A Systematic Review of Short-Term Outcomes of Minimally Invasive Thoracoscopic Surgery for Lung Cancer after Neoadjuvant Systemic Therapy
Abstract
Simple Summary
Abstract
1. Introduction
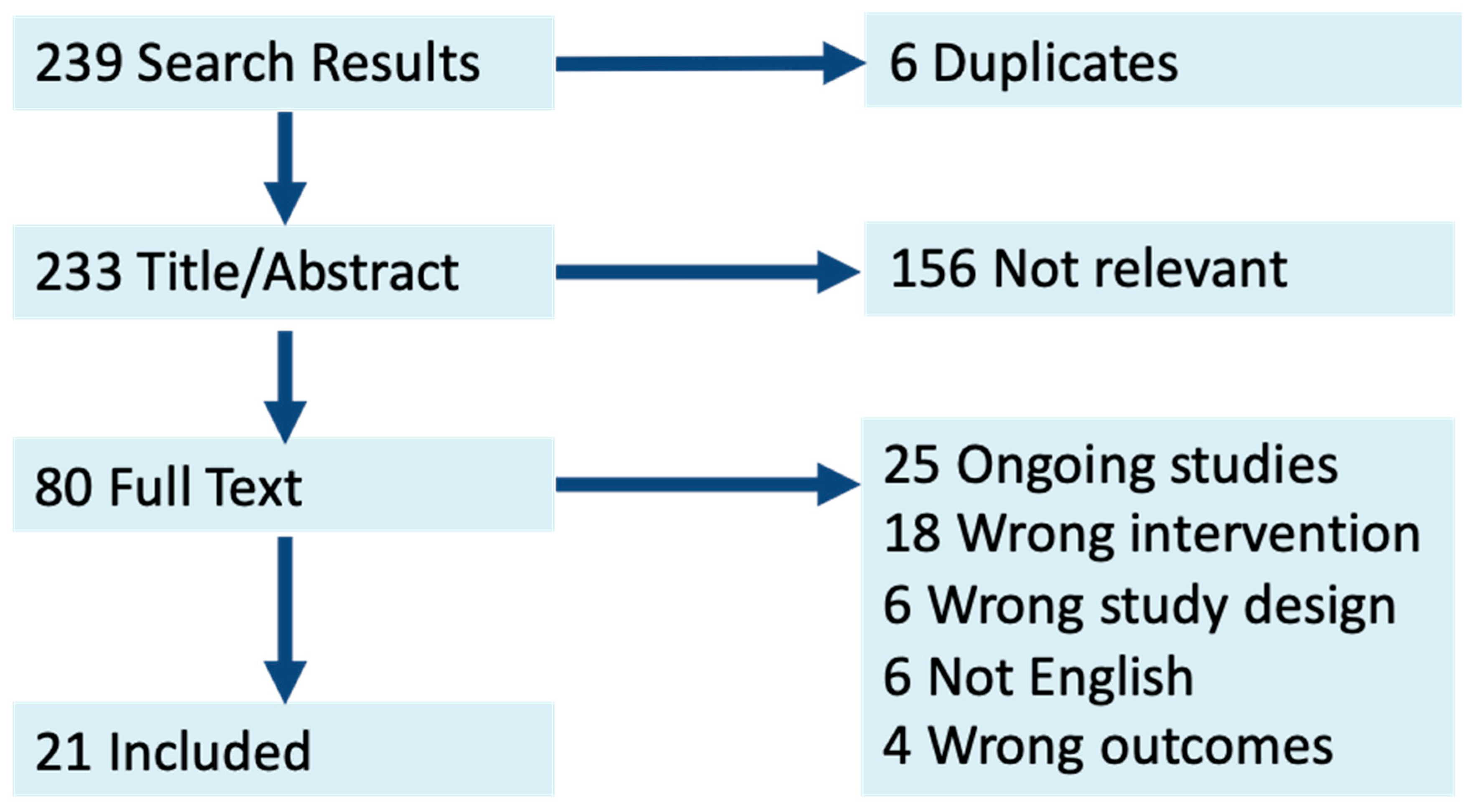
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed]
- Provencio, M.; Calvo, V.; Romero, A.; Spicer, J.D.; Cruz-Bermúdez, A. Treatment Sequencing in Resectable Lung Cancer: The Good and the Bad of Adjuvant Versus Neoadjuvant Therapy. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [CrossRef]
- Kang, J.; Zhang, C.; Zhong, W.Z. Neoadjuvant immunotherapy for non-small cell lung cancer: State of the art. Cancer Commun. 2021, 41, 287–302. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Feng, H.; Yao, Z.; Teng, J.; Wei, D.; Liu, D. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2013, 44, 407–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, T.D.; Black, D.; Bannon, P.G.; McCaughan, B.C. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 2553–2562. [Google Scholar] [CrossRef]
- Ujiie, H.; Gregor, A.; Yasufuku, K. Minimally invasive surgical approaches for lung cancer. Expert Rev. Respir. Med. 2019, 13, 571–578. [Google Scholar] [CrossRef]
- Pless, M.; Stupp, R.; Ris, H.B.; Stahel, R.A.; Weder, W.; Thierstein, S.; Gerard, M.A.; Xyrafas, A.; Früh, M.; Cathomas, R.; et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015, 386, 1049–1056. [Google Scholar] [CrossRef]
- Hanna, J.M.; Berry, M.F.; D’Amico, T.A. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J. Thorac. Dis. 2013, 5 (Suppl. 3), S182–S189. [Google Scholar]
- Huang, J.; Xu, X.; Chen, H.; Yin, W.; Shao, W.; Xiong, X.; He, J. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. 3), S267–S273. [Google Scholar] [PubMed]
- Kamel, M.K.; Nasar, A.; Stiles, B.M.; Altorki, N.K.; Port, J.L. Video-Assisted Thoracoscopic Lobectomy Is the Preferred Approach Following Induction Chemotherapy. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 495–500. [Google Scholar] [CrossRef]
- Hireche, K.; Canaud, L.; Lounes, Y.; Aouinti, S.; Molinari, N.; Alric, P. Thoracoscopic Versus Open Lobectomy After Induction Therapy for Nonsmall Cell Lung Cancer: New Study Results and Meta-analysis. J. Surg. Res. 2022, 276, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, C. Uniportal video-assisted thoracoscopic surgery following neoadjuvant chemotherapy for locally-advanced lung cancer. J. Cardiothorac. Surg. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Meyerhoff, R.R.; Mayne, N.R.; Singhapricha, T.; Toomey, C.B.; Speicher, P.J.; Hartwig, M.G.; Tong, B.C.; Onaitis, M.W.; Harpole, D.H., Jr.; et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2016, 49, 1615–1623. [Google Scholar] [CrossRef]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef]
