Cancer-Associated Thrombosis on Bevacizumab: Risk of Recurrence and Bleeding When Bevacizumab Is Stopped or Continued
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
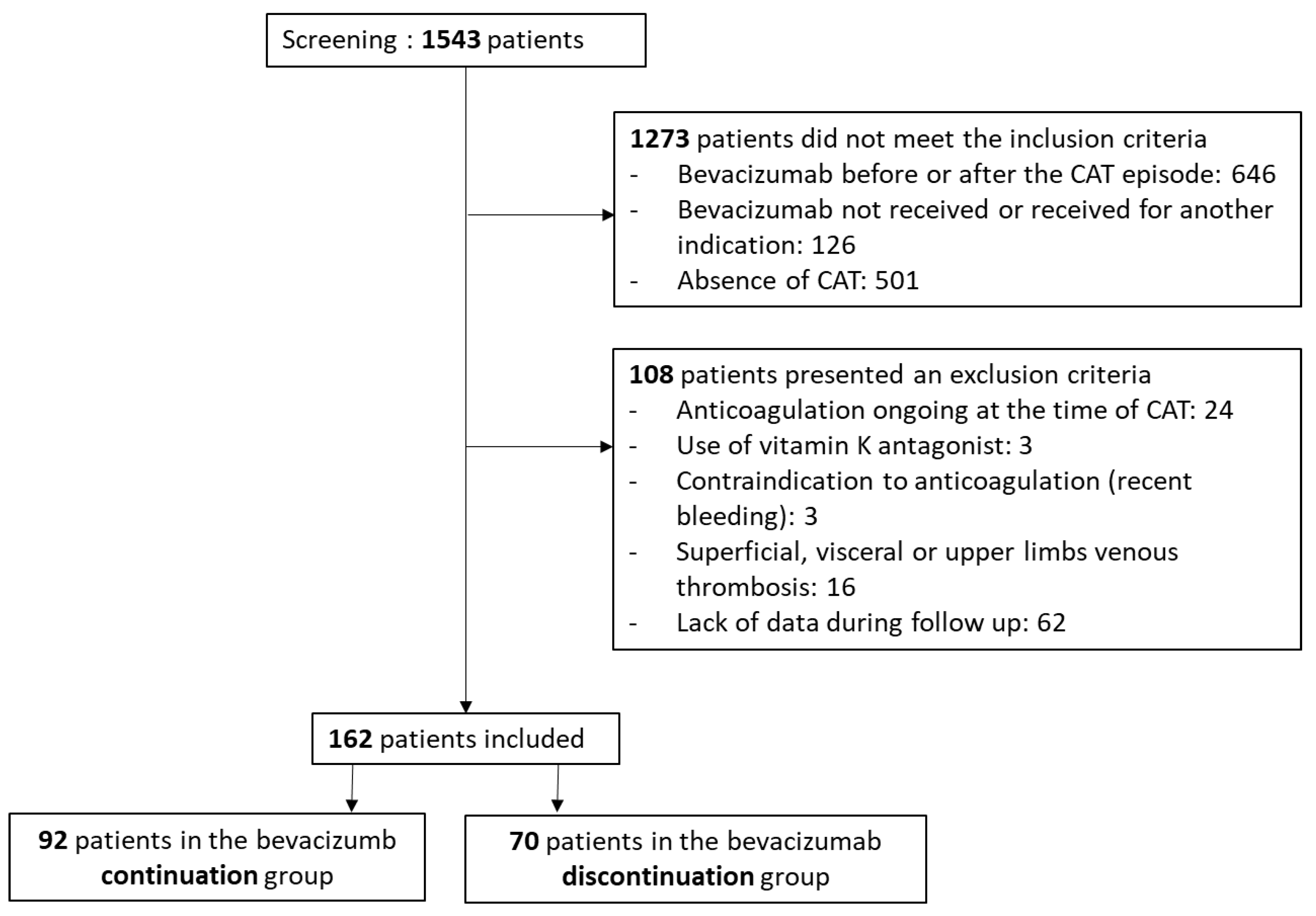
2.2. Patients, Inclusion Criteria, and Non-Inclusion Criteria
2.3. Surveillance and Follow-up
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
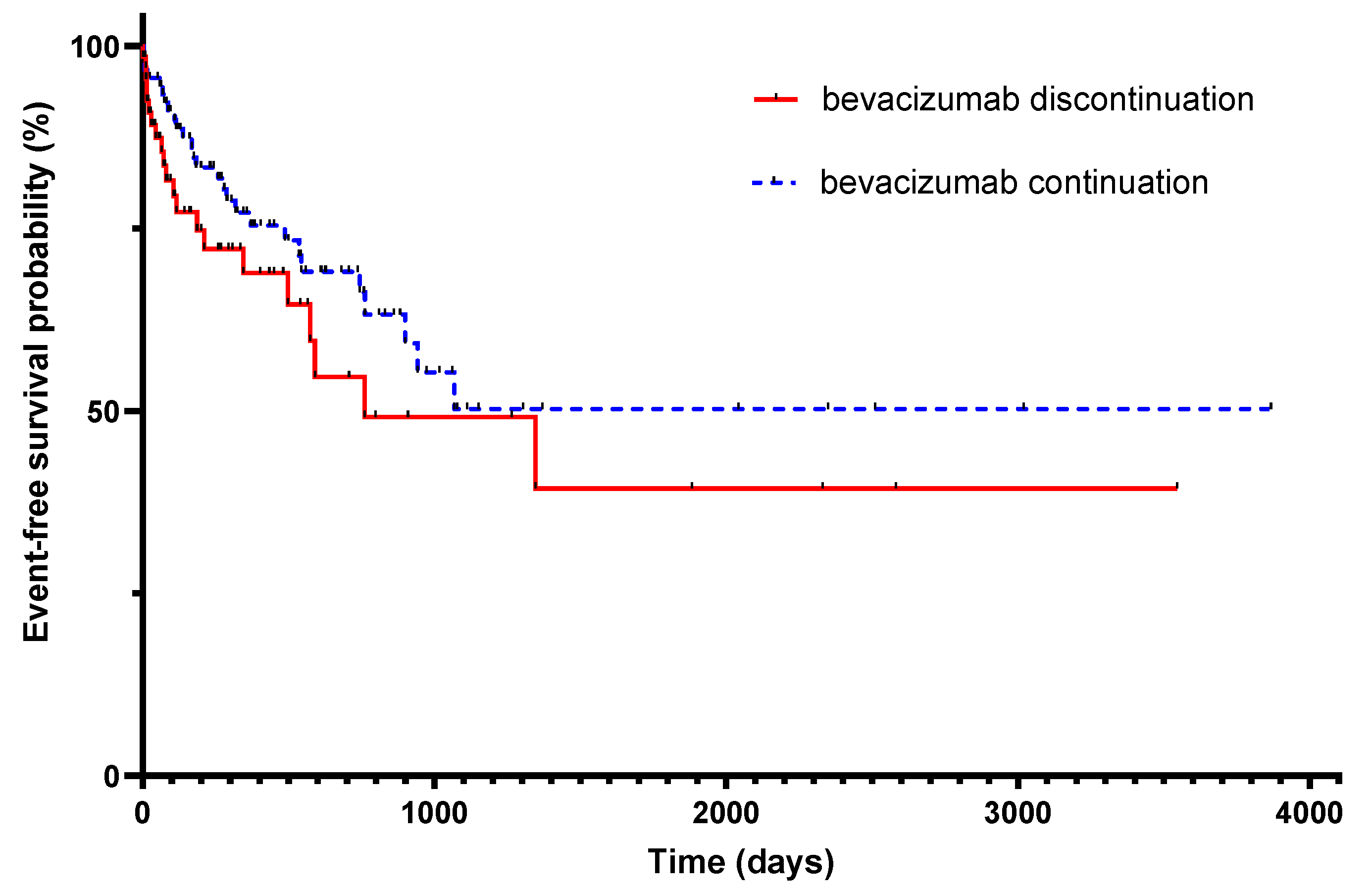
3.2. Primary Endpoint
3.3. Bleedings
3.4. Recurrences
3.5. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2012, 119, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Hisada, Y.M.; Kasthuri, R.S.; Reeves, B.N.; Mackman, N. Cancer Therapy–Associated Thrombosis. Arter. Thromb. Vasc. Biol. 2021, 41, 1291–1305. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Farge, D.; Trujillo-Santos, J.; Debourdeau, P.; Bura-Riviere, A.; Rodriguez-Beltrán, E.M.; Nieto, J.A.; Peris, M.L.; Zeltser, D.; Mazzolai, L.; Hij, A.; et al. Fatal Events in Cancer Patients Receiving Anticoagulant Therapy for Venous Thromboembolism. Medicine 2015, 94, e1235. [Google Scholar] [CrossRef] [Green Version]
- Prandoni, P.; Lensing, A.W.A.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef] [Green Version]
- Angelini, D.E.; Radivoyevitch, T.; McCrae, K.R.; Khorana, A.A. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am. J. Hematol. 2019, 94, 780–785. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Roviello, G.; Bachelot, T.; Hudis, C.A.; Curigliano, G.; Reynolds, A.R.; Petrioli, R.; Generali, D. The role of bevacizumab in solid tumours: A literature based meta-analysis of randomised trials. Eur. J. Cancer 2017, 75, 245–258. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Wang, W.; Zhang, D. Risk of bleeding associated with antiangiogenic monoclonal antibodies bevacizumab and ramucirumab: A meta-analysis of 85 randomized controlled trials. OncoTargets Ther. 2018, 11, 5059–5074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, X.F.; Xu, W.S.; Wang, J.X.; Wang, L.; Xin, H.G.; Zhang, R.Q.; Ni, W. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: A meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2011, 67, 613–623. [Google Scholar] [CrossRef]
