The Risk of Venous Thromboembolism in Korean Patients with Breast Cancer: A Single-Center Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Procedures
2.3. Outcome Variables
2.4. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics of the Patients
3.2. Factors Associated with VTE Occurrence
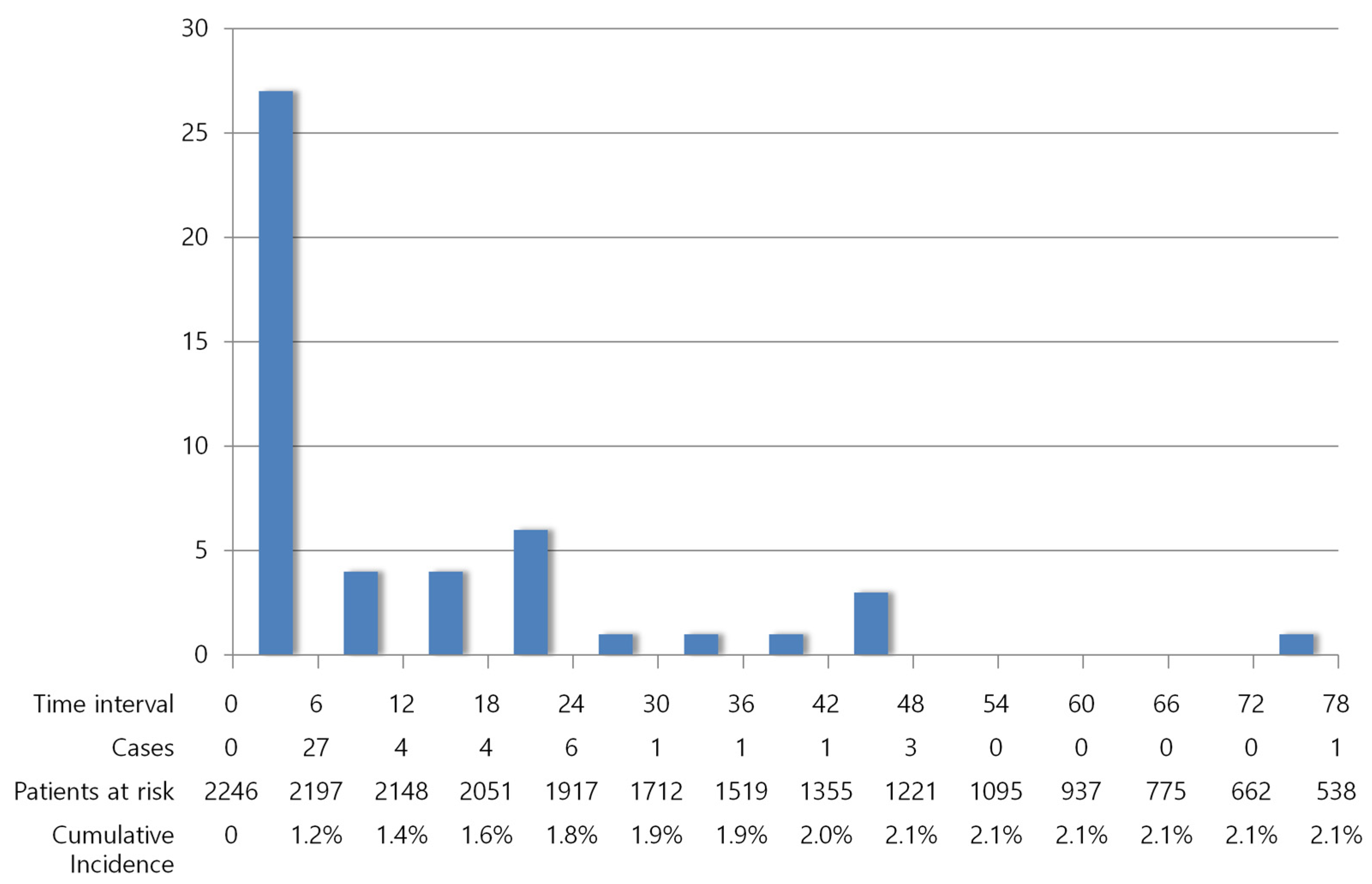
3.3. Occurrence of VTE over Time
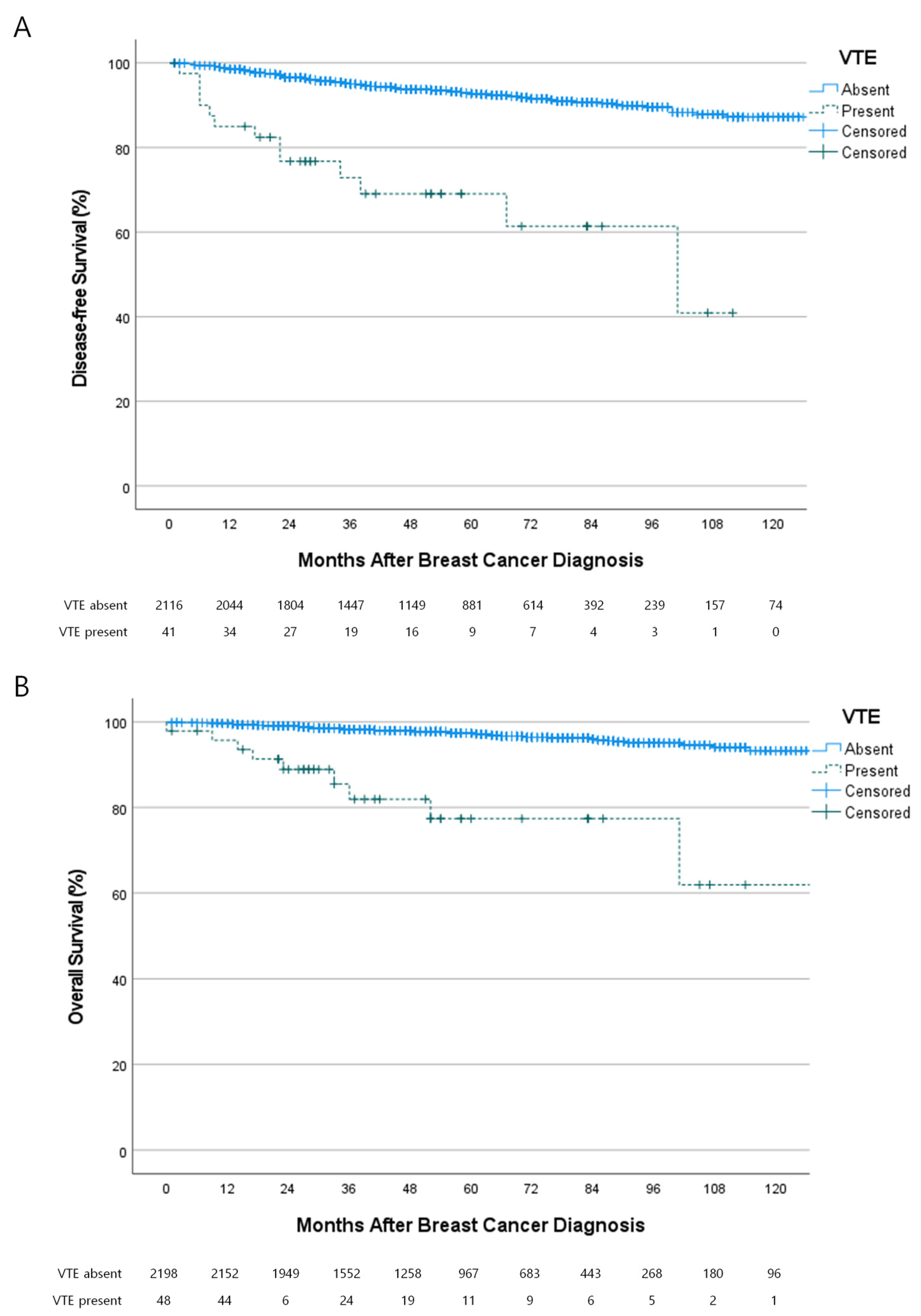
3.4. Oncologic Outcomes of the Patients with VTE
3.5. Clinical Presentations of the Patients with VTE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liederman, Z.; Chan, N.; Bhagirath, V. Current Challenges in Diagnosis of Venous Thromboembolism. J. Clin. Med. 2020, 9, 3509. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, C.C.; Blower, E.L. Contemporary breast cancer treatment-associated thrombosis. Thromb. Res. 2022, 213, S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Mandala, M.; Tondini, C. Adjuvant therapy in breast cancer and venous thromboembolism. Thromb. Res. 2012, 130 (Suppl. S1), S66–S70. [Google Scholar] [CrossRef]
- Nasser, N.J.; Fox, J.; Agbarya, A. Potential Mechanisms of Cancer-Related Hypercoagulability. Cancers 2020, 12, 566. [Google Scholar] [CrossRef]
- Lyman, G.H. Venous thromboembolism in the patient with cancer: Focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011, 117, 1334–1349. [Google Scholar] [CrossRef]
- Angchaisuksiri, P.; Goto, S.; Farjat, A.E.; Fryk, H.; Bang, S.M.; Chiang, C.E.; Jing, Z.C.; Kondo, K.; Sathar, J.; Tse, E.; et al. Venous thromboembolism in Asia and worldwide: Emerging insights from GARFIELD-VTE. Thromb. Res. 2021, 201, 63–72. [Google Scholar] [CrossRef]
- Oh, H.; Boo, S.; Lee, J.A. Clinical nurses’ knowledge and practice of venous thromboembolism risk assessment and prevention in South Korea: A cross-sectional survey. J. Clin. Nurs. 2017, 26, 427–435. [Google Scholar] [CrossRef]
- Lee, L.H.; Nagarajan, C.; Tan, C.W.; Ng, H.J. Epidemiology of Cancer-Associated Thrombosis in Asia: A Systematic Review. Front. Cardiovasc. Med. 2021, 8, 669288. [Google Scholar] [CrossRef]
