Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Inhibition of Proliferative Signaling of Cancer Cells
3. Cancer Cell Death Induction
3.1. Apoptosis
3.2. Autophagy
3.3. Ferroptosis
3.4. Necroptosis
4. Inhibition of Replicative Immortality
5. Inhibition of Tumor Promoting Inflammation
6. Inhibition of Tumor Invasion and Metastasis
7. Inhibition of Abnormal Metabolism
8. Targeting Cancer’s Evasion of Anti-Growth Signaling
9. Inhibition of Angiogenesis
10. Anti-Tumor Immune Response Activation
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Iwamoto, T. Clinical application of drug delivery systems in cancer chemotherapy: Review of the efficacy and side effects of approved drugs. Biol. Pharm. Bull. 2013, 36, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Hosseini, A.; Ghorbani, A. Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar]
- Shu, L.; Cheung, K.L.; Khor, T.O.; Chen, C.; Kong, A.N. Phytochemicals: Cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010, 29, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L.; Brockmueller, A.; Zhai, K.; Abdellatif, B.; Siddiqui, M.; Biringer, K.; Kudela, E.; Pec, M.; et al. Flavonoids as an effective sensitizer for anti-cancer therapy: Insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021, 12, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass spectrometry analysis of saponins. Mass Spectrom. Rev. 2023, 42, 954–983. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Ghanadian, S.M.; Bahrami, G.; Mansouri, K.; Iranpanah, A.; Farzaei, M.H.; Mojarrab, M. Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways. Metabolites 2023, 13, 323. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Wang, M.; Xia, X.; Dai, R.; Zhao, Y. Steroidal saponins and sapogenins from fenugreek and their inhibitory activity against alpha-glucosidase. Steroids 2020, 161, 108690. [Google Scholar] [CrossRef]
- Passos, F.R.S.; Araujo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araujo, A.A.S.; Almeida, J.; Thangaraj, P.; Junior, L.J.Q.; Quintans, J.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Lin, X.; Wang, Y.; He, X. A steroidal saponin isolated from Allium chinense simultaneously induces apoptosis and autophagy by modulating the PI3K/Akt/mTOR signaling pathway in human gastric adenocarcinoma. Steroids 2020, 161, 108672. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Wang, Y.; Wang, Z.; He, X. A-24, a steroidal saponin from Allium chinense, induced apoptosis, autophagy and migration inhibition in p53 wild-type and p53-deficient gastric cancer cells. Chem. Biol. Interact. 2021, 348, 109648. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Nguyen, H.T.; Seephan, S.; Do, H.B.; Nguyen, H.T.; Ho, D.V.; Pongrakhananon, V. Antitumor activities of Aspiletrein A, a steroidal saponin from Aspidistra letreae, on non-small cell lung cancer cells. BMC Complement. Med. Ther. 2021, 21, 87. [Google Scholar] [CrossRef]
- Iksen, I.; Witayateeraporn, W.; Wirojwongchai, T.; Suraphan, C.; Pornputtapong, N.; Singharajkomron, N.; Nguyen, H.M.; Pongrakhananon, V. Identifying molecular targets of Aspiletrein-derived steroidal saponins in lung cancer using network pharmacology and molecular docking-based assessments. Sci. Rep. 2023, 13, 1545. [Google Scholar] [CrossRef]
- Ho, D.V.; Hoang, H.N.T.; Vo, H.Q.; Nguyen, K.V.; Pham, T.V.; Le, A.T.; Van Phan, K.; Nguyen, H.M.; Morita, H.; Nguyen, H.T. Three new steroidal saponins from Aspidistra letreae plants and their cytotoxic activities. J. Nat. Med. 2020, 74, 591–598. [Google Scholar] [CrossRef]