- Nalluri, S.R.; Chu, D.; Keresztes, R.; Zhu, X.; Wu, S. Risk of Venous Thromboembolism With the Angiogenesis Inhibitor Bevacizumab in Cancer Patients: A meta-analysis. JAMA 2008, 300, 2277–2285. [Google Scholar] [CrossRef]
- Scappaticci, F.A.; Skillings, J.R.; Holden, S.N.; Gerber, H.-P.; Miller, K.; Kabbinavar, F.; Bergsland, E.; Ngai, J.; Holmgren, E.; Wang, J.; et al. Arterial Thromboembolic Events in Patients with Metastatic Carcinoma Treated with Chemotherapy and Bevacizumab. J. Natl. Cancer Inst. 2007, 99, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, H.I.; Saltz, L.B.; Van Cutsem, E.; Cassidy, J.; Wiedemann, J.; Sirzén, F.; Lyman, G.H.; Rohr, U.-P. Venous Thromboembolic Events With Chemotherapy Plus Bevacizumab: A Pooled Analysis of Patients in Randomized Phase II and III Studies. J. Clin. Oncol. 2011, 29, 1757–1764. [Google Scholar] [CrossRef]
- Leighl, N.B.; Bennouna, J.; Yi, J.; Moore, N.; Hambleton, J.; Hurwitz, H. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br. J. Cancer 2011, 104, 413–418. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 1901647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug, Administration. ABP215, a Proposed Biosimilar to Avastin (Bevacizumab); FDA Briefing Document; FDA: Silver Spring, MD, USA, 2017. [Google Scholar]
- Schulman, S.; Kearon, C. The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Saerens, M.; De Jaeghere, E.A.; Kanervo, H.; Vandemaele, N.; Denys, H.; Naert, E. Risk of Thrombo-Embolic Events in Ovarian Cancer: Does Bevacizumab Tilt the Scale? A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4603. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.; Al-Samkari, H. Thrombotic and bleeding risk of angiogenesis inhibitors in patients with and without malignancy. J. Thromb. Haemost. 2021, 19, 1852–1863. [Google Scholar] [CrossRef]