- Fang, L.; Wang, L.; Wang, Y.; Lv, W.; Hu, J. Video assisted thoracic surgery vs. thoracotomy for locally advanced lung squamous cell carcinoma after neoadjuvant chemotherapy. J. Cardiothorac. Surg. 2018, 13, 128. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Choi, Y.S.; Lee, K.J.; Lee, S.H.; Pyo, H.; Choi, J.Y. Outcomes of Pulmonary Resection and Mediastinal Node Dissection by Video-Assisted Thoracoscopic Surgery Following Neoadjuvant Chemoradiation Therapy for Stage IIIA N2 Non-Small Cell Lung Cancer. Korean J. Thorac. Cardiovasc. Surg. 2018, 51, 29–34. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yamada, T.; Matsuoka, T.; Nagai, S.; Ueda, M.; Miyamoto, Y. Video-assisted thoracoscopic surgery for lung cancer after induction therapy. Asian Cardiovasc. Thorac. Ann. 2018, 26, 608–614. [Google Scholar] [CrossRef]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A.; et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Nwosu, A.; Mayne, N.R.; Wang, Y.Y.; Raman, V.; Meyerhoff, R.R.; D’Amico, T.A.; Berry, M.F. A Minimally Invasive Approach to Lobectomy After Induction Therapy Does Not Compromise Survival. Ann. Thorac. Surg. 2020, 109, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, T.; Luo, Z.; Tong, L.; Dong, X.; Zhang, Y.; Afzal, M.Z.; Correale, P.; Liu, H.; Jiang, T.; et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: An open-label, multicenter, single-arm study. Transl. Lung Cancer Res. 2021, 10, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Romero Román, A.; Campo-Cañaveral de la Cruz, J.L.; Macía, I.; Escobar Campuzano, I.; Figueroa Almánzar, S.; Delgado Roel, M.; Gálvez Muñoz, C.; García Fontán, E.M.; Muguruza Trueba, I.; Romero Vielva, L.; et al. Outcomes of surgical resection after neoadjuvant chemoimmunotherapy in locally advanced stage IIIA non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2021, 60, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cabañero Sánchez, A.; Muñoz Molina, G.M.; Fra Fernández, S.; Muriel García, A.; Cilleruelo Ramos, A.; Martínez Hernández, N.; Hernando Trancho, F.; Moreno Mata, N. Impact of neoadjuvant therapy on postoperative complications in non-small-cell lung cancer patients subjected to anatomic lung resection. Eur. J. Surg. Oncol. 2022, 48, 1947–1953. [Google Scholar] [CrossRef]
- Dell’Amore, A.; Lomangino, I.; Tamburini, N.; Bongiolatti, S.; Parri, N.S.F.; Grossi, W.; Catelli, C.; Lorenzoni, G.; Gregori, D.; Nicotra, S.; et al. Video-assisted thoracoscopic lobectomy after neoadjuvant chemotherapy for non-small cell lung cancer: A multicenter propensity-matched study. Surg. Endosc. 2022, 36, 1466–1475. [Google Scholar] [CrossRef]