- Qian, L.; Su, H.; Wang, G.; Li, B.; Shen, G.; Gao, Q. Anti-tumor Activity of Bufalin by Inhibiting c-MET Mediated MEK/ERK and PI3K/AKT Signaling Pathways in Gallbladder Cancer. J. Cancer 2020, 11, 3114–3123. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Nie, L.; Yao, S.; Bi, A.; Ye, Y.; Wu, Y.; Tan, Z.; Wu, Z. Bufalin exerts antitumor effects in neuroblastoma via the induction of reactive oxygen species-mediated apoptosis by targeting the electron transport chain. Int. J. Mol. Med. 2020, 46, 2137–2149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, X.; Yang, Q.; Zhou, X.; Wu, J.; Yang, X.; Zhao, Y.; Lin, R.; Xie, Y.; et al. Bufalin induces mitochondrial dysfunction and promotes apoptosis of glioma cells by regulating Annexin A2 and DRP1 protein expression. Cancer Cell Int. 2021, 21, 424. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, G. Dioscin induces ferroptosis and synergistic cytotoxicity with chemotherapeutics in melanoma cells. Biochem Biophys. Res. Commun. 2021, 557, 213–220. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, X.; Li, M.; Gong, G.; Liu, W.; Li, T.; Zuo, H.; Li, W.; Gao, F.; Liu, H. Cdh1-mediated Skp2 degradation by dioscin reprogrammes aerobic glycolysis and inhibits colorectal cancer cells growth. EBioMedicine 2020, 51, 102570. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.D.; Mahanta, S.; Das, S.G.; Das, S.K.; Paul, D.; Tag, H.; Hui, P.K. Identification of phytocompounds from Paris polyphylla Smith as potential inhibitors against two breast cancer receptors (ERα and EGFR tyrosine kinase) through chromatographic and In silico approaches. J. Appl. Biol. Biotechnol. 2022, 10, 60–80. [Google Scholar] [CrossRef]
- Shalayel, M.H.F.; Al-Mazaideh, G.M.; Alanezi, A.A.; Almuqati, A.F.; Alotaibi, M. Diosgenin and Monohydroxy Spirostanol from Prunus amygdalus var amara Seeds as Potential Suppressors of EGFR and HER2 Tyrosine Kinases: A Computational Approach. Pharmaceuticals 2023, 16, 704. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wang, H.; Tang, X.; Zhang, L.; Wang, T.; Cheng, J. Study on the Mechanism of Diosgenin Targeting STAT3 to Inhibit Colon Cancer Proliferation and Migration. Dis. Markers 2022, 2022, 7494887. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.; Shanmugam, M.K.; Siveen, K.S.; Ong, T.H.; Yang, M.H.; Lee, J.H.; Rajendran, P.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; et al. Diosgenin attenuates tumor growth and metastasis in transgenic prostate cancer mouse model by negatively regulating both NF-kappaB/STAT3 signaling cascades. Eur. J. Pharmacol. 2021, 906, 174274. [Google Scholar] [CrossRef] [PubMed]
- Khathayer, F.; Ray, S.K. Diosgenin as a Novel Alternative Therapy for Inhibition of Growth, Invasion, and Angiogenesis Abilities of Different Glioblastoma Cell Lines. Neurochem. Res. 2020, 45, 2336–2351. [Google Scholar] [CrossRef]
- Tsukayama, I.; Mega, T.; Hojo, N.; Toda, K.; Kawakami, Y.; Takahashi, Y.; Suzuki-Yamamoto, T. Diosgenin suppresses COX-2 and mPGES-1 via GR and improves LPS-induced liver injury in mouse. Prostaglandins Other Lipid Mediat. 2021, 156, 106580. [Google Scholar] [CrossRef] [PubMed]
- Rahmati-Yamchi, M.; Ghareghomi, S.; Haddadchi, G.; Milani, M.; Aghazadeh, M.; Daroushnejad, H. Fenugreek extract diosgenin and pure diosgenin inhibit the hTERT gene expression in A549 lung cancer cell line. Mol. Biol. Rep. 2014, 41, 6247–6252. [Google Scholar] [CrossRef]
- Li, T.Y.; Du, Y.; Wang, M.C.; Liu, K.; Liu, Y.; Cao, Y.; Wang, Y.Y.; Chen, W.W.; Qian, X.Y.; Qiu, P.C.; et al. Cytotoxic Steroidal Saponins Containing a Rare Fructosyl from the Rhizomes of Paris polyphylla var. latifolia. Int. J. Mol. Sci. 2023, 24, 7149. [Google Scholar] [CrossRef]
- Min, H.Y.; Pei, H.; Hyun, S.Y.; Boo, H.J.; Jang, H.J.; Cho, J.; Kim, J.H.; Son, J.; Lee, H.Y. Potent Anticancer Effect of the Natural Steroidal Saponin Gracillin Is Produced by Inhibiting Glycolysis and Oxidative Phosphorylation-Mediated Bioenergetics. Cancers 2020, 12, 913. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, H.; Liu, X.; Xiao, B.; Zhang, M.; Luo, Y.; Li, M.; Yang, J. Gracillin Shows Potential Efficacy Against Non-Small Cell Lung Cancer Through Inhibiting the mTOR Pathway. Front. Oncol. 2022, 12, 851300. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; You, L.; Sun, M.; Qu, C.; Dong, X.; Yin, X.; Ni, J. Inhibitory effects of Paris saponin I, II, Ⅵ and Ⅶ on HUVEC cells through regulation of VEGFR2, PI3K/AKT/mTOR, Src/eNOS, PLCgamma/ERK/MERK, and JAK2-STAT3 pathways. Biomed. Pharmacother. 2020, 131, 110750. [Google Scholar] [CrossRef]
