Mixed-Method Systematic Review and Meta-Analysis of Shared Decision-Making Tools for Cancer Screening
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- Study population: Studies involving eligible, asymptomatic participants for cancer screening; legal guardians of vulnerable patients; clinicians, including gastroenterologists, general practitioners, primary care providers, nurses, or professionals that are either licensed, in practice, or training.
- Intervention: SDM tools that were developed and well defined for cancer screening.
- Comparator: Usual care, simple decision aids, aids, or attention control materials. For before–after study designs, ‘before exposure to any SDM tool’ was considered a sufficient comparator.
- Outcome: Knowledge, decisional conflict, self-efficacy, the intention to screen, screening uptake/test ordered, decision regret, readiness to decide, and satisfaction with the SDM tool.
- Study design: Randomized controlled trials, controlled before–after, or quasi-experimental study designs.
- Other considerations: Studies published between 2010 and 2022, and those that presented baseline information on the study populations making a distinction between vulnerable and non-vulnerable people. Studies were identified to have included vulnerable people when more than 50% of participants had: (1) educational attainment lower than a college degree, (2) immigrant background, or (3) membership of a racial minority [16].
- An attrition rate of more than 40%.
- Small sample sizes (n < 30).
- Studies that investigated associations relevant to SDM tools but not effectiveness.
- Developmental, pilot testing, or feasibility studies.
- Population: Studies involving vulnerable people or clinicians who had experience or were familiar with SDM related to the general patient population.
- Intervention: No specific intervention, but studies that captured information relevant to any SDM tool for cancer screening.
- Outcome: Studies that reported findings relevant to vulnerable people’s or clinicians’ preferences in terms of SDM tool content, format, or delivery strategies.
- Study design: Qualitative or mixed-method studies that used qualitative analysis as part of the methodology, including but not limited to thematic, content, grounded theory analysis, phenomenology, or discourse analysis.
2.3. Selection Process
2.4. Data Extraction and Management
2.5. Risk of Bias Assessment
2.6. Measures of Intervention Effect
2.7. Meta-Analyses
2.8. Subgroup Analysis
2.9. Meta-Aggregation