- Wang, K.L.; Yap, E.S.; Goto, S.; Zhang, S.; Siu, C.W.; Chiang, C.E. The diagnosis and treatment of venous thromboembolism in asian patients. Thromb. J. 2018, 16, 4. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chang, S.A.; Kim, K.H.; Kim, J.Y.; Seo, W.K.; Kim, H.; Seo, J.S.; Shin, S.H.; Rhee, S.J.; Lee, S.H.; et al. Epidemiology of Venous Thromboembolism and Treatment Pattern of Oral Anticoagulation in Korea, 2009–2016: A Nationwide Study Based on the National Health Insurance Service Database. J. Cardiovasc. Imaging 2021, 29, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-G.; Lee, J.H.; Hong, J.; Kim, S.-A.; Kim, Y.-K.; Kim, M.S.; Bang, S.-M. Recurrence of Cancer-associated Venous Thromboembolism between 2009 and 2013: A Nationwide Korean Study. Clin. Exp. Thromb. Hemost. 2021, 7, 14–19. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007, 25, 70–76. [Google Scholar] [CrossRef]
- Londero, A.P.; Bertozzi, S.; Cedolini, C.; Neri, S.; Bulfoni, M.; Orsaria, M.; Mariuzzi, L.; Uzzau, A.; Risaliti, A.; Barillari, G. Incidence and Risk Factors for Venous Thromboembolism in Female Patients Undergoing Breast Surgery. Cancers 2022, 14, 988. [Google Scholar] [CrossRef]
- Kang, M.J.; Ryoo, B.-Y.; Ryu, M.-H.; Koo, D.-H.; Chang, H.M.; Lee, J.-L.; Kim, T.W.; Kang, Y.-K. Venous thromboembolism (VTE) in patients with advanced gastric cancer: An Asian experience. Eur. J. Cancer 2012, 48, 492–500. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, J.H.; Lee, K.-W.; Bang, S.-M.; Hwang, J.-H.; Oh, D.; Lee, J.S. Venous thromboembolism in patients with pancreatic adenocarcinoma: Lower incidence in Asian ethnicity. Thromb. Res. 2008, 122, 485–490. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Kim, I.; Lee, E.; Bang, S.-M.; Kang, C.H.; Kim, Y.T.; Kim, H.J.; Wu, H.-G.; Kim, Y.W.; Kim, T.M. Risk factors and prognostic impact of venous thromboembolism in Asian patients with non-small cell lung cancer. Thromb. Haemost. 2014, 111, 1112–1120. [Google Scholar] [CrossRef]
- Walker, A.J.; West, J.; Card, T.R.; Crooks, C.; Kirwan, C.C.; Grainge, M.J. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood 2016, 127, 849–857. [Google Scholar] [CrossRef]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e227S–e277S. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Jun, K.W.; Hwang, J.K.; Kim, S.D.; Kim, J.Y.; Park, S.C.; Won, Y.S.; Yun, S.S.; Moon, I.S.; Kim, J.I. Venous Thromboembolism following Abdominal Cancer Surgery in the Korean Population: Incidence and Validation of a Risk Assessment Model. Ann. Surg. Oncol. 