- Xiang, Y.C.; Peng, P.; Liu, X.W.; Jin, X.; Shen, J.; Zhang, T.; Zhang, L.; Wan, F.; Ren, Y.L.; Yu, Q.Q.; et al. Paris saponin VII, a Hippo pathway activator, induces autophagy and exhibits therapeutic potential against human breast cancer cells. Acta Pharmacol. Sin. 2022, 43, 1568–1580. [Google Scholar] [CrossRef]
- Yao, M.; Li, R.; Yang, Z.; Ding, Y.; Zhang, W.; Li, W.; Liu, M.; Zhao, C.; Wang, Y.; Tang, H.; et al. PP9, a steroidal saponin, induces G2/M arrest and apoptosis in human colorectal cancer cells by inhibiting the PI3K/Akt/GSK3beta pathway. Chem. Biol. Interact. 2020, 331, 109246. [Google Scholar] [CrossRef]
- Chen, Y.R.; Wang, S.C.; Huang, S.P.; Su, C.C.; Liu, P.L.; Cheng, W.C.; Chuu, C.P.; Chen, J.K.; Bao, B.Y.; Lee, C.H.; et al. Protodioscin inhibits bladder cancer cell migration and growth, and promotes apoptosis through activating JNK and p38 signaling pathways. Biomed. Pharmacother. 2022, 156, 113929. [Google Scholar] [CrossRef]
- Huang, D.; Dong, X.; Li, J.; Chen, Y.; Zhou, Y.; Chen, Q.; Sun, Y. Steroidal saponin SSPH I induces ferroptosis in HepG2 cells via regulating iron metabolism. Med. Oncol. 2023, 40, 132. [Google Scholar] [CrossRef]
- Dai, Z.; Zhu, P.F.; Liu, H.; Li, X.C.; Zhu, Y.Y.; Liu, Y.Y.; Shi, X.L.; Chen, W.D.; Liu, Y.P.; Zhao, Y.L.; et al. Discovery of potent immune-modulating molecule taccaoside A against cancers from structures-active relationships of natural steroidal saponins. Phytomedicine 2022, 104, 154335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, T.; Zhu, R.; Lu, J.; Ouyang, X.; Zhang, Z.; Chen, Q.; Li, J.; Cui, J.; Jiang, F.; et al. Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation. Int. J. Biol. Sci. 2023, 19, 1471–1489. [Google Scholar] [CrossRef]
- Zhan, G.; Hu, J.; Xiao, B.; Wang, X.; Yang, Z.; Yang, G.; Lu, L. Trillin prevents proliferation and induces apoptosis through inhibiting STAT3 nuclear translocation in hepatoma carcinoma cells. Med. Oncol. 2020, 37, 44. [Google Scholar] [CrossRef]
- Lone, B.A.; Tabassum, M.; Bhushan, A.; Dhiman, U.; Rani, D.; Gupta, P.N.; Mondhe, D.M.; Gairola, S.; Gupta, P. Trilliumosides A-B, two novel steroidal saponins isolated from Rhizomes of Trillium govanianum as potent anticancer agents targeting apoptosis in A-549 cancer cell line. bioRxiv 2023. [Google Scholar] [CrossRef]
- Pang, D.; Yang, C.; Li, C.; Zou, Y.; Feng, B.; Li, L.; Liu, W.; Luo, Q.; Chen, Z.; Huang, C. Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway. Biology Open 2020, 9, bio046854. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; Xu, L.; Li, J.; Zhai, Y.; Sun, Z.; Shi, W.; Jiang, Y.; Ma, C.; Lin, H.; Guo, Y.; et al. Polyphyllin II inhibits human bladder cancer migration and invasion by regulating EMT-associated factors and MMPs. Oncol. Lett. 2020, 20, 2928–2936. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Kai, H.; Han, Y.; Huang, M.; Gao, L.; Qiao, H. Polyphyllin E Inhibits Proliferation, Migration and Invasion of Ovarian Cancer Cells by Down-Regulating the AKT/NF-κB Pathway. Biol. Pharm. Bull. 2022, 45, 561–568. [Google Scholar] [CrossRef]
- Valayil, J.M.; Kuriakose, G.C.; Jayabaskaran, C. Isolation, Purification and Characterization of a Novel Steroidal Saponin Cholestanol Glucoside from Lasiodiplodia theobromae that Induces Apoptosis in A549 Cells. Anti-Cancer Agents Med. Chem. 2016, 16, 865–874. [Google Scholar] [CrossRef]
- He, H.; Xu, C.; Zheng, L.; Wang, K.; Jin, M.; Sun, Y.; Yue, Z. Polyphyllin VII induces apoptotic cell death via inhibition of the PI3K/Akt and NF-kappaB pathways in A549 human lung cancer cells. Mol. Med. Rep. 2020, 21, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, J.; Wang, Y.; Xiang, L.; He, X. S-20, a steroidal saponin from the berries of black nightshade, exerts anti-multidrug resistance activity in K562/ADR cells through autophagic cell death and ERK activation. Food Funct. 2022, 13, 2200–2215. [Google Scholar] [CrossRef]