2.10. Assessment of Reporting Biases
2.11. Data Synthesis
3. Results
3.1. Literature Search
3.2. Study Characteristics
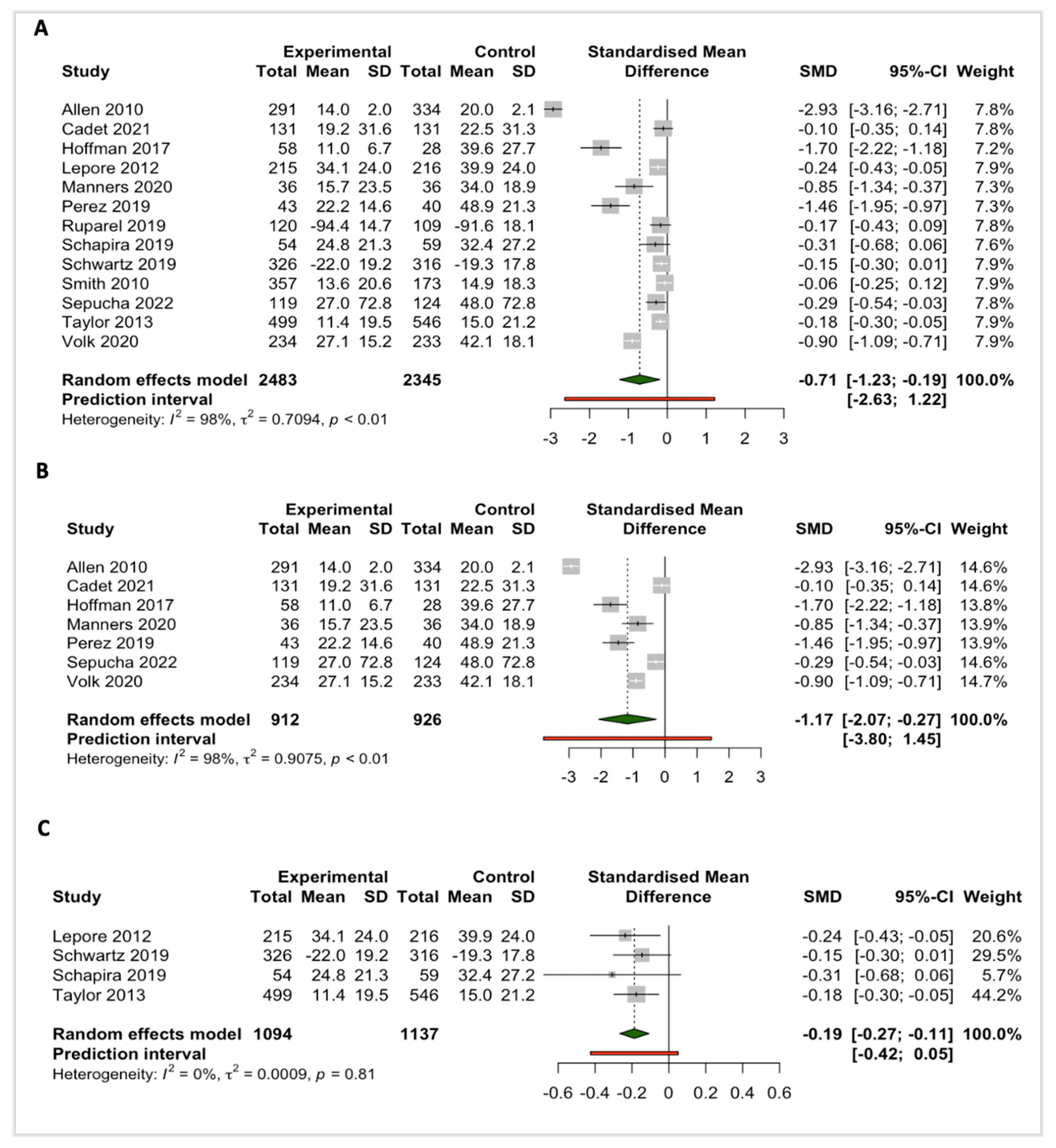
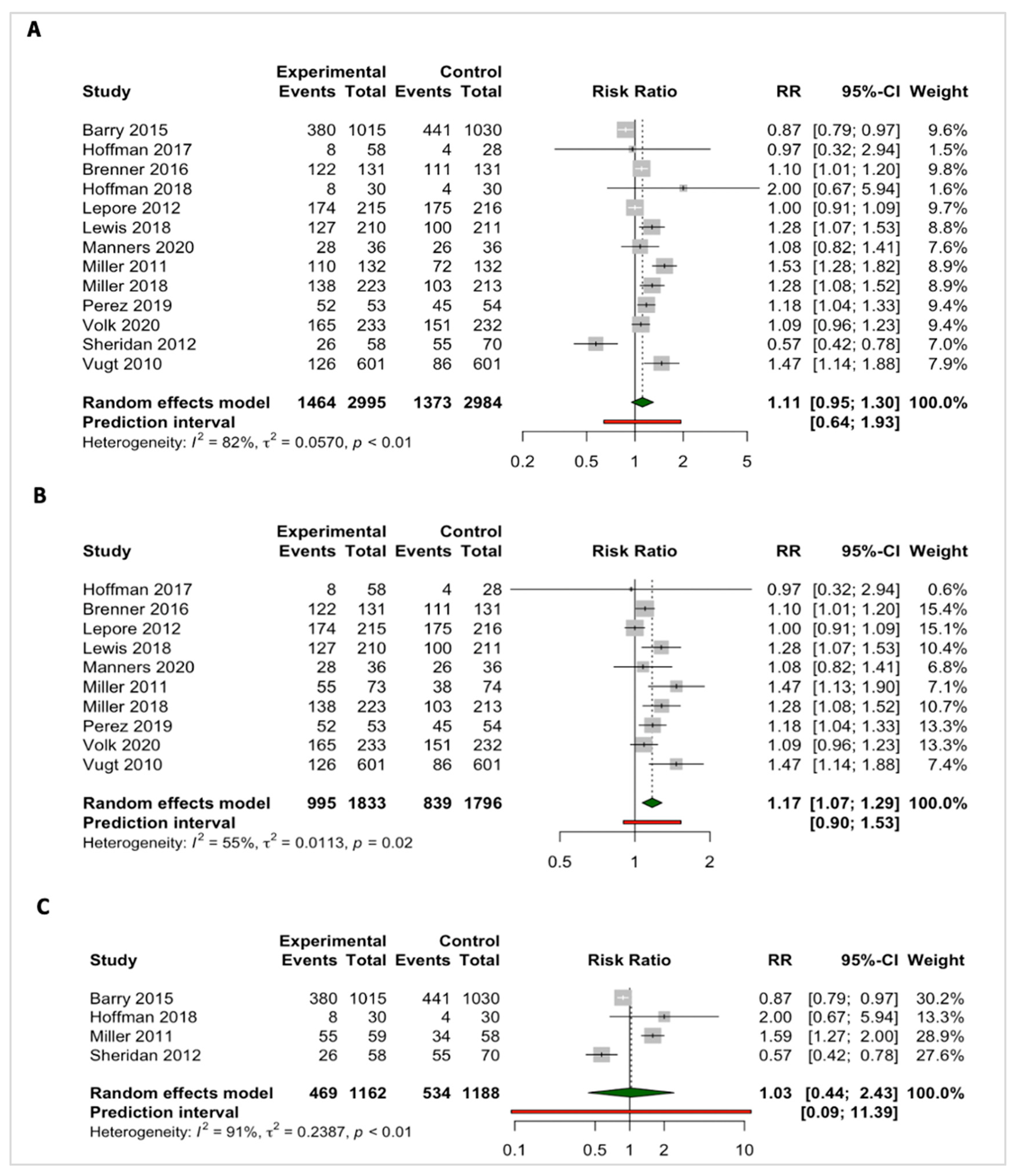
3.3. Meta-Analyses
3.3.1. Knowledge
3.3.2. Decision Conflict
3.3.3. Intention to Screen
3.4. Narrative Synthesis
3.4.1. Patient–Clinician Screening Discussions
3.4.2. Decisional Regret
3.4.3. Self-Efficacy
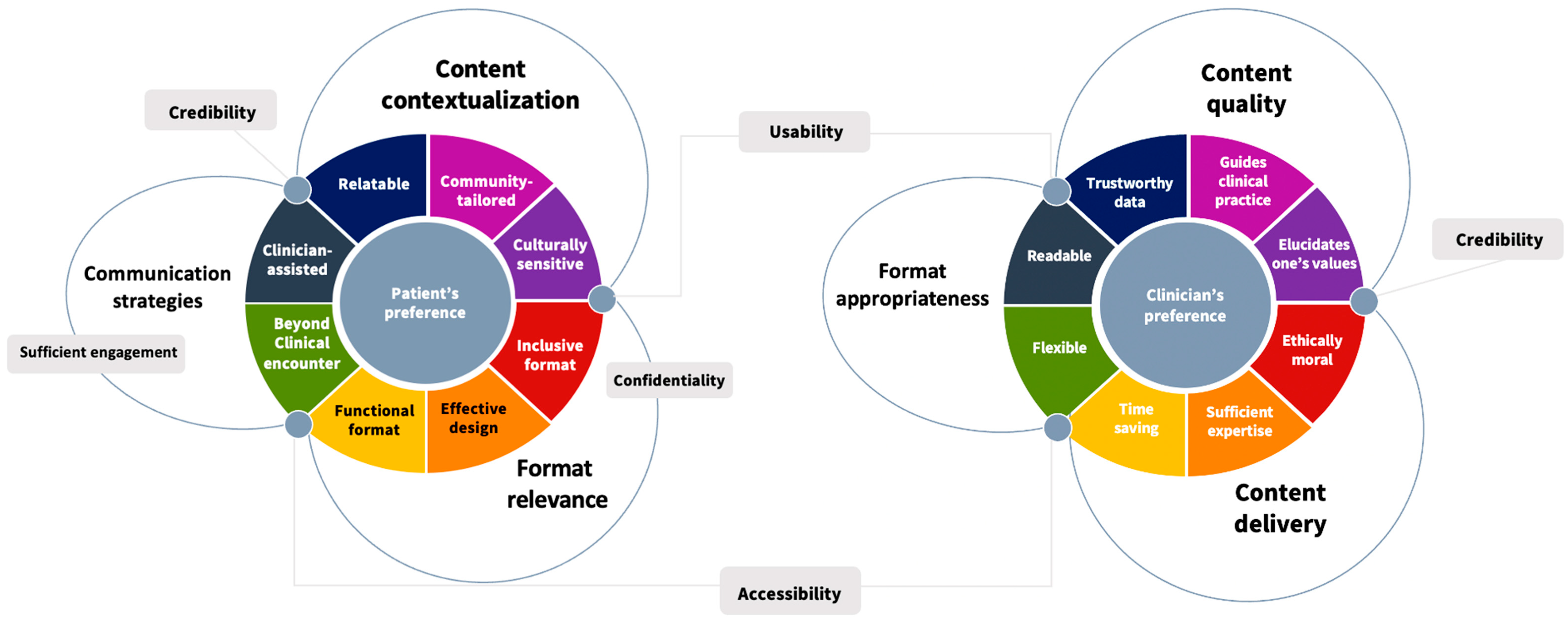
3.5. Theoretical Framework Based on the Thematic Analysis
3.6. Vulnerable People’s Preferences
3.7. Clinicians’ Preferences
4. Discussion
4.1. Summary of the Main Findings
4.2. Strengths and Limitations
4.3. Implications for Clinical Practice
4.4. Implications for Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinsky, P.F. Principles of Cancer Screening. Surg. Clin. North Am. 2015, 95, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Habbema, D.; De Kok, I.M.; Brown, M.L. Cervical Cancer Screening in the United States and the Netherlands: A Tale of two countries. Milbank Q. 2012, 90, 5–37. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cervical Cancer Screening. World Health Organization, 2022. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3240 (accessed on 8 November 2022).