- Mahé, I.; Chidiac, J.; Bertoletti, L.; Font, C.; Trujillo-Santos, J.; Peris, M.; Ductor, C.P.; Nieto, S.; Grandone, E.; Monreal, M.; et al. The Clinical Course of Venous Thromboembolism May Differ According to Cancer Site. Am. J. Med. 2017, 130, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2019, 173, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-Molecular-Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Cohen, A.T.; Katholing, A.; Rietbrock, S.; Bamber, L.; Martinez, C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb. Haemost. 2017, 117, 57–65. [Google Scholar] [CrossRef]
- Abdulla, A.; Davis, W.M.; Ratnaweera, N.; Szefer, E.; Scott, B.B.; Lee, A.Y.Y. A Meta-Analysis of Case Fatality Rates of Recurrent Venous Thromboembolism and Major Bleeding in Patients with Cancer. Thromb. Haemost. 2020, 120, 702–713. [Google Scholar] [CrossRef]

| Population n = 162 | Bevacizumab Continuation Group, n = 92 | Bevacizumab Discontinuation Group, n = 70 | p-Value | |
|---|---|---|---|---|
| Age at cancer diagnosis | 64 [55–71] | 64 [54–71] | 64 [57–1.5] | 0.82 |
| Sex | ||||
| Female | 96 (59%) | 58 (63%) | 38 (54%) | 0.33 |
| Male | 66 (41%) | 34 (37%) | 32 (46%) | |
| BMI (n = 156) | 24.6 [21–28] | 24 [20–27] | 24.6 [22–28] | 0.24 |
| Performance Status | ||||
| 0–1 | 130/153 (85%) | 75/85 (88%) | 55/68 (81%) | 0.18 |
| 2–3 | 23 (15%) | 10 (12%) | 13 (19%) | 0.26 |
| Renal failure (eGFR < 60 mL/mn) | 27/144 (19%) | 15/92 (16%) | 12/63 (19%) | >0.99 |
| Anemia (Hb < 100 g/L) | 11/153 5 (7%) | 4/86 (5%) | 7/67 (10%) | 0.21 |
| Thrombocytosis (>300 G/L) | 38/148 (26%) | 19/85 (22%) | 19/63 (30%) | 0.34 |
| Antiaplatelet therapy | 17 (11%) | 11 (12%) | 6 (9%) | 0.61 |
| Cancer type | ||||
| Colorectal | 78 (48%) | 39 (42%) | 39 (56%) | 0.11 |
| Ovarian, endometrial | 28 (17%) | 20 (21%) | 8 (11%) | 0.10 |
| Breast | 15 (9%) | 10 (11%) | 5 (7%) | 0.59 |
| Lung | 18 (11%) | 13 (14%) | 5 (7%) | 0.21 |
| Central nervous system | 17 (11%) | 7 (8%) | 10 (14%) | 0.20 |
| Others | 6 (4%) | 3 (3%) | 3 (4%) | 0.99 |
| Histological subtype | ||||
| Adénocarcinome | 119 (73%) | 67 (73%) | 52 (74%) | 0.86 |
| Current treatment line | ||||