2019, 26, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Laws, A.; Anderson, K.; Hu, J.N.; McLean, K.; Novak, L.; Dominici, L.S.; Nakhlis, F.; Carty, M.; Caterson, S.; Chun, Y.; et al. Implementation of a Venous Thromboembolism Prophylaxis Protocol Using the Caprini Risk Assessment Model in Patients Undergoing Mastectomy. Ann. Surg. Oncol. 2018, 25, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.M.; Jang, M.J.; Kim, K.H.; Yhim, H.Y.; Kim, Y.K.; Nam, S.H.; Hwang, H.G.; Bae, S.H.; Kim, S.H.; Mun, Y.C.; et al. Prevention of Venous Thromboembolism, 2nd Edition: Korean Society of Thrombosis and Hemostasis Evidence-Based Clinical Practice Guidelines. J. Korean Med. Sci. 2014, 29, 164–171. [Google Scholar] [CrossRef]
- Lovely, J.K.; Nehring, S.A.; Boughey, J.C.; Degnim, A.C.; Donthi, R.; Harmsen, W.S.; Jakub, J.W. Balancing venous thromboembolism and hematoma after breast surgery. Ann. Surg. Oncol. 2012, 19, 3230–3235. [Google Scholar] [CrossRef]
- Momeni, A.; Fox, J.P. Venous Thromboembolism after Surgical Treatment of Breast Cancer. Ann. Plast. Surg. 2018, 80, 188–192. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; Jara-Palomares, L.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline(dagger). Ann. Oncol. 2023, 21, v274–v276. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 1953, Erratum in Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Pastori, D.; Marang, A.; Bisson, A.; Menichelli, D.; Herbert, J.; Lip, G.Y.; Fauchier, L. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: A nationwide cohort study. Cancer 2021, 127, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K. Epidemiological characteristics of breast cancer in Koreans. J. Korean Med. Assoc. 2019, 62, 424–436. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, S.W.; Yu, J.H.; Lee, J.E.; Kim, J.Y.; Woo, J.; Lee, S.; Kim, E.K.; Moon, H.G.; Ko, S.S.; et al. Is the high proportion of young age at breast cancer onset a unique feature of Asian breast cancer? Breast Cancer Res. Treat. 2019, 173, 189–199. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Zhao, H.; Kroll, M.; Fang, S.; Zhang, N.; Hortobagyi, G.N.; Buchholz, T.A.; Shih, Y.C.; Giordano, S.H. Risk factors and incidence of thromboembolic events (TEEs) in older men and women with breast cancer. Ann. Oncol. 