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E. The Impact of Mammographic Screening on Breast Cancer Mortality in Europe: A Review of Observational Studies. J. Med. Screen 2012, 19 (Suppl. 1), 14–25. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Byers, T.; Wender, R.C.; Jemal, A.; Baskies, A.M.; Ward, E.E.; Brawley, O.W. The American Cancer Society challenge goal to reduce US cancer mortality by 50% between 1990 and 2015: Results and reflections. CA A Cancer J. Clin. 2016, 66, 359–369. [Google Scholar] [CrossRef]
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21st Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef]
- Molassiotis, A.; Tyrovolas, S.; Giné-Vázquez, I.; Yeo, W.; Aapro, M.; Herrstedt, J. Organized breast cancer screening not only reduces mortality from breast cancer but also significantly decreases disability-adjusted life years: Analysis of the Global Burden of Disease Study and screening programme availability in 130 countries. ESMO Open 2021, 6, 100111. [Google Scholar] [CrossRef]
- Marcus, P.M.; Prorok, P.C.; Miller, A.B.; DeVoto, E.J.; Kramer, B.S. Conceptualizing overdiagnosis in cancer screening. J. Natl. Cancer Inst. 2015, 107, djv014. [Google Scholar] [CrossRef]
- Welch, H.G.; Black, W.C. Overdiagnosis in Cancer. J. Natl. Cancer Inst. 2010, 102, 605–613. [Google Scholar] [CrossRef]
- Dreier, M.; Borutta, B.; Seidel, G.; Münch, I.; Kramer, S.; Töppich, J.; Dierks, M.-L.; Walter, U. Communicating the benefits and harms of colorectal cancer screening needed for an informed choice: A systematic evaluation of leaflets and booklets. PLoS ONE 2014, 9, e107575. [Google Scholar] [CrossRef]
- Levit, L.A. Institute of Medicine (U.S.). Committee on Improving the Quality of Cancer Care, and Institute of Medicine (U.S.). Board on Health Care Services, Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK202148/pdf/Bookshelf_NBK202148.pdf (accessed on 8 November 2022).
- Joseph-Williams, N.; Newcombe, R.; Politi, M.; Durand, M.-A.; Sivell, S.; Stacey, D.; O’connor, A.; Volk, R.J.; Edwards, A.; Bennett, C.; et al. Toward minimum standards for certifying patient decision aids: A modified delphi consensus process. Med. Decis. Mak. 2014, 34, 699–710. [Google Scholar] [CrossRef]
- European Commission Initiative on Breast Cancer. Decision Aid Use for Informing Women About Screening. European Union, 2022. Available online: https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines/Invitation-to-screening-and-decision-aid/decision-aid (accessed on 21 September 2022).
- Stacey, D.; Légaré, F.; Lewis, K.; Barry, M.J.; Bennett, C.L.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017, 2017, CD001431. [Google Scholar] [CrossRef]
- Muscat, D.M.; Shepherd, H.L.; Morony, S.; Smith, S.K.; Dhillon, H.M.; Trevena, L.; Hayen, A.; Luxford, K.; Nutbeam, D.; McCaffery, K. Can adults with low literacy understand shared decision making questions? A qualitative investigation. Patient Educ. Couns. 2016, 99, 1796–1802. [Google Scholar] [CrossRef]
- Lee, Y.J.; Brazile, T.; Galbiati, F.; Hamm, M.; Bryce, C.; Jain, S.; Kraschnewski, J.; McTigue, K. Understanding shared decision-making experience among vulnerable population: Focus group with food bank clients. J. Clin. Transl. Sci. 2021, 5, e37. [Google Scholar] [CrossRef]
- McCaffery, K.J.; Holmes-Rovner, M.; Smith, S.K.; Rovner, D.; Nutbeam, D.; Clayman, M.L.; Kelly-Blake, K.; Wolf, M.S.; Sheridan, S.L. Addressing health literacy in patient decision aids. BMC Med. Inform. Decis. Mak. 2013, 13 (Suppl. 2), S10. [Google Scholar] [CrossRef]
- Moodley, J.; Constant, D.; Mwaka, A.D.; Scott, S.E.; Walter, F.M. Anticipated help seeking behaviour and barriers to seeking care for possible breast and cervical cancer symptoms in Uganda and South Africa. Ecancermedicalscience 2020, 15, 1171. [Google Scholar] [CrossRef]