| 1st line | 74/152 (49%) | 44/85 (52%) | 30/67 (45%) | 0.43 |
| 2nd line | 50 (33%) | 24 (28%) | 26 (39%) | 0.22 |
| 3rd line | 28 (18%) | 17 (20%) | 11 (16%) | 0.68 |
| Platinum salt treatment | 62 (38%) | 36 (39%) | 26 (37%) | 0.87 |
| Metastatic disease | 123/146 (84%) | 77/85 (91%) | 46/61 (75%) | 0.02 |
| Metastases | ||||
| Cerebral | 9 (6%) | 6 (6%) | 3 (4%) | 0.73 |
| Hepatic | 57 (35%) | 29 (32%) | 28 (40%) | 0.32 |
| Population n = 162 | Bevacizumab Continuation Group, n = 92 | Bevacizumab Discontinuation Group, n = 70 | p-Value | |
|---|---|---|---|---|
| CAT localization | ||||
| DVT | 58 (36%) | 34 (37%) | 24 (34%) | 0.74 |
| PE | 81 (50%) | 48 (52%) | 33 (47%) | 0.63 |
| DVT and PE | 23 (14%) | 10 (11%) | 13 (19%) | 0.18 |
| Most proximal obstruction of PE | ||||
| Segmental or more proximal | 79/97 (81%) | 46/54 (85%) | 33/43 (76%) | 0.31 |
| Subsegmental | 18 (19%) | 8 (15%) | 10 (23%) | |
| Unilateral | 55/98 (56%) | 32/56 (57%) | 23/42 (55%) | 0.84 |
| Bilateral | 43 (44%) | 24 (43%) | 19 (45%) | |
| Discovery mode Clinically suspected | 82/160 (51%) | 44/91 (48%) | 38/69 (55%) | 0.43 |
| Incidental asymptomatic | 65 (41%) | 42 (46%) | 23 (33%) | 0.11 |
| Incidental symptomatic | 13 (8%) | 5 (6%) | 8 (12%) | 0.24 |
| Time between CAT and first inclusion (days) | 3080 [1947–4089] | 3239 [2931–3554] | 2528 [2313–3505] | 0.03 |
| LMWH therapy | 152 (94%) | 87(95%) | 65 (93%) | 0.75 |
| LMWH discontinuation during the follow-up | 52/145 (36%) | 31/79(39%) | 21/66 (32%) | 0.23 |
| Time between bevacizumab initiation and CAT (days) | 79 [39–154] | 77 [41–141] | 84 [35–173] | 0.55 |
| Bevacizumab posology at CAT diagnosis (mg/kg) (n = 135) | 7.5 [5–10] | 7.5 [5–10] | 5 [5–10] | 0.20 |
| Other risk factor of CAT | 37 (23%) | 18 (20%) | 19 (21%) | 0.26 |
| Response to oncologic treatment Response | 40/154 (26%) | 27/87 (31%) | 13/67 (19%) | 0.14 |
| Stability | 76 (49%) | 50 (57%) | 26 (38%) | 0.02 |
| Progression | 38 (25%) | 10 (11%) | 28 (42%) | 0.0001 |
| Population n = 162 | Bevacizumab Continuation Group, n = 92 | Bevacizumab Discontinuation Group, n = 70 | |