2011, 22, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Eggemann, H.; Bernreiter, A.L.; Reinisch, M.; Loibl, S.; Taran, F.A.; Costa, S.D.; Ignatov, A. Tamoxifen treatment for male breast cancer and risk of thromboembolism: Prospective cohort analysis. Br. J. Cancer 2019, 120, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Bouchard, B.A.; Cushman, M. Venous thromboembolism, factor VIII and chronic kidney disease. Thromb. Res. 2018, 170, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Szeki, I.; Nash, M.J.; Thachil, J. Anticoagulation in chronic kidney disease patients—The practical aspects. Clin. Kidney J. 2014, 7, 442–449. [Google Scholar] [CrossRef]
- Chen, T.W.W.; Chen, H.M.; Lin, C.H.; Huang, C.S.; Cheng, A.L.; Lai, M.S.; Lu, Y.S. No increased venous thromboembolism risk in Asian breast cancer patients receiving adjuvant tamoxifen. Breast Cancer Res. Treat. 2014, 148, 135–142. [Google Scholar] [CrossRef]
- Lin, H.F.; Liao, K.F.; Chang, C.M.; Lin, C.L.; Lai, S.W.; Hsu, C.Y. Correlation of the tamoxifen use with the increased risk of deep vein thrombosis and pulmonary embolism in elderly women with breast cancer A case-control study. Medicine 2018, 97, e12842. [Google Scholar] [CrossRef]
- Nam, G.E.; Kim, Y.-H.; Han, K.; Jung, J.-H.; Rhee, E.-J.; Lee, S.-S.; Kim, D.J.; Lee, K.-W.; Lee, W.-Y. Obesity fact sheet in Korea, 2019: Prevalence of obesity and abdominal obesity from 2009 to 2018 and social factors. J. Obes. Metab. Syndr. 2020, 29, 124. [Google Scholar] [CrossRef]
- Almasi-Sperling, V.; Hieber, S.; Lermann, J.; Strahl, O.; Beckmann, M.; Lang, W.; Sagban, T. Femoral placement of totally implantable venous access ports in patients with bilateral breast cancer. Geburtshilfe Frauenheilkd. 2016, 76, 53–58. [Google Scholar] [CrossRef]
- Hanna-Sawires, R.G.; Groen, J.V.; Hamming, A.; Tollenaar, R.; Mesker, W.E.; Luelmo, S.A.C.; Vahrmeijer, A.L.; Bonsing, B.A.; Versteeg, H.H.; Klok, F.A.; et al. Incidence, timing and risk factors of venous thromboembolic events in patients with pancreatic cancer. Thromb. Res. 2021, 207, 134–139. [Google Scholar] [CrossRef]
- Lee, K.W.; Bang, S.M.; Kim, S.; Lee, H.J.; Shin, D.Y.; Koh, Y.; Lee, Y.G.; Cha, Y.; Kim, Y.J.; Kim, J.H.; et al. The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J. Thromb. Haemost. 2010, 8, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, A.; Wun, T.; Khatri, V.; Chew, H.K.; Harvey, D.; Zhou, H.; White, R.H. Venous thromboembolism in patients with colorectal cancer: Incidence and effect on survival. J. Clin. Oncol. 2006, 24, 1112–1118. [Google Scholar] [CrossRef]