- Habtu, Y.; Yohannes, S.; Laelago, T. Health seeking behavior and its determinants for cervical cancer among women of childbearing age in Hossana Town, Hadiya zone, Southern Ethiopia: Community based cross sectional study. BMC Cancer 2018, 18, 298. [Google Scholar] [CrossRef]
- Shen, H.-N.; Lin, C.-C.; Hoffmann, T.; Tsai, C.-Y.; Hou, W.-H.; Kuo, K.N. The relationship between health literacy and perceived shared decision making in patients with breast cancer. Patient Educ. Couns. 2019, 102, 360–366. [Google Scholar] [CrossRef]
- Newsome, M. We Must Improve Equity in Cancer Screening. Nature 2021. [Google Scholar] [CrossRef]
- Waisel, D.B. Vulnerable populations in healthcare. Curr. Opin. Anaesthesiol. 2013, 26, 186–192. [Google Scholar] [CrossRef]
- Hoeck, S.; Van Roy, K.; Willems, S. Barriers and facilitators to participate in the colorectal cancer screening programme in Flanders (Belgium): A focus group study. Acta Clin. Belg. Int. J. Clin. Lab. Med. 2022, 77, 37–44. [Google Scholar] [CrossRef]
- Yen, R.W.; Smith, J.; Engel, J.; Muscat, D.M.; Smith, S.K.; Mancini, J.; Perestelo-Pérez, L.; Elwyn, G.; O’malley, A.J.; Leyenaar, J.K.; et al. A Systematic Review and Meta-Analysis of Patient Decision Aids for Socially Disadvantaged Populations: Update from the International Patient Decision Aid Standards (IDPAS). Med. Decis. Mak. 2021, 41, 870–896. [Google Scholar] [CrossRef]
- Stacey, D.; Légaré, F.; Col, N.F.; Bennett, C.L.; Barry, M.J.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. In Cochrane Database of Systematic Reviews; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2014; Volume 2014, p. CD001431. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Volk, R.J.; Linder, S.K.; Lopez-Olivo, M.A.; Kamath, G.R.; Reuland, D.S.; Saraykar, S.S.; Leal, V.B.; Pignone, M.P. Patient Decision Aids for Colorectal Cancer Screening: A Systematic Review and Meta-analysis. Am. J. Prev. Med. 2016, 51, 779–791. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savovic, J.; Page, M.J.; Sterne, J.A.; RoB Development Group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). 2019, pp. 1–72. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 27 July 2023).
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Wieseler, B.; Kerekes, M.F.; Vervoelgyi, V.; McGauran, N.; Kaiser, T. Impact of document type on reporting quality of clinical drug trials: A comparison of registry reports, clinical study reports, and journal publications. BMJ 2012, 344, d8141. [Google Scholar] [CrossRef]
- Légaré, F.; Adekpedjou, R.; Stacey, D.; Turcotte, S.; Kryworuchko, J.; Graham, I.D.; Lyddiatt, A.; Politi, M.C.; Thomson, R.; Elwyn, G.; et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst. Rev. 2018, 2018, CD006732. [Google Scholar] [CrossRef]
- Grimshaw, J.M.; Thomas, R.E.; MacLennan, G.; Fraser, C.; Ramsay, C.; Vale, L.; Whitty, P.; Eccles, M.P.; Matowe, L.; Shirran, L.; et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Int. J. Technol. Assess. Health Care 2005, 21, 149. [Google Scholar] [CrossRef]
- Friedrich, J.O.; Adhikari, N.K.; Beyene, J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: A simulation study. BMC Med. Res. Methodol. 2008, 8, 32. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: https://scholar.google.com/scholar_lookup?title=Cochrane%20Handbook%20for%20Systematic%20Reviews%20of%20Interventions&publication_year=2019&author=J.P.T.%20Higgins (accessed on 28 May 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the meta for package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Kossmeier, M.; Tran, U.S.; Voracek, M. Forest Plots, Funnel Plots, and Visual Funnel Plot Inference for Meta-Analysis. 2020. Available online: https://CRAN.R-project.org/package=metaviz (accessed on 30 May 2023).