- Deng, H.; Liu, J.; Cai, X.; Chen, J.; Rocco, G.; Petersen, R.H.; Brunelli, A.; Ng, C.S.H.; D’Amico, T.A.; Liang, W.; et al. Radical Minimally Invasive Surgery After Immuno-chemotherapy in Initially-unresectable Stage IIIB Non-small cell Lung Cancer. Ann. Surg. 2022, 275, e600–e602. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Choi, Y.S.; Cho, J.H.; Kim, H.K.; Kim, J.; Zo, J.I.; Shim, Y.M. Thoracoscopic Vs Open Surgery Following Neoadjuvant Chemoradiation for Clinical N2 Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 300–308. [Google Scholar] [CrossRef]
- Kamel, M.K.; Sholi, A.N.; Harrison, S.W.; Lee, B.; Port, J.L.; Altorki, N.K.; Stiles, B.M. Minimally Invasive Surgery for Lung Cancer Following Neoadjuvant Therapy in the United States. J. Laparoendosc. Adv. Surg. Tech. A 2022, 32, 860–865. [Google Scholar] [CrossRef]
- Tian, Z.; Sui, X.; Yang, F.; Wang, J. Is video-assisted thoracoscopy a sufficient approach for mediastinal lymph node dissection to treat lung cancer after neoadjuvant therapy? Thorac. Cancer 2019, 10, 782–790. [Google Scholar] [CrossRef]
- Tong, B.C.; Gu, L.; Wang, X.; Wigle, D.A.; Phillips, J.D.; Harpole, D.H., Jr.; Klapper, J.A.; Sporn, T.; Ready, N.E.; D’Amico, T.A. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 427–436. [Google Scholar] [CrossRef]
- Yao, Y.; Tang, D.; Gao, W.; Zhang, H. Neoadjuvant Immuno-Chemotherapy: A New Perspective for Stage III NSCLC? Front. Surg. 2022, 9, 843987. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, Q.; Xiao, H.; Wu, J.; Yang, D.; Tang, J.; Li, X.; Wu, Z.; Zhou, Y.; Wang, W. Perioperative Outcomes of Video-Assisted Thoracoscopic Surgery Versus Open Thoracotomy After Neoadjuvant Chemoimmunotherapy in Resectable NSCLC. Front. Oncol. 2022, 12, 858189. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Zhang, X.N.; Huang, L. Neoadjuvant chemotherapy followed by surgery versus upfront surgery in non-metastatic non-small cell lung cancer: Systematic review and meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 90327–90337. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Lu, J.; Zhang, S.; Yang, X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer 2017, 17, 75. [Google Scholar]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J. Thorac. Oncol. 2020, 15, 1281–1297. [Google Scholar] [CrossRef]
- Muslim, Z.; Stroever, S.; Poulikidis, K.; Weber, J.F.; Connery, C.P.; Herrera, L.J.; Bhora, F.Y. Conversion to Thoracotomy in Non-Small Cell Lung Cancer: Risk Factors and Perioperative Outcomes. Innovations 2022, 17, 148–155. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Messina, J.L.; Stein, J.E.; Xu, X.; Amaria, R.N.; Blank, C.U.; van de Wiel, B.A.; Ferguson, P.M.; Rawson, R.V.; Ross, M.I.; et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 2018, 29, 1861–1868. [Google Scholar] [CrossRef]
- Bongiolatti, S.; Gonfiotti, A.; Viggiano, D.; Borgianni, S.; Politi, L.; Crisci, R.; Curcio, C.; Voltolini, L. Risk factors and impact of conversion from VATS to open lobectomy: Analysis from a national database. Surg. Endosc. 2019, 33, 3953–3962. [Google Scholar] [CrossRef]