- Ul Alam, A.; Karkhaneh, M.; Sun, H.W.; Wu, C. Survival patterns among venous thromboembolism patients with hematologic malignancies in Alberta, Canada from 2003 to 2015. Thromb. Res. 2021, 199, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Black, K.A.; Ghosh, S.; Singh, N.; Chu, P.; Pin, S. Venous Thromboembolism in Patients Receiving Neoadjuvant Chemotherapy for Advanced Ovarian Cancer and Impact on Survival. J. Obstet. Gynaecol. Can. 2021, 43, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Pin, S.; Mateshaytis, J.; Ghosh, S.; Batuyong, E.; Easaw, J.C. Risk factors for venous thromboembolism in endometrial cancer. Curr. Oncol. 2020, 27, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Sussman, T.A.; Li, H.; Hobbs, B.; Funchain, P.; McCrae, K.R.; Khorana, A.A. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J. Immunother. Cancer 2021, 9, e001719. [Google Scholar] [CrossRef]
- Kirwan, C.C.; Descamps, T.; Castle, J. Circulating tumour cells and hypercoagulability: A lethal relationship in metastatic breast cancer. Clin. Transl. Oncol. 2020, 22, 870–877. [Google Scholar] [CrossRef]
- Dunbar, A.; Bolton, K.L.; Devlin, S.M.; Sanchez-Vega, F.; Gao, J.J.; Mones, J.V.; Wills, J.; Kelly, D.; Farina, M.; Cordner, K.B.; et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood 2021, 137, 2103–2113. [Google Scholar] [CrossRef]
- Mahe, I.; Scotte, F.; Elalamy, I. Tumor Genetics Are Thrombogenic: The Need for Action. JACC Cardio Oncol. 2023, 5, 256–258. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, M.J.; Seo, J.; Kim, H.Y.; Min, S.K.; Kim, L.S. Somatic Mutations of TP53 Identified by Targeted Next-Generation Sequencing Are Poor Prognostic Factors for Primary Operable Breast Cancer: A Single-Center Study. J. Breast Cancer 2022, 25, 379–386. [Google Scholar] [CrossRef]
- Cote, L.P.; Greenberg, S.; Caprini, J.A.; Tafur, A.; Choi, C.; Munoz, F.J.; Skride, A.; Valero, B.; Porras, J.A.; Ciammaichella, M.; et al. Comparisons Between Upper and Lower Extremity Deep Vein Thrombosis: A Review of the RIETE Registry. Clin. Appl. Thromb.-Hemost. 2017, 23, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, M.; Carrier, M. Incidental venous thromboembolism: Is anticoagulation indicated? Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 121–127. [Google Scholar] [CrossRef] [PubMed]