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Harden, A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med. Res. Methodol. 2008, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Schünemann, H.J.; Oxman, A.D.; Pottie, K.; Meerpohl, J.J.; Coello, P.A.; Rind, D.; Montori, V.M.; Brito, J.P.; Norris, S.; et al. GRADE guidelines: 15. Going from evidence to recommendation—Determinants of a recommendation’s direction and strength. J. Clin. Epidemiol. 2013, 66, 726–735. [Google Scholar] [CrossRef]
- Lewin, S.; Booth, A.; Glenton, C.; Munthe-Kaas, H.; Rashidian, A.; Wainwright, M.; Bohren, M.A.; Tunçalp, Ö.; Colvin, C.J.; Garside, R.; et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: Introduction to the series. Implement. Sci. 2018, 13, 2. [Google Scholar] [CrossRef]
- Halley, M.C.; Rendle, K.A.; Gillespie, K.A.; Stanley, K.M.; Frosch, D.L. An exploratory mixed-methods crossover study comparing DVD- vs. Web-based patient decision support in three conditions: The importance of patient perspectives. Health Expect. 2015, 18, 2880–2891. [Google Scholar] [CrossRef]
- Allen, J.D.; Othus, M.K.; Hart, A.; Tom, L.; Li, Y.; Berry, D.; Bowen, D. A Randomized Trial of a Computer-Tailored Decision Aid to Improve Prostate Cancer Screening Decisions: Results from the Take the Wheel Trial. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2172–2186. [Google Scholar] [CrossRef]
- Barry, M.J.; Wexler, R.M.; Brackett, C.D.; Sepucha, K.R.; Simmons, L.H.; Gerstein, B.S.; Stringfellow, V.L.; Fowler, F.J. Responses to a Decision Aid on Prostate Cancer Screening in Primary Care Practices. Am. J. Prev. Med. 2015, 49, 520–525. [Google Scholar] [CrossRef]
- Brenner, A.T.; Hoffman, R.; McWilliams, A.; Pignone, M.P.; Rhyne, R.L.; Tapp, H.; Weaver, M.A.; Callan, D.; de Hernandez, B.U.; Harbi, K.; et al. Colorectal Cancer Screening in Vulnerable Patients: Promoting Informed and Shared Decisions. Am. J. Prev. Med. 2016, 51, 454–462. [Google Scholar] [CrossRef]
- Cadet, T.; Aliberti, G.; Karamourtopoulos, M.; Jacobson, A.; Gilliam, E.A.; Primeau, S.; Davis, R.; Schonberg, M.A. Evaluation of a mammography decision aid for women 75 and older at risk for lower health literacy in a pretest-posttest trial. Patient Educ. Couns. 2021, 104, 2344–2350. [Google Scholar] [CrossRef]
- Eden, K.B.; Scariati, P.; Klein, K.; Watson, L.; Remiker, M.; Hribar, M.; Forro, V.; Michaels, L.; Nelson, H.D. Mammography Decision Aid Reduces Decisional Conflict for Women in Their Forties Considering Screening. J. Women’s Health 2015, 24, 1013–1020. [Google Scholar] [CrossRef]
- Gökce, M.I.; Wang, X.; Frost, J.; Roberson, P.; Volk, R.J.; Brooks, D.; Canfield, S.E.; Pettaway, C.A. Informed decision making before prostate-specific antigen screening: Initial results using the American Cancer Society (ACS) Decision Aid (DA) among medically underserved men. Cancer 2017, 123, 583–591. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Lowenstein, L.M.; Kamath, G.R.; Housten, A.J.; Leal, V.B.; Linder, S.K.; Jibaja-Weiss, M.L.; Raju, G.S.; Volk, R.J. An entertainment-education colorectal cancer screening decision aid for African American patients: A randomized controlled trial. Cancer 2017, 123, 1401–1408. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Hempstead, A.P.; Housten, A.J.; Richards, V.F.; Lowenstein, L.M.; Leal, V.B.; Volk, R.J. Using a Patient Decision Aid Video to Assess Current and Former Smokers’ Values About the Harms and Benefits of Lung Cancer Screening with Low-Dose Computed Tomography. MDM Policy Pract. 2018, 3, 2381468318769886. [Google Scholar] [CrossRef]
- Housten, A.J.; Kamath, G.R.; Bevers, T.B.; Cantor, S.B.; Dixon, N.; Hite, A.; Kallen, M.A.; Leal, V.B.; Li, L.; Volk, R.J. Does Animation Improve Comprehension of Risk Information in Patients with Low Health Literacy? A Randomized Trial. Med. Decis. Mak. 2020, 40, 17–28. [Google Scholar] [CrossRef]
