | 1.00–8.26 | 0.05 | 4.56 | 1.36–15.34 | 0.014 | |
| CMV status alloHCT1 | 3.19 | 0.90–11.29 | 0.072 | 6.13 | 1.34–28.06 | 0.019 | |
| CIR, n = 37 CR/CRi FU90 | MRC Grimwade, adverse vs. favorable + intermediate | 2.68 | 0.84–8.53 | 0.095 | 4.02 | 1.14–14.22 | 0.031 |
| FU90 MRD+ vs. MRD− | 3.02 | 1.02–8.94 | 0.047 | 4.66 | 1.42–15.31 | 0.011 | |
| NRM, n = 37 CR/CRi FU90 | Pre DLI median age, ≤54 vs. >54 | 5.79 | 1.24–27.10 | 0.026 | 8.4 | 1.6–44.04 | 0.012 |
| Extramedullary manifestation at diagnosis, yes vs. no | 4.78 | 1.10–20.87 | 0.037 | 8.71 | 3.22–23.57 | <0.001 | |
| RFS, n = 37 CR/CRi FU90 | Extramedullary manifestation at diagnosis, yes vs. no | 3.59 | 1.19–10.86 | 0.024 | 7.36 | 1.38–39.1 | 0.019 |
| Complex karyotype, yes vs. no | 3.17 | 1.02–9.90 | 0.047 | 6.79 | 1.86–24.81 | 0.004 | |
| FU90 MRD+ vs. MRD | 2.27 | 0.87–5.92 | 0.095 | 4.53 | 1.93–10.66 | 0.001 | |
| OS, n = 37 CR/CRi FU90 | Extramedullary manifestation at diagnosis, yes vs. no | 3.91 | 1.27–12.03 | 0.017 | 9.67 | 2.54–36.79 | 0.001 |
| MRC Grimwade, adverse vs. favorable + intermediate | 2.64 | 1.02–6.82 | 0.045 | 4.27 | 1.46–12.48 | 0.008 | |
| CMV status alloHCT1, other vs. D + P neg. | 3.01 | 0.99–9.11 | 0.052 | 4.26 | 1.29–14.07 | 0.017 | |
| Remission + MRD Status pre-DLI | n = 76 | Remission and MRD Status FU30 | Remission and MRD Status FU90 | ||
|---|---|---|---|---|---|
| CR/CRi MRD− | 23 (30%) | CR/CRi MRD− | 15 (65%) | CR/CRi MRD− | 12 (52%) |
| CR/CRi MRD+ | 0 (0%) | CR/CRi MRD+ | 1 (4.5%) | ||
| MRD not assessable | 8 (35%) | MRD not assessable | 9 (39%) | ||
| No CR/CRi | 0 (0%) | No CR/CRi | 1 (4.5%) | ||
| CR/CRi MRD+ | 15 (20%) | CR/CRi MRD− | 6 (40%) | CR/CRi MRD− | 11 (73.3%) |
| CR/CRi MRD+ | 7 (47%) | CR/CRi MRD+ | 2 (13.3%) | ||
| MRD not assessable | 0 (0%) | MRD not assessable | 0 (0) | ||
| No CR/CRi | 2 (13%) | No CR/CRi | 2 (13.3%) | ||
| Non-CR/CRi | 38 (50%) | CR/CRi MRD− | 4 (11%) | CR/CRi MRD− | 8 (21%) |
| CR/CRi MRD+ | 2 (5%) | CR/CRi MRD+ | 3 (8%) | ||
| CR/CRi MRD not assessed | 0 (0%) | CR/CRi MRD not assessed | 1 (3%) | ||
| No CR/CRi | 32 (84%) | No CR/CRi | 26 (68%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teich, K.; Stadler, M.; Gabdoulline, R.; Kandarp, J.; Wienecke, C.; Heida, B.; Klement, P.; Büttner, K.; Venturini, L.; Wichmann, M.; et al. MRD as Biomarker for Response to Donor Lymphocyte Infusion after Allogeneic Hematopoietic Cell Transplantation in Patients with AML. Cancers 2023, 15, 3911. https://doi.org/10.3390/cancers15153911
Teich K, Stadler M, Gabdoulline R, Kandarp J, Wienecke C, Heida B, Klement P, Büttner K, Venturini L, Wichmann M, et al. MRD as Biomarker for Response to Donor Lymphocyte Infusion after Allogeneic Hematopoietic Cell Transplantation in Patients with AML. Cancers. 2023; 15(15):3911. https://doi.org/10.3390/cancers15153911
Chicago/Turabian StyleTeich, Katrin, Michael Stadler, Razif Gabdoulline, Jyoti Kandarp, Clara Wienecke, Bennet Heida, Piroska Klement, Konstantin Büttner, Letizia Venturini, Martin Wichmann, and et al. 2023. "MRD as Biomarker for Response to Donor Lymphocyte Infusion after Allogeneic Hematopoietic Cell Transplantation in Patients with AML" Cancers 15, no. 15: 3911. https://doi.org/10.3390/cancers15153911
APA StyleTeich, K., Stadler, M., Gabdoulline, R., Kandarp, J., Wienecke, C., Heida, B., Klement, P., Büttner, K., Venturini, L., Wichmann, M., Puppe, W., Schultze-Florey, C., Koenecke, C., Beutel, G., Eder, M., Ganser, A., Heuser, M., & Thol, F. (2023). MRD as Biomarker for Response to Donor Lymphocyte Infusion after Allogeneic Hematopoietic Cell Transplantation in Patients with AML. Cancers, 15(15), 3911. https://doi.org/10.3390/cancers15153911