| Patients without VTE (n = 2198) | Patients with VTE (n = 48) | p | |
|---|---|---|---|
| Median age (range), years | 51 (21–91) | 61.5 (42–91) | <0.001 |
| Age group | <0.001 | ||
| ≤44 | 505 (23.0%) | 3 (6.3%) | |
| 45–59 | 1170 (53.2%) | 17 (35.4%) | |
| ≥60 | 523 (23.8%) | 28 (58.3%) | |
| Male sex | 4 (0.2%) | 2 (4.2%) | 0.006 |
| BMI, kg/m2 | 23.9 (14.1–45.4) | 24.8 (18.6–35.8) | 0.193 |
| Comorbidity | 0.113 | ||
| Absent | 1892 (86.1%) | 37 (77.1%) | |
| Present | 306 (13.9%) | 11 (22.9%) | |
| Operation | 0.002 | ||
| Breast-conserving surgery | 1650 (75.1%) | 30 (62.5%) | |
| Total mastectomy | 536 (24.4%) | 16 (33.3%) | |
| Mastectomy with reconstruction | 12 (0.5%) | 2 (4.2%) | |
| Bilaterality | 89 (4.0%) | 3 (6.3%) | 0.445 |
| Stage | <0.001 | ||
| 0 | 327 (14.9%) | 0 | |
| I | 859 (39.1%) | 11 (22.9%) | |
| II | 746 (33.9%) | 23 (47.9%) | |
| III | 222 (10.1%) | 10 (20.8%) | |
| IV | 44 (2.0%) | 4 (8.3%) | |
| Histology | 0.075 | ||
| DCIS or microIDC | 385 (17.5%) | 1 (2.1%) | |
| IDC | 1605 (73.0%) | 41 (85.4%) | |
| ILC | 66 (3.0%) | 2 (4.2%) | |
| Mucinous | 50 (2.3%) | 0 | |
| Medullary | 15 (0.7%) | 1 (2.1%) | |
| Metaplastic | 16 (0.7%) | 1 (2.1%) | |
| Others | 61 (2.8%) | 2 (4.2%) | |
| Hormonal receptor | 0.171 | ||
| Negative | 526 (23.9%) | 16 (33.3%) | |
| Positive | 1672 (76.1%) | 32 (66.7%) | |
| HER2 status | 0.062 | ||
| Negative | 1652 (75.2%) | 30 (62.5%) | |
| Positive | 546 (24.8%) | 18 (37.5%) | |
| Chemotherapy | <0.001 | ||
| Not done | 675 (30.7%) | 6 (12.5%) | |
| Adjuvant | 1376 (62.6%) | 32 (66.7%) | |
| Neoadjuvant | 105 (4.8%) | 6 (12.5%) | |
| Palliative | 42 (1.9%) | 4 (8.3%) | |
| Endocrine treatment | 0.004 | ||
| Not done | 499 (22.7%) | 19 (39.6%) | |
| Tamoxifen ± OFS | 1039 (47.3%) | 12 (25.0%) | |
| Aromatase inhibitor | 660 (30.0%) | 17 (35.4%) |
| Variables | Cumulative Incidence | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age group | |||||
| ≤44 | 0.6% | Reference | Reference | ||
| 45–59 | 1.4% | 2.450 (0.718–8.361) | 0.152 | 2.289 (0.669–7.825) | 0.187 |
| ≥60 | 5.1% | 9.204 (2.797–30.290) | <0.001 | 8.468 (2.562–27.984) | <0.001 |
| Sex | |||||
| Female | 2.1% | Reference | Reference | ||
| Male | 33.3% | 20.225 (4.907–83.358) | <0.001 | 9.953 (2.170–45.647) | 0.003 |
| Operation | |||||
| Breast-conserving surgery | 1.8% | Reference | Reference | ||