- Lau, Y.K.; Caverly, T.J.; Cao, P.; Cherng, S.T.; West, M.; Gaber, C.; Arenberg, D.; Meza, R. Evaluation of a Personalized, Web-Based Decision Aid for Lung Cancer Screening. Am. J. Prev. Med. 2015, 49, e125–e129. [Google Scholar] [CrossRef]
- Lau, Y.K.; Bhattarai, H.; Caverly, T.J.; Hung, P.-Y.; Jimenez-Mendoza, E.; Patel, M.R.; Coté, M.L.; Arenberg, D.A.; Meza, R. Lung Cancer Screening Knowledge, Perceptions, and Decision Making Among African Americans in Detroit, Michigan. Am. J. Prev. Med. 2021, 60, e1–e8. [Google Scholar] [CrossRef]
- Lepore, S.J.; Wolf, R.L.; Basch, C.E.; Godfrey, M.; McGinty, E.; Shmukler, C.; Ullman, R.; Thomas, N.; Weinrich, S. Informed decision making about prostate cancer testing in predominantly immigrant black men: A randomized controlled trial. Ann. Behav. Med. 2012, 44, 320–330. [Google Scholar] [CrossRef]
- Lewis, C.L.; Adams, J.; Tai-Seale, M.; Huang, Q.; Knowles, S.B.; Nielsen, M.E.; Pignone, M.P.; Walter, L.C.; Frosch, D.L. A Randomized Controlled Effectiveness Trial for PSA Screening Decision Support Interventions in Two Primary Care Settings. J. Gen. Intern. Med. 2015, 30, 810–816. [Google Scholar] [CrossRef]
- Lewis, C.L.; Kistler, C.E.; Dalton, A.F.; Morris, C.; Ferrari, R.; Barclay, C.; Brewer, N.T.; Dolor, R.; Harris, R.; Vu, M.; et al. A Decision Aid to Promote Appropriate Colorectal Cancer Screening among Older Adults: A Randomized Controlled Trial. Med. Decis. Mak. 2018, 38, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Manners, D.; Pettigrew, S.; Lake, F.R.; Piccolo, F.; McWilliams, A.M.; Brims, F.J.H. Development and evaluation of a consumer information resource, including Patient Decision Aid, for lung cancer screening: A quasi-experimental study. Transl. Behav. Med. 2020, 10, 404–412. [Google Scholar] [CrossRef]
- Miller, D.P.; Denizard-Thompson, N.; Weaver, K.E.; Case, L.D.; Troyer, J.L.; Spangler, J.G.; Lawler, D.; Pignone, M.P. Effect of a digital health intervention on receipt of colorectal cancer screening in vulnerable patients a randomized controlled trial. Ann. Intern. Med. 2018, 168, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Perestelo-Perez, L.; Rivero-Santana, A.; Torres-Castaño, A.; Ramos-Garcia, V.; Alvarez-Perez, Y.; Gonzalez-Hernandez, N.; Buron, A.; Pignone, M.; Serrano-Aguilar, P. Effectiveness of a decision aid for promoting colorectal cancer screening in Spain: A randomized trial. BMC Med. Inform. Decis. Mak. 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Reuland, D.S.; Cubillos, L.; Brenner, A.T.; Harris, R.P.; Minish, B.; Pignone, M.P. A pre-post study testing a lung cancer screening decision aid in primary care. BMC Med. Informatics Decis. Mak. 2018, 18, 5. [Google Scholar] [CrossRef]
- Ruparel, M.; Quaife, S.L.; Ghimire, B.; Dickson, J.L.; Bhowmik, A.; Navani, N.; Baldwin, D.R.; Duffy, S.; Waller, J.; Janes, S.M.; et al. Impact of a lung cancer screening information film on informed decision-making: A randomized trial. Ann. Am. Thorac. Soc. 2019, 16, 744–751. [Google Scholar] [CrossRef]
- Rubel, S.K.; Miller, J.W.; Stephens, R.L.; Xu, Y.; Scholl, L.E.; Holden, E.W.; Stroud, L.A.; Volk, R.J. Testing the effects of a decision aid for prostate cancer screening. J. Health Commun. 2010, 15, 307–321. [Google Scholar] [CrossRef]
- Salkeld, G.; Cunich, M.; Dowie, J.; Howard, K.; Patel, M.I.; Mann, G.; Lipworth, W. The role of personalised choice in decision support: A randomized controlled trial of an online decision aid for prostate cancer screening. PLoS ONE 2016, 11, e0152999. [Google Scholar] [CrossRef]
- Schapira, M.M.; Hubbard, R.A.; Seitz, H.H.; Conant, E.F.; Schnall, M.; Cappella, J.N.; Harrington, T.; Inge, C.; Armstrong, K. The Impact of a Risk-Based Breast Cancer Screening Decision Aid on Initiation of Mammography Among Younger Women: Report of a Randomized Trial. MDM Policy Pract. 2019, 4, 2381468318812889. [Google Scholar] [CrossRef]