- Hu, C.; Zu, D.; Xu, J.; Xu, H.; Yuan, L.; Chen, J.; Wei, Q.; Zhang, Y.; Han, J.; Lu, T.; et al. Polyphyllin B Suppresses Gastric Tumor Growth by Modulating Iron Metabolism and Inducing Ferroptosis. Int. J. Biol. Sci. 2023, 19, 1063–1079. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Chen, C.; Li, Z.; Chen, Y.; Zhang, X.; Wang, L.; Zhou, J. Polyphyllin Ⅲ-Induced Ferroptosis in MDA-MB-231 Triple-Negative Breast Cancer Cells can Be Protected Against by KLF4-Mediated Upregulation of xCT. Front. Pharmacol. 2021, 12, 670224. [Google Scholar] [CrossRef]
- Watanabe, S.; Suzuki, T.; Hara, F.; Yasui, T.; Uga, N.; Naoe, A. Polyphyllin D, a steroidal saponin in Paris polyphylla, induces apoptosis and necroptosis cell death of neuroblastoma cells. Pediatr. Surg. Int. 2017, 33, 713–719. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, P.; Liu, X.; Xiang, Y.; Zhang, T.; Wu, Y.; Xu, J.; Sun, Z.; Zhen, W.; Zhang, L.; et al. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J. Pharmacol. Sci. 2018, 137, 305–312. [Google Scholar] [CrossRef]
- Chang, J.; Li, Y.; Wang, X.; Hu, S.; Wang, H.; Shi, Q.; Wang, Y.; Yang, Y. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/beta-catenin pathway in vitro and in vivo. Sci. Rep. 2017, 7, 7605. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, Z.; Deng, J.; Liu, J.; Ma, K.; Si, Y.; Zhang, T.; Feng, T.; Liu, Y.; Tan, Y. Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int. J. Oncol. 2018, 53, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lu, Y.; Li, H.; Ji, Y.; Fang, F.; Tang, H.; Qiu, P. A steroidal saponin form Paris vietnamensis (Takht.) reverses temozolomide resistance in glioblastoma cells via inducing apoptosis through ROS/PI3K/Akt pathway. Biosci. Trends 2020, 14, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Zhang, Y.; Zhao, W.; Zhou, X.; Wang, C.; Deng, F. The cardiac glycoside oleandrin induces apoptosis in human colon cancer cells via the mitochondrial pathway. Cancer Chemother. Pharmacol. 2017, 80, 91–100. [Google Scholar] [CrossRef]
- Li, X.X.; Wang, D.Q.; Sui, C.G.; Meng, F.D.; Sun, S.L.; Zheng, J.; Jiang, Y.H. Oleandrin induces apoptosis via activating endoplasmic reticulum stress in breast cancer cells. Biomed. Pharmacother. 2020, 124, 109852. [Google Scholar] [CrossRef]
- Eroglu Gunes, C.; Secer Celik, F.; Secme, M.; Elmas, L.; Dodurga, Y.; Kurar, E. Glycoside oleandrin downregulates toll-like receptor pathway genes and associated miRNAs in human melanoma cells. Gene 2022, 843, 146805. [Google Scholar] [CrossRef]
- Bao, Z.; Tian, B.; Wang, X.; Feng, H.; Liang, Y.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Oleandrin induces DNA damage responses in cancer cells by suppressing the expression of Rad51. Oncotarget 2016, 7, 59572–59579. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; Grimaldi, A.; Chece, G.; Porzia, A.; Morrone, S.; Mainiero, F.; D’Alessandro, G.; Esposito, V.; Cortese, B.; Di Angelantonio, S.; et al. The Glycoside Oleandrin Reduces Glioma Growth with Direct and Indirect Effects on Tumor Cells. J. Neurosci. 2017, 37, 3926–3939. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Fukuhara, H.; Chen, G.; Kawada, C.; Kurabayashi, A.; Furihata, M.; Inoue, K.; Shuin, T. Terrestrosin D, a steroidal saponin from Tribulus terrestris L., inhibits growth and angiogenesis of human prostate cancer in vitro and in vivo. Pathobiology 2014, 81, 123–132. [Google Scholar] [CrossRef]
- XiaoJiao, Y.; CaiHong, B.; Kun, Z.; HaiBo, H.; XiaoQin, Y.; HuiLin, Q.; YongFeng, Z.; JunZhi, W. Steroidal saponin RCE-4 from Reineckia cornea (Andr.) Kunth inhibits growth of human cervical cancer xenograft in nude mice. J. Third Mil. Med. Univ. 2016, 38, 476–482. [Google Scholar]
- Iancu, G.; Serban, D.; Badiu, C.D.; Tanasescu, C.; Tudosie, M.S.; Tudor, C.; Costea, D.O.; Zgura, A.; Iancu, R.; Vasile, D. Tyrosine kinase inhibitors in breast cancer (Review). Exp. Ther. Med. 2022, 23, 114. [Google Scholar] [CrossRef]