| Simple mastectomy | 2.9% | 1.673 (0.912–3.069) | 0.097 | 0.889 (0.446–1.772) | 0.738 |
| Mastectomy with reconstruction | 14.3% | 10.059 (2.399–42.174) | 0.002 | 7.209 (1.478–35.167) | 0.009 |
| Stage | |||||
| 0 | 0 | 0 | 0.954 | 0 | 0.954 |
| I | 1.3% | Reference | Reference | ||
| II | 3.0% | 2.377 (1.159–4.875) | 0.018 | 2.250 (1.085–4.667) | 0.029 |
| III | 4.3% | 3.617 (1.536–8.519) | 0.003 | 3.506 (1.415–8.686) | 0.007 |
| IV | 8.3% | 7.367 (2.344–23.157) | 0.001 | 5.766 (1.618–20.548) | 0.007 |
| HER2 status | |||||
| Negative | 1.8% | Reference | |||
| Positive | 3.2% | 1.805 (1.006–3.237) | 0.048 | ||
| Chemotherapy | |||||
| Not done | 0.9% | Reference | |||
| Performed | 2.7% | 3.054 (1.298–7.183) | 0.011 | ||
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age group | ||||
| ≤44 | Reference | |||
| 45–59 | 0.827 (0.575–1.191) | 0.308 | ||
| ≥60 | 0.812 (0.515–1.282) | 0.372 | ||
| T stage | ||||
| Tis | Reference | Reference | ||
| 1 | 6.016 (1.456–24.860) | 0.013 | 4.582 (1.102–19.061) | 0.036 |
| 2 | 22.697 (5.594–92.098) | <0.001 | 12.015 (2.907–49.665) | 0.001 |
| 3 | 35.723 (7.827–163.046) | <0.001 | 15.294 (3.258–71.803) | 0.001 |
| 4 | 67.903 (14.875–309.981) | <0.001 | 17.674 (3.716–84.069) | <0.001 |
| N stage | ||||
| 0 | Reference | Reference | ||
| 1 | 2.393 (1.616–3.543) | <0.001 | 1.757 (1.173–2.633) | 0.006 |
| 2 | 4.423 (2.766–7.075) | <0.001 | 2.341 (1.423–3.852) | 0.001 |
| 3 | 15.062 (9.831–23.074) | <0.001 | 9.006 (5.696–14.238) | <0.001 |
| Hormonal receptor | ||||
| Negative | Reference | Reference | ||
| Positive | 0.380 (0.278–0.519) | <0.001 | 0.377 (0.271–0.525) | <0.001 |
| HER2 status | ||||
| Negative | Reference | Reference | ||
| Positive | 1.229 (0.875–1.727) | 0.234 | 0.652 (0.454–0.935) | 0.020 |
| VTE | ||||
| Absent | Reference | Reference | ||
| Present | 6.140 (3.480–10.835) | <0.001 | 3.426 (1.909–6.149) | <0.001 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age group | ||||
| ≤44 | Reference | |||
| 45–59 | 1.213 (0.642–2.293) | 0.552 | ||
| ≥60 | 2.058 (1.041–4.071) | 0.038 | ||
| T stage | ||||
| Tis | Reference | |||
| 1 | 1.637 (0.482–5.565) | 0.430 | ||
| 2 | 4.919 (1.510–16.029) | 0.008 | ||
| 3 | 11.336 (2.835–45.339) | 0.001 | ||
| 4 | 28.803 (7.796–106.418) | <0.001 | ||
| N stage | ||||
| 0 | Reference | Reference | ||