- Schroy, P.C.; Emmons, K.; Peters, E.; Glick, J.T.; Robinson, P.A.; Lydotes, M.A.; Mylvanaman, S.; Evans, S.; Chaisson, C.; Pignone, M.; et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: A randomized trial. Med. Decis. Mak. 2011, 31, 93–107. [Google Scholar] [CrossRef]
- Schwartz, P.H.; Imperiale, T.; Perkins, S.M.; Schmidt, K.K.; Althouse, S.; Rawl, S.M. Impact of including quantitative information in a decision aid for colorectal cancer screening: A randomized controlled trial. Patient Educ. Couns. 2019, 102, 726–734. [Google Scholar] [CrossRef]
- Sepucha, K.R.; Valentine, K.D.; Atlas, S.J.; Chang, Y.; Fairfield, K.M.; Ha, J.; Leavitt, L.; Lee, V.; Percac-Lima, S.; Richter, J.M.; et al. Getting patients back for routine colorectal cancer screening: Randomized controlled trial of a shared decision-making intervention. Cancer Med. 2022, 12, 3555–3566. [Google Scholar] [CrossRef]
- Sferra, S.R.; Cheng, J.S.; Boynton, Z.; DiSesa, V.; Kaiser, L.R.; Ma, G.X.; Erkmen, C.P. Aiding shared decision making in lung cancer screening: Two decision tools. J. Public Health 2021, 43, 673–680. [Google Scholar] [CrossRef]
- Sheridan, S.L.; Golin, C.; Bunton, A.; Lykes, J.B.; Schwartz, B.; McCormack, L.; Driscoll, D.; I Bangdiwala, S.; Harris, R.P. Shared decision making for prostate cancer screening: The results of a combined analysis of two practice-based randomized controlled trials. BMC Med. Inform. Decis. Mak. 2012, 12, 130. [Google Scholar] [CrossRef]
- Sheridan, S.L.; Sutkowi-Hemstreet, A.; Barclay, C.; Brewer, N.T.; Dolor, R.J.; Gizlice, Z.; Lewis, C.L.; Reuland, D.S.; Golin, C.E.; Kistler, C.E.; et al. A comparative effectiveness trial of alternate formats for presenting benefits and harms information for low-value screening services a randomized clinical trial. JAMA Intern. Med. 2016, 176, 31–41. [Google Scholar] [CrossRef]
- Smith, S.K.; Trevena, L.; Simpson, J.M.; Barratt, A.; Nutbeam, D.; McCaffery, K.J. A decision aid to support informed choices about bowel cancer screening among adults with low education: Randomised controlled trial. BMJ 2010, 341, c5370. [Google Scholar] [CrossRef]
- Taylor, K.L.; Williams, R.M.; Davis, K.; Luta, G.; Penek, S.; Barry, S.; Kelly, S.; Tomko, C.; Schwartz, M.; Krist, A.H.; et al. Decision making in prostate cancer screening using decision aids vs. usual care a randomized clinical trial. JAMA Intern. Med. 2013, 173, 1704–1712. [Google Scholar] [CrossRef]
- van Vugt, H.A.; Roobol, M.J.; Venderbos, L.D.; Zwanenburg, E.J.-V.; Essink-Bot, M.-L.; Steyerberg, E.W.; Bangma, C.H.; Korfage, I.J. Informed decision making on PSA testing for the detection of prostate cancer: An evaluation of a leaflet with risk indicator. Eur. J. Cancer 2010, 46, 669–677. [Google Scholar] [CrossRef]
- Volk, R.J.; Lowenstein, L.M.; Leal, V.B.; Escoto, K.H.; Cantor, S.B.; Munden, R.F.; Rabius, V.A.; Bailey, L.; Cinciripini, P.M.; Lin, H.; et al. Effect of a Patient Decision Aid on Lung Cancer Screening Decision-Making by Persons Who Smoke: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e1920362. [Google Scholar]
- Williams, R.M.; Davis, K.M.; Luta, G.; Edmond, S.N.; Dorfman, C.S.; Schwartz, M.D.; Lynch, J.; Ahaghotu, C.; Taylor, K.L. Fostering informed decisions: A randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Educ. Couns. 2013, 91, 329–336. [Google Scholar] [CrossRef]
- Akanuwe, J.N.A.; Black, S.; Owen, S.; Siriwardena, A.N. Communicating cancer risk in the primary care consultation when using a cancer risk assessment tool: Qualitative study with service users and practitioners. Health Expect. 2020, 23, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Amélie, A.-E.; Ruelle, Y.; Frèche, B.; Houllemare, M.; Bonillo, A.; Bouaziz, L.; Rat, C.; Gocko, X.; Cerisey, C.; Aubin-Auger, I.; et al. What do women and healthcare professionals expect of decision aids for breast cancer screening? A qualitative study in France. BMJ