- Li, C.J.; Li, Y.C.; Zhang, D.R.; Pan, J.H. Signal transducers and activators of transcription 3 function in lung cancer. J. Cancer Res. Ther. 2013, 9 (Suppl. 2), S67–S73. [Google Scholar] [CrossRef]
- Yue, P.; Turkson, J. Targeting STAT3 in cancer: How successful are we? Expert Opin. Investig. Drugs 2009, 18, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, C.; Xiao, D.; Han, J.; Yue, Z.; Sun, Y.; Fan, L.; Zhang, F.; Meng, J.; Zhang, R.; et al. Trillium tschonoskii steroidal saponins suppress the growth of colorectal Cancer cells in vitro and in vivo. J. Ethnopharmacol. 2015, 168, 136–145. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, H.; Li, J.; Xu, J.; Fei, Z. Anticancer Effects of Paris Saponins by Apoptosis and PI3K/AKT Pathway in Gefitinib-Resistant Non-Small Cell Lung Cancer. Med. Sci. Monit. 2016, 22, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Ji, Z.; Xu, C.; Zhu, J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: A systematic review and meta-analysis. Medicine 2018, 97, e12912. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, J.; Huang, B.; Liu, S.; Zhu, H.; Xu, T. Emerging role of the Hippo pathway in autophagy. Cell Death Dis. 2020, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, Y.P.; Zheng, L.; Yue, Z.G. Steroidal saponins from Paris polyphylla induce apoptotic cell death and autophagy in A549 human lung cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 1169–1173. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, S.; Xu, J.; Wang, Y.; Xiang, L.; He, X. Total steroidal saponins from black nightshade (Solanum nigrum L.) overcome tumor multidrug resistance by inducing autophagy-mediated cell death in vivo and in vitro. Phytother. Res. 2023, 37, 3009–3024. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, J.; Zhang, Z.; Dong, D.; Shen, Y.; Fang, Y.; Hu, L.; Liu, M.; Dai, C.; Peng, S.; et al. Iron and magnetic: New research direction of the ferroptosis-based cancer therapy. Am. J. Cancer Res. 2018, 8, 1933–1946. [Google Scholar] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, G.; Ye, J.; Yao, Y.; Lu, G.; Chen, J.; Fang, L.; Lu, S.; Zhou, J. Dioscin elicits anti-tumour immunity by inhibiting macrophage M2 polarization via JNK and STAT3 pathways in lung cancer. J. Cell. Mol. Med. 2020, 24, 9217–9230. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- He, H.; Zheng, L.; Sun, Y.P.; Zhang, G.W.; Yue, Z.G. Steroidal saponins from Paris polyphylla suppress adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 10911–10916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, C.; Bellance, N.; Rossignol, R. Choosing between glycolysis and oxidative phosphorylation: A tumor’s dilemma? Biochim. Biophys. Acta 2011, 1807, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.H.; Li, C.F.; Yang, W.L.; Gao, Y.; Lee, S.W.; Feng, Z.; Huang, H.Y.; Tsai, K.K.C.; Flores, L.G.; Shao, Y.; et al. The Skp2-SCF E3 Ligase Regulates Akt Ubiquitination, Glycolysis, Herceptin Sensitivity, and Tumorigenesis. Cell 2012, 151, 913–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, M.; Oshikawa, K.; Shimizu, H.; Yoshioka, S.; Takahashi, M.; Izumi, Y.; Bamba, T.; Tateishi, C.; Tomonaga, T.; Matsumoto, M.; et al. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat. Commun. 2020, 11, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.X.; Yu, P.C.; Li, J. High-Throughput Metabolomics for Identification of Metabolic Pathways and Deciphering the Effect Mechanism of Dioscin on Rectal Cancer From Cell Metabolic Profiles Coupled With Chemometrics Analysis. Front. Pharmacol. 2020, 11, 68. [Google Scholar] [CrossRef]
- Jaramillo, S.; Muriana, F.; Guillen, R.; Jimenez-Araujo, A.; Rodriguez-Arcos, R.; Lopez, S. Saponins from edible spears of wild asparagus inhibit AKT, p70S6K, and ERK signalling, and induce apoptosis through G0/G1 cell cycle arrest in human colon cancer HCT-116 cells. J. Funct. Foods 2016, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, F.; Zhuang, Y.; Liu, Y.; Xu, L.; Zhao, H.; Xiang, Y.; Dai, X.; Liu, Z.; Huang, X.; et al. Acetyl-bufalin shows potent efficacy against non-small-cell lung cancer by targeting the CDK9/STAT3 signalling pathway. Br. J. Cancer 2021, 124, 645–657. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Z.; Wang, Y.; Li, W.; Su, Q.; Jia, Q.; Zhang, J.; Zhang, X.; Shen, J.; Yin, J. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/β-catenin signaling pathway. Cell Death Dis. 2018, 9, 343. [Google Scholar] [CrossRef]