- Herb, J.N.; Kindell, D.G.; Strassle, P.D.; Stitzenberg, K.B.; Haithcock, B.E.; Mody, G.N.; Long, J.M. Trends and Outcomes in Minimally Invasive Surgery for Locally Advanced Non-Small-Cell Lung Cancer with N2 Disease. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, H. Selective lymph node dissection in early-stage non-small cell lung cancer. J. Thorac. Dis. 2017, 9, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Koulaxouzidis, G.; Karagkiouzis, G.; Konstantinou, M.; Gkiozos, I.; Syrigos, K. Sampling versus systematic full lymphatic dissection in surgical treatment of non-small cell lung cancer. Oncol. Rev. 2013, 7, e2. [Google Scholar] [CrossRef] [PubMed]
- Darling, G.E.; Allen, M.S.; Decker, P.A.; Ballman, K.; Malthaner, R.A.; Inculet, R.I.; Jones, D.R.; McKenna, R.J.; Landreneau, R.J.; Rusch, V.W.; et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J. Thorac. Cardiovasc. Surg. 2011, 141, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, Z.F.; Wang, S.Y.; Yang, X.N.; Ou, W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002, 36, 1–6. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Smith, C.; Mhango, G.; Wisnivesky, J.P. The number of lymph node metastases as a prognostic factor in patients with N1 non-small cell lung cancer. Chest 2011, 140, 433–440. [Google Scholar] [CrossRef]
- Wen, Y.S.; Xi, K.X.; Zhang, R.S.; Wang, G.M.; Huang, Z.R.; Zhang, L.J. The number of resected lymph nodes is associated with the long-term survival outcome in patients with T2 N0 non-small cell lung cancer. Cancer Manag. Res. 2018, 10, 6869–6877. [Google Scholar] [CrossRef]
- Nissen, A.P.; Vreeland, T.J.; Teshome, M.; Archer, M.A.; Francescatti, A.B.; Katz, M.H.G.; Hunt, K.K.; Zheng, L.; Mullett, T.W. American College of Surgeons Commission on Cancer Standard for Curative-intent Pulmonary Resection. Ann. Thorac. Surg. 2022, 113, 5–8. [Google Scholar] [CrossRef]
- Toker, A.; Özyurtkan, M.O.; Kaba, E. Nodal upstaging: Effects of instrumentation and three-dimensional view in clinical stage I lung cancer. J. Vis. Surg. 2017, 3, 76. [Google Scholar] [CrossRef]
- Nachira, D.; Meacci, E.; Congedo, M.T.; Chiappetta, M.; Petracca-Ciavarella, L.; Vita, M.L.; Margaritora, S. Upstaging, centrality and survival in early stage non-small cell lung cancer video-assisted surgery: Lymph nodal upstaging in lung cancer surgery: Is it really a surgical technique problem? Lung Cancer 2020, 144, 85–86. [Google Scholar] [CrossRef]
- Van Houtte, P.; Moretti, L.; Charlier, F.; Roelandts, M.; Van Gestel, D. Preoperative and postoperative radiotherapy (RT) for non-small cell lung cancer: Still an open question. Transl. Lung Cancer Res. 2021, 10, 1950–1959. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H.; Song, X.; Yue, J.; Yu, J. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non-small-cell lung cancer patients: A propensity score matching-based analysis from surveillance, epidemiology, and end results database. Cancer Med. 2018, 7, 4354–4360. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; De Castro Carpeño, J.; et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Cancer Res. 2017, 77, 1322–1330. [Google Scholar] [CrossRef]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017, 170, 1120–1133.e17. [Google Scholar] [CrossRef]