|---|---|---|---|
| CAT recurrence or bleeding | 48 (30%) | 27 (29%) | 21 (30%) |
| Recurrence | 21 (13%) | 13 (14%) | 8 (11%) |
| Bleeding | 27 (17%) | 14 (15%) | 13 (19%) |
| Major bleeding | 10 (6%) | 6 (7%) | 4 (6%) |
| Clinically relevant non major bleeding | 17 (11%) | 8 (8%) | 9 (13%) |
| Population n = 162 | Bevacizumab Continuation Group, n = 92 | Bevacizumab Discontinuation Group, n = 70 | |
|---|---|---|---|
| Death | 139 (90%) | 80 (87%) | 59 (82%) |
| Cancer related deaths | 115 (83%) | 65 (81%) | 50 (85%) |
| Fatal bleeding | 4 (3%) | 3 (4%) | 1 (2%) |
| Other cause of death | 12 (9%) | 6 (8%) | 6 (10%) |
| Unknown cause of death | 4 (3%) | 6 (8%) | 2 (3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayenga, M.; Falvo, N.; Mahé, I.; Jannot, A.-S.; Gazeau, B.; Meyer, G.; Gendron, N.; Sanchez, O.; Djennaoui, S.; Planquette, B. Cancer-Associated Thrombosis on Bevacizumab: Risk of Recurrence and Bleeding When Bevacizumab Is Stopped or Continued. Cancers 2023, 15, 3893. https://doi.org/10.3390/cancers15153893
Mayenga M, Falvo N, Mahé I, Jannot A-S, Gazeau B, Meyer G, Gendron N, Sanchez O, Djennaoui S, Planquette B. Cancer-Associated Thrombosis on Bevacizumab: Risk of Recurrence and Bleeding When Bevacizumab Is Stopped or Continued. Cancers. 2023; 15(15):3893. https://doi.org/10.3390/cancers15153893
Chicago/Turabian StyleMayenga, Marie, Nicolas Falvo, Isabelle Mahé, Anne-Sophie Jannot, Benoit Gazeau, Guy Meyer, Nicolas Gendron, Olivier Sanchez, Sadji Djennaoui, and Benjamin Planquette. 2023. "Cancer-Associated Thrombosis on Bevacizumab: Risk of Recurrence and Bleeding When Bevacizumab Is Stopped or Continued" Cancers 15, no. 15: 3893. https://doi.org/10.3390/cancers15153893
APA StyleMayenga, M., Falvo, N., Mahé, I., Jannot, A.-S., Gazeau, B., Meyer, G., Gendron, N., Sanchez, O., Djennaoui, S., & Planquette, B. (2023). Cancer-Associated Thrombosis on Bevacizumab: Risk of Recurrence and Bleeding When Bevacizumab Is Stopped or Continued. Cancers, 15(15), 3893. https://doi.org/10.3390/cancers15153893