- Okawara, M.; Hashimoto, F.; Todo, H.; Sugibayashi, K.; Tokudome, Y. Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. Int. J. Pharm. 2014, 472, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Tang, Y.; Fawcett, J.P.; Gu, J.; Zhong, D. Characterization of the pharmacokinetics of dioscin in rat. Steroids 2005, 70, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, B.N.; Le, H.T.; Hong, H.N.; Lim, C.W.; Park, K.H.; Kim, T.W.; Kang, T.H. Small molecular weight PEGylation of diosgenin in an in vivo animal study for diabetic auditory impairment treatment. Bioorganic Med. Chem. Lett. 2012, 22, 4609–4612. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Baishya, D.; Sarkar, T.; Edinur, H.A.; Pati, S. Synthesis and Characterization of Diosgenin Encapsulated Poly-ε-Caprolactone-Pluronic Nanoparticles and Its Effect on Brain Cancer Cells. Polymers 2021, 13, 1322. [Google Scholar] [CrossRef]
- Chen, L.; Lan, J.; Li, Z.; Zeng, R.; Wang, Y.; Zhen, L.; Jin, H.; Ding, Y.; Zhang, T. A Novel Diosgenin-Based Liposome Delivery System Combined with Doxorubicin for Liver Cancer Therapy. Pharmaceutics 2022, 14, 1685. [Google Scholar] [CrossRef] [PubMed]
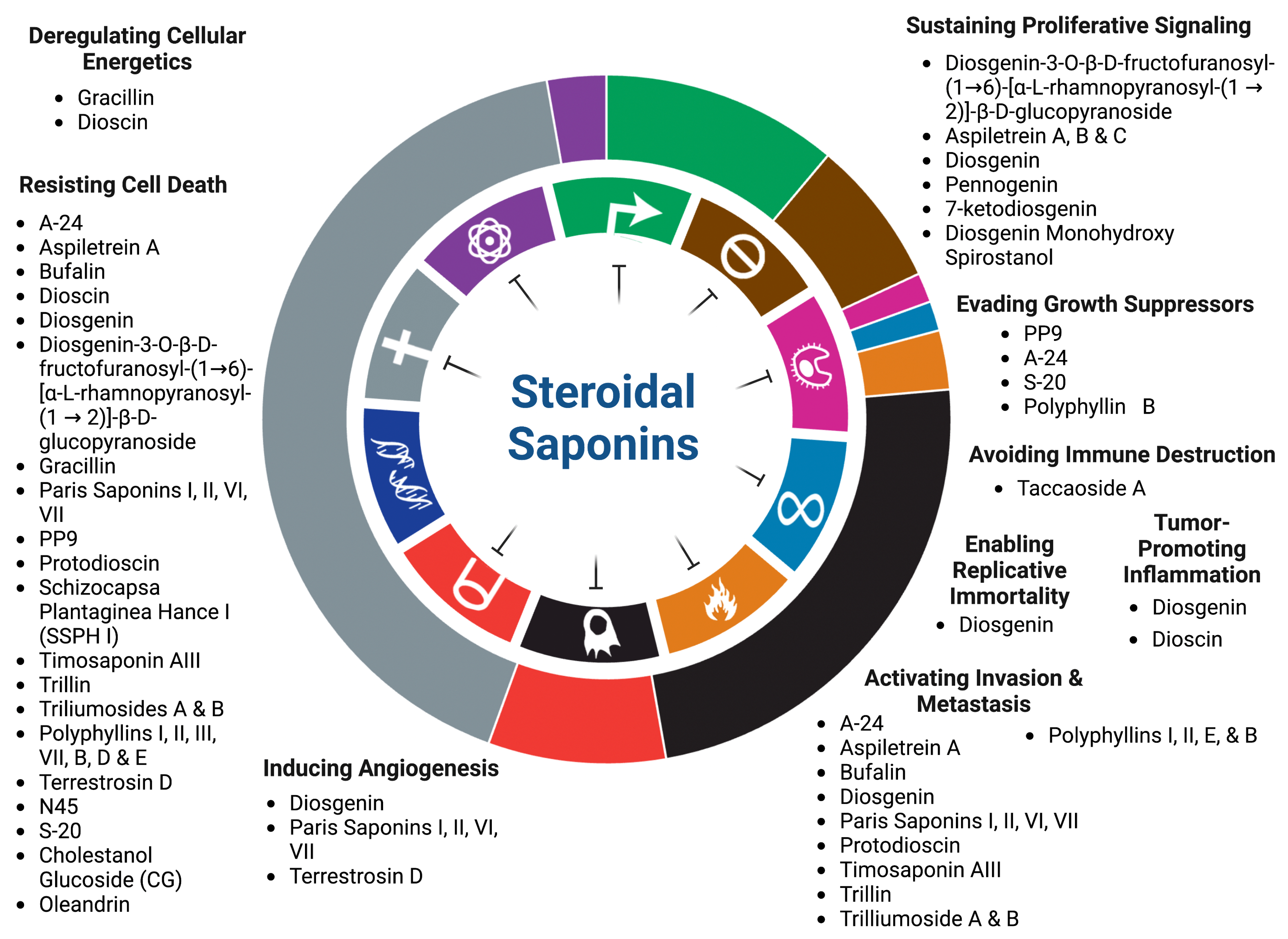
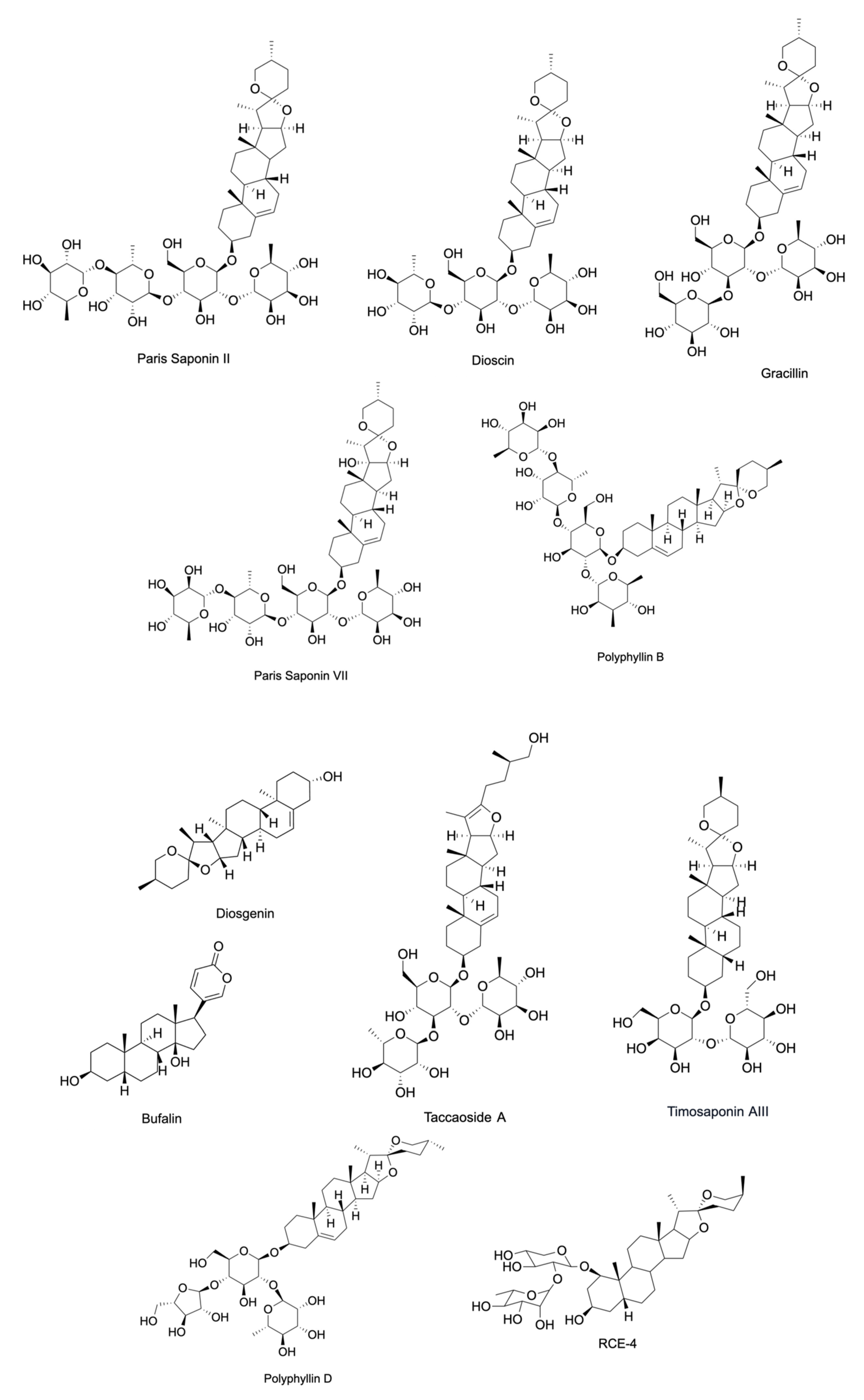
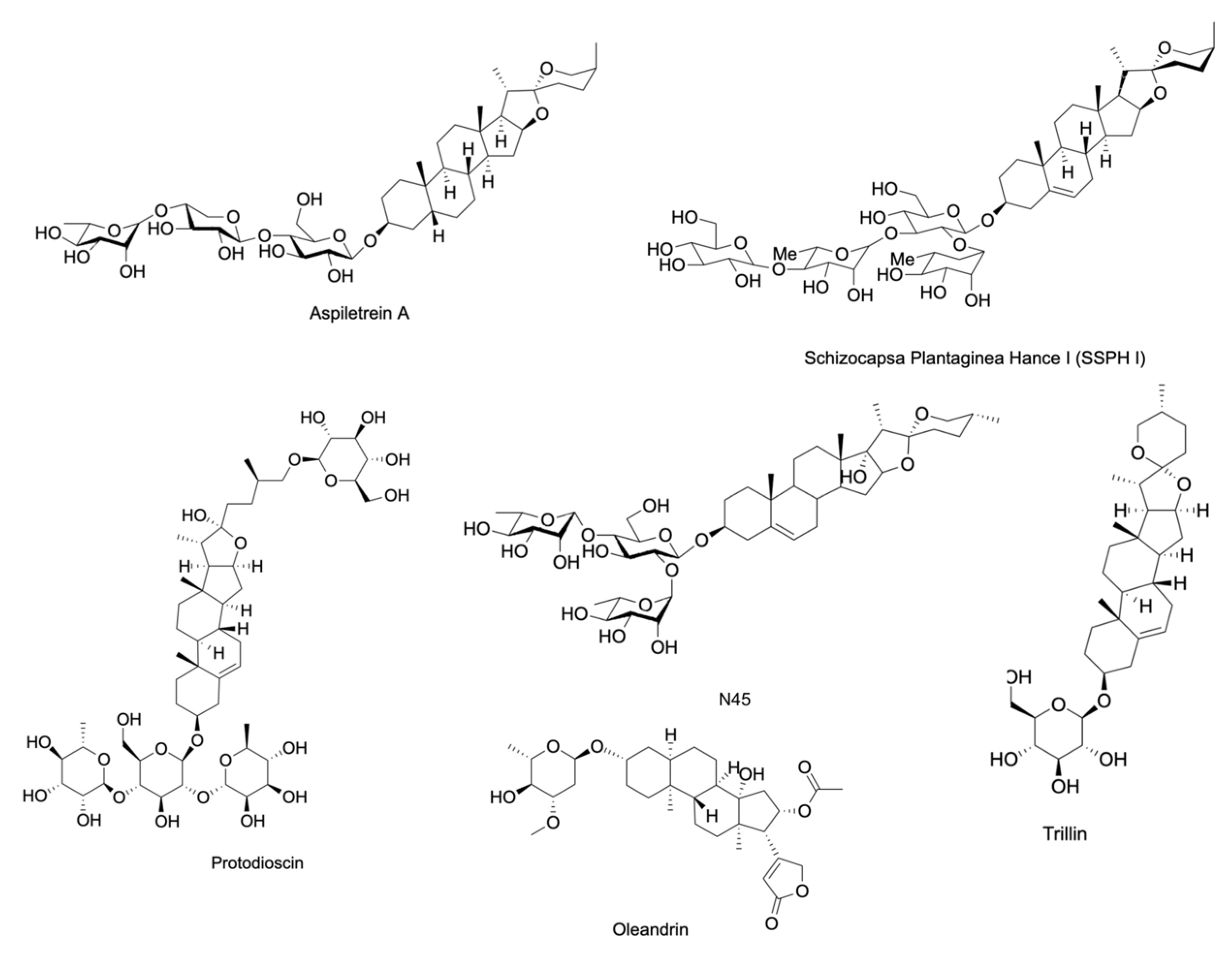

| Compound | Source | Cells | IC50 | Targeted Hallmark of Cancer | References |
|---|---|---|---|---|---|
| A-24 | Allium chinense | SGC-7901, AGS, MGC-803, NCI-N87, BGC-823, and KATO-III | 3.03 * µM (SGC-7901), 2.18 * µM (AGS), 4.10 * µM (MGC-803), 4.53 * µM (NCI-N87), and 5.11 * µM (BGC-823). | Resisting Cell Death, Evading Growth Suppressors, Activating Tumor Invasion and Metastasis | [20,21] |
| Aspiletrein A | Aspidistra letreae | LU-1, HeLa, MDA-MB-231, HepG2, MKN-7, H460, H23 and A549 | 9.94 ‡ µM (LU-1), 7.69 ‡ µM (HeLa), 7.75 ‡ µM (MDA-MB-231), 9.19 ‡ µM (HepG2), 9.39 ‡ µM (MKN-7), 15.13 * µM (H460), 10.10 * µM (H23), and 8.78 * µM (A549) | Sustaining Proliferative Signaling, Activating Invasion and Metastasis, Resisting Cell Death | [22,23,24] |
| Aspiletrein B | Aspidistra letreae | LU-1, HeLa, MDA-MB-231, HepG2, MKN-7, and H460 | 20.27 ‡ µM (LU-1), 12.54 ‡ µM (HeLa), 20.46 ‡ µM (MDA-MB-231), 16.07 ‡ µM (HepG2), 18.15 ‡ µM (MKN-7), and 6.82 * µM (H460) | Sustaining Proliferative Signaling | [23,24] |
| Aspiletrein C | Aspidistra letreae | LU-1, HeLa, MDA-MB-231, HepG2, MKN-7, and H460 | 10.10 ‡ µM (LU-1), 9.03 ‡ µM (HeLa), 9.09 ‡ µM (MDA-MB-231), 8.84 ‡ µM (HepG2), 11.82 ‡ µM (MKN-7), and 15.75 * µM (H460) | Sustaining Proliferative Signaling | [23,24] |
| Bufalin | Bufo gargarizans | U251, SK-N-BE, SH-SY5Y, A549, GBC-SD | 90 ‡ nM (SK-N-BE) and 30 ‡ nM (SH-SY5Y) | Resisting Cell Death, Activating Invasion and Metastasis | [25,26,27] |
| Dioscin | Dioscorea zingiberensis and Dioscorea nipponica | HUVEC, A375, G361, and WM115 | - | Tumor Promoting Inflammation, Resisting Cell Death, and Deregulating Cellular Energetics | [28,29] |
| Diosgenin | Prunus dulcis, Trigonella foenum-graecum, Dioscorea villosa, and Dioscorea japonica | SW480, DU145, LnCaP, T98G, C6, and A549 | 47 * µM (A549) | Sustaining Proliferative Signaling, Resisting Cell Death, Activating Invasion and Metastasis, Inducing Angiogenesis, Enabling Replicative Immortality, and Tumor Promoting Inflammation | [30,31,32,33,34,35,36] |
| Diosgenin-3-O-β-D-fructofuranosyl-(1→6)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside | Paris polyphylla | LN229, U251, Capan-2, HeLa, and HepG2 | 4.18 * µM (LN229), 3.85 * µM (U251), 3.26 * µM (Capan-2), 3.30 * µM (HeLa), and 4.32 * µM (HepG2) | Sustaining Proliferative Signaling and Resisting Cell Death | [37] |