- Dougan, M. Understanding and Overcoming the Inflammatory Toxicities of Immunotherapy. Cancer Immunol. Res. 2020, 8, 1230–1235. [Google Scholar] [CrossRef]
- Yin, J.; Wu, Y.; Yang, X.; Gan, L.; Xue, J. Checkpoint Inhibitor Pneumonitis Induced by Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer: Occurrence and Mechanism. Front. Immunol. 2022, 13, 830631. [Google Scholar] [CrossRef]
- Wu, J.; Hong, D.; Zhang, X.; Lu, X.; Miao, J. PD-1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose-independent manner: A meta-analysis. Sci. Rep. 2017, 7, 44173. [Google Scholar] [CrossRef]
- Chen, M.; Lu, H.; Copley, S.J.; Han, Y.; Logan, A.; Viola, P.; Cortellini, A.; Pinato, D.J.; Power, D.; Aboagye, E.O. A Novel Radiogenomics Biomarker for Predicting Treatment Response and Pneumotoxicity From Programmed Cell Death Protein or Ligand-1 Inhibition Immunotherapy in NSCLC. J. Thorac. Oncol. 2023, 18, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Etienne, H.; Fournel, L.; Mordant, P.; Delatour, B.R.; Pfeuty, K.; Frey, G.; Seguin-Givelet, A.; Fourdrain, A.; Lancelin, C.; Berna, P.; et al. Anatomic lung resection after immune checkpoint inhibitors for initially unresectable advanced-staged non-small cell lung cancer: A retrospective cohort analysis. J. Thorac. Dis. 2023, 15, 270–280. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Stage | Neoadjuvant | Open N(%) | VATS N(%) | Conversion N(%) |
|---|---|---|---|---|---|---|
| Huang [12] | 2013 | IIA-IIIB | CT, IT, RT | 0 (0%) | 42 (100%) | 7 (17%) |
| Yang, C [16] | 2016 | IA-IV | CT, RT | 203 (74%) | 69 (25%) | 7 (10%) |
| Kamel [13] | 2017 | I-IV | CT, IT | 74 (64%) | 40 (35%) | 5 (13%) |
| Bott [17] | 2018 | I-IIIA | IT | 7 (35%) | 10 (50%) | 7 (54%) |
| Fang [18] | 2018 | IIB-IIIB | CT | 67 (80%) | 14 (17%) | NR |
| Jeon [19] | 2018 | IIIA | CT, RT | 18 (54%) | 17 (48%) | 5 (28%) |
| Matsuoka [20] | 2018 | NR | CT, RT | 31 (28%) | 79 (72%) | 4 (5%) |
| Yang, Z [15] | 2018 | IIB-IIIB | CT | 0 (0%) | 29 (100%) | 1 (3%) |
| Shu [21] | 2020 | IB-IIIA | CT, IT | 14 (53%) | 12 (46%) | NR |
| Yang, C [22] | 2020 | NR | CT, RT | 2221 (76%) | 676 (23%) | 152 (22%) |
| Duan [23] | 2021 | IIA-IIIB | CT, IT | 4 (17%) | 14 (61%) | 2 (9%) |
| RomeroRoman [24] | 2021 | IIIA | CT, IT | 20 (48%) | 21 (51%) | 4 (19%) |
| Cabanero sanchez [25] | 2022 | NR | CT, IT, RT | 135 (51%) | 74 (28%) | 21 (8%) |
| Dell’Amore [26] | 2022 | IIA-IIIB | CT, IT | 93 (60%) | 62 (40%) | 8 (5%) |