| 1 | 0.890 (0.364–2.179) | <0.799 | 0.906 (0.370–2.221) | 0.830 |
| 2 | 5.787 (2.945–11.374) | <0.001 | 4.032 (2.016–8.061) | <0.001 |
| 3 | 24.380 (14.098–42.161) | <0.001 | 13.495 (7.265–25.065) | <0.001 |
| M stage | ||||
| 0 | Reference | Reference | ||
| 1 | 19.667 (11.159–34.661) | <0.001 | 4.971 (2.600–9.503) | <0.001 |
| Hormonal receptor | ||||
| Negative | Reference | Reference | ||
| Positive | 0.293 (0.184–0.466) | <0.001 | 0.282 (0.174–0.457) | <0.001 |
| HER2 status | ||||
| Negative | Reference | |||
| Positive | 1.780 (1.102–2.874) | 0.018 | ||
| VTE | ||||
| Absent | Reference | Reference | ||
| Present | 8.842 (4.386–17.824) | <0.001 | 4.863 (2.367–9.989) | <0.001 |
| Classification | Frequency |
|---|---|
| Presentation | |
| DVT | 18 (37.5%) |
| PTE | 25 (52.1%) |
| DVT and PTE | 5 (10.4%) |
| Symptom | |
| Absent | 25 (52.1%) |
| Present | 23 (47.9%) |
| Timing of VTE diagnosis | |
| Neoadjuvant chemotherapy | 4 (8.3%) |
| Within 1 month after surgery | 3 (6.3%) |
| Adjuvant chemotherapy | 16 (33.3%) |
| Follow up | 17 (35.4%) |
| After metastasis | 8 (16.7%) |
| Treatment | |
| Observation | 11 (22.9%) |
| Unfractionated heparin | 2 (4.2%) |
| Warfarin ± LMWH | 11 (22.9%) |
| DOAC | 24 (50.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Ahn, S.E.; Kwon, L.M.; Ko, H.H.; Kim, S.; Suh, Y.J.; Kim, H.Y.; Park, K.-H.; Kim, D. The Risk of Venous Thromboembolism in Korean Patients with Breast Cancer: A Single-Center Experience. Cancers 2023, 15, 3124. https://doi.org/10.3390/cancers15123124
Park JH, Ahn SE, Kwon LM, Ko HH, Kim S, Suh YJ, Kim HY, Park K-H, Kim D. The Risk of Venous Thromboembolism in Korean Patients with Breast Cancer: A Single-Center Experience. Cancers. 2023; 15(12):3124. https://doi.org/10.3390/cancers15123124
Chicago/Turabian StylePark, Jung Ho, So Eun Ahn, Lyo Min Kwon, Ho Hyun Ko, Sanghwa Kim, Yong Joon Suh, Ho Young Kim, Kyoung-Ha Park, and Doyil Kim. 2023. "The Risk of Venous Thromboembolism in Korean Patients with Breast Cancer: A Single-Center Experience" Cancers 15, no. 12: 3124. https://doi.org/10.3390/cancers15123124
APA StylePark, J. H., Ahn, S. E., Kwon, L. M., Ko, H. H., Kim, S., Suh, Y. J., Kim, H. Y., Park, K.-H., & Kim, D. (2023). The Risk of Venous Thromboembolism in Korean Patients with Breast Cancer: A Single-Center Experience. Cancers, 15(12), 3124. https://doi.org/10.3390/cancers15123124