| Gracillin | Pairs polyphylla, Dioscorea villosa, Aconitum carmichaeli, Solanum incanum, and Solanum virginianum | MDA-MB-231, MCF7, H460, H226B, T47D, MDA-MB-453, and A549 | 2.421 * μmol/L. (A549) | Deregulating Cellular Energetics and Resisting Cell Death | [38,39] |
| Paris Saponin I | Paris polyphylla | HUVEC and PC-9-ZD | 0.643 † µM (HUVEC) and 2.51 * µM (PC-9-ZD) | Resisting Cell Death, Inducing Angiogenesis, Activating Invasion and Metastasis | [40] |
| Paris Saponin II | Paris polyphylla | HUVEC and PC-9-ZD | 0.994 † µM (HUVEC) and 3.12 * µM (PC-9-ZD) | Resisting Cell Death, Inducing Angiogenesis, Activating Invasion and Metastasis | [40] |
| Paris Saponin VI | Paris polyphylla | HUVEC and PC-9-ZD | 2.204 † µM (HUVEC) and 4.21 * µM (PC-9-ZD) | Resisting Cell Death, Inducing Angiogenesis, Activating Invasion and Metastasis | [40] |
| Paris Saponin VII | Paris polyphylla | MDA-MB-231, MDA-MB-436, MCF-7, HUVEC, and PC-9-ZD | 3.16 * µM (MDA-MB-231), 3.45 * µM (MDA-MB-436), 2.86 * µM (MCF-7), 6.212 † µM (HUVEC), and 3.57 * µM (PC-9-ZD) | Resisting Cell Death, Inducing Angiogenesis, Activating Invasion and Metastasis | [40,41] |
| PP9 | Paris polyphylla | HT-29 and HCT116 | 1.08 † µM (HT-29) and 1.97 † µM (HCT116) | Resisting Cell Death, Evading Growth Suppressors | [42] |
| Protodioscin | Dioscorea villosa, Trigonella foenum-graecum, and Asparagus officinalis | 5637 and T24 cells | 72.6 * µM (5637) and 63.4 * µM (T24) | Resisting Cell Death, Activating Invasion and Metastasis | [43] |
| Schizocapsa Plantaginea Hance I (SSPH I) | Tacca plantaginea | HepG2 | 3.395 * µM | Resisting Cell Death | [44] |
| Taccaoside A | Tacca plantaginea and Tacca subflabellata | H1299, MHCC97H, BT549, SW620, and HUVEC | - | Avoiding Immune Destruction | [45] |
| Timosaponin AIII | Anemarrhena asphodeloides | H1266 and A549 | 1.55 † µM (H1266) and 2.16 † µM (A549) | Resisting Cell Death, Activating Invasion and Metastasis | [46] |
| Trillin | Trillium tschonoskii | HepG2 and PLC/PRF | - | Resisting Cell Death, Activating Invasion and Metastasis | [47] |
| Trilliumoside A | Trillium govanianum | A549 and SW-620 | 1.83 † µM (A-549) and 1.85 † µM (SW-620) | Resisting Cell Death, Activating Invasion and Metastasis | [48] |
| Trilliumoside B | Trillium govanianum | A549 and SW-620 | 1.79 † µM (A-549) and 3.18 † µM (SW-620) | Resisting Cell Death, Activating Invasion and Metastasis | [48] |
| Polyphyllin II | Paris polyphylla | HepG2, BEL7402, T24, and 5637 | 4.8351 * μM (HepG2), 4.4765 * μM (BEL7402), 4.43 ± 0.08 µg/mL (T24), and 7.87 ± 0.39 µg/mL (5637) | Resisting Cell Death, Activating Invasion and Metastasis | [49,50] |
| Polyphyllin E | Paris polyphylla | SK-OV-3 and OVCAR-3 | 7.462 * µM (SK-OV-3) and 5.053 * µM (OVCAR-3) | Resisting Cell Death, Activating Invasion and Metastasis | [51] |
| Cholestanol Glucoside (CG) | Lasiodiplodia theobromae | A549, PC3, HepG2, U251, MCF7, and OVCAR3 | - | Resisting Cell Death | [52] |
| Polyphyllin VII | Paris polyphylla | A549 | 0.41 * µM | Resisting Cell Death | [53] |
| S-20 | Solanum americanum | K562, K562/ADR, HL-60, and U937 | 8.35 µM ± 0.57 (K562), 10.74 µM ± 0.92 (K562/ADR), 22.14 µM ± 0.54 (HL-60), and 29.52 µM ± 1.99 (U937) | Evading Growth Suppressors and Resisting Cell Death | [54] |
| Polyphyllin B | Paris polyphylla | NUGC-3, MKN-1, MKN-45, HGC-27, and NUGC-4 | 1.447 * µM (NUGC-3), 2.734 * µM (MKN-1), 3.378 * µM (MKN-45), 3.318 * µM (HGC-27), and 2.579 * µM (NUGC-4) | Activating Invasion and Metastasis, Resisting Cell Death, and Evading Growth Suppressors | [55] |
| Polyphyllin III | Paris polyphylla | MDA-MB-231, HS-578T, HBL-100, MCF-7, and T47D | 7.96 * µM (MDA-MB-231), 2.59 * µM (HS-578T), 7.74 * µM (HBL-100), 3.89 * µM (MCF-7), and 9.85 * µM (T47D) | Resisting Cell Death | [56] |
| Polyphyllin D | Paris polyphylla | IMR-32, LA-N-2, and NB-69 | 25 * µM (IMR-32), 20 * µM (LA-N-2), and 5 * µM (NB-69) | Resisting Cell Death | [57] |