| Deng [27] | 2022 | IIIB | CT, IT | 0 (0%) | 31 (100%) | 0 (0) |
| Jeon [28] | 2022 | IIIA | CT, RT | 350 (90%) | 35 (9%) | 6 (17%) |
| Kamel [29] | 2022 | NR | CT, IT, RT | 7894 (70%) | 2753 (24%) | 557 (16%) |
| Tian [30] | 2022 | IIB-IIIA | CT, RT | 71 (56%) | 56 (44%) | 6 (11%) |
| Tong [31] | 2022 | IB-IIIA | IT | 2 (8%) | 18 (72%) | 5 (20) |
| Yao [32] | 2022 | IIIA-IIIB | CT, IT | 0 (0%) | 11 (100%) | 1 (9%) |
| Zhang [33] | 2022 | IB-IIIB | CT, IT | 78 (59%) | 53 (40%) | 42 (54%) |
| Forde [34] | 2022 | IB-IIIA | CT, IT | 173 (70%) | 73 (26%) | 38 (13%) |
| Author | Year | LN Open (Median N) | LN VATS (Median N) | p-Value |
|---|---|---|---|---|
| Huang [12] | 2013 | NR | 16.88 | |
| Kamel [13] | 2017 | 15 | 12 | 0.945 |
| Fang [18] | 2018 | 20 | 16 | 0.011 |
| Jeon [19] | 2018 | 13.5 | 24 | 0.004 |
| Yang, Z [15] | 2018 | NR | 21.9 | |
| Yang, C [22] | 2020 | 11 | 12 | 0.38 |
| Dell’Amore [26] | 2022 | 26 | 20 | 0.022 |
| Deng [27] | 2022 | NR | 16 | |
| Jeon [28] | 2022 | 22.5 | 22 | 0.217 |
| Kamel [29] | 2022 | 10 | 9 | <0.001 |
| Tian [30] | 2022 | 19 | 17 | 0.337 |
| Zhang [33] | 2022 | 23 | 19.5 | 0.013 |
| Open | VATS | |
|---|---|---|
| Complication | Freq Range (%) | Freq Range (%) |
| Prolonged air leak | 0–22 | 0–12 |
| Arrythmia | 0–22 | 0–23 |
| Pneumonia | 0–10 | 0–6 |
| Wound infection | 0–11 | 0 |
| Cardiac complication | 0–14 | 0–3 |
| Atelectasis | 0–6 | 0–6 |
| ARDS | 0–6 | 0–6 |
| Pneumothorax or effusion | 0–1 | 0 |
| Fistula | 0–1 | 0 |
| Empyema | 0–1 | 0–3 |
| Pulmonary Embolism | 0–1 | 0–12 |
| Respiratory failure | 0–3 | 0 |
| Chylothorax | 0–1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedighim, S.; Frank, M.I.; Heutlinger, O.; Lee, C.; Hachey, S.J.; Keshava, H.B. A Systematic Review of Short-Term Outcomes of Minimally Invasive Thoracoscopic Surgery for Lung Cancer after Neoadjuvant Systemic Therapy. Cancers 2023, 15, 3908. https://doi.org/10.3390/cancers15153908
Sedighim S, Frank MI, Heutlinger O, Lee C, Hachey SJ, Keshava HB. A Systematic Review of Short-Term Outcomes of Minimally Invasive Thoracoscopic Surgery for Lung Cancer after Neoadjuvant Systemic Therapy. Cancers. 2023; 15(15):3908. https://doi.org/10.3390/cancers15153908
Chicago/Turabian StyleSedighim, Shaina, Madelyn I. Frank, Olivia Heutlinger, Carlin Lee, Stephanie J. Hachey, and Hari B. Keshava. 2023. "A Systematic Review of Short-Term Outcomes of Minimally Invasive Thoracoscopic Surgery for Lung Cancer after Neoadjuvant Systemic Therapy" Cancers 15, no. 15: 3908. https://doi.org/10.3390/cancers15153908
APA StyleSedighim, S., Frank, M. I., Heutlinger, O., Lee, C., Hachey, S. J., & Keshava, H. B. (2023). A Systematic Review of Short-Term Outcomes of Minimally Invasive Thoracoscopic Surgery for Lung Cancer after Neoadjuvant Systemic Therapy. Cancers, 15(15), 3908. https://doi.org/10.3390/cancers15153908