| Polyphyllin I | Paris polyphylla | SGC7901/DDP, SGC7901, 143-B, HOS, DU145, and PC3 | 2.48 * μM (SGC7901), 0.93 * μM (SGC7901/DDP), 0.3942 † µM (143-B), 0.8145 † µM (HOS), 1.03 * µM (DU145), and 2.13 * μM (PC3) | Resisting Cell Death, Activating Invasion and Metastasis | [58,59,60] |
| Pennogenin-3α-L-rhamnopyranosyl-(1→4)-[α-Lrhamno-pyranosyl-(1→2)]-β-D-glucopyranoside (N45) | Paris vietnamensis | U251 and U87 | 3.808 * μg/mL (U251) and 3.39 * (U87) μg/mL | Resisting Cell Death | [61] |
| Oleandrin | Nerium oleander | A549, SW480, HCT116, RKO, A375, GL261, U87MG, MCF7, SK-BR-3, and MDA-MB-231 | 47 † nM (A375), 6.07 † nM (MCF7), 1.42 † nM (SK-BR-3), and 11.47 † nM (MDA-MB-231) | Resisting Cell Death | [62,63,64,65,66] |
| Terrestrosin D | Tribulus terrestris | HUVEC and PC-3 | - | Resisting Cell Death, Evading Growth Suppressors, and Inducing Angiogenesis | [67] |
| Compound | Source | In Vivo Model | Targeted Hallmark of Cancer | References |
|---|---|---|---|---|
| Bufalin | Bufo gargarizans | Neuroblastoma, Gallbladder Cancer | Resisting Cell Death, Activating Invasion and Metastasis | [37,51] |
| Dioscin | Dioscorea zingiberensis and Dioscorea nipponica | Lung Adenocarcinoma and Colorectal Cancer | Tumor Promoting InflammationTumor Microenvironment, Activating Invasion and Metastasis, and Deregulating Cellular Energetics | [55,62] |
| Diosgenin | Prunus dulcis, Prunus amygdalus, Trigonella foenum-graecum, Dioscorea villosa, and Dioscorea japonica | Colorectal Cancer and Prostate Cancer | Sustaining Proliferative Signaling, Resisting Cell Death, Activating Invasion and Metastasis, and Resisting Cell Death | [28,29] |
| Gracillin | Rhizoma paridis, Pairs polyphylla, Dioscorea villosa, AconitumAcontum carmichaeli, Solanum incanum, Solanum virginianum, and Solanum xanthocarpum | Breast Cancer and Non-small Cell Lung Cancer | Deregulating Cellular Energetics and Resisting Cell Death | [39,54] |
| Paris Saponin VII | Paris polyphylla | Breast Cancer | Resisting Cell Death | [42,60] |
| Polyphyllin B | Paris polyphylla | Gastric Cancer | Resisting Cell Death | [55] |
| Polyphyllin I | Paris polyphylla | Osteosarcoma, Prostate Cancer, and Gastric Cancer | Resisting Cell Death, Activating Invasion and Metastasis | [58,59,60] |
| Polyphyllin III | Paris polyphylla | Breast Cancer | Resisting Cell Death | [56] |
| PP9 | Paris polyphylla | Colorectal Cancer | Resisting Cell Death, and Evading Growth Suppressors | [33] |
| Protodioscin | Dioscorea villosa, Trigonella foenum-graecum, and Asparagus officinalis | Bladder Cancer | Resisting Cell Death, Activating Invasion and Metastasis | [35] |
| RCE-4 | Reineckea carnea | Cervical Cancer | Resisting Cell Death, and Tumor Promoting Inflammation | [68] |
| Taccaoside A | Tacca plantaginea and Tacca subflabellata | Non-small Cell Lung Cancer | Avoiding Immune Destruction | [61] |
| Terrestrosin D | Tribulus terrestris | Prostate Cancer | Resisting Cell Death, Evading Growth Suppressors, and Inducing Angiogenesis | [67] |
| Timosaponin AIII | Anemarrhena asphodeloides | Non-small Cell Lung Cancer | Resisting Cell Death, Activating Invasion and Metastasis | [46] |
| Oleandrin | Nerium oleander | Glioma | Resisting Cell Death | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouabdallah, S.; Al-Maktoum, A.; Amin, A. Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer. Cancers 2023, 15, 3900. https://doi.org/10.3390/cancers15153900
Bouabdallah S, Al-Maktoum A, Amin A. Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer. Cancers. 2023; 15(15):3900. https://doi.org/10.3390/cancers15153900
Chicago/Turabian StyleBouabdallah, Salwa, Amna Al-Maktoum, and Amr Amin. 2023. "Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer" Cancers 15, no. 15: 3900. https://doi.org/10.3390/cancers15153900
APA StyleBouabdallah, S., Al-Maktoum, A., & Amin, A. (2023). Steroidal Saponins: Naturally Occurring Compounds as Inhibitors of the Hallmarks of Cancer. Cancers, 15(15), 3900. https://doi.org/10.3390/cancers15153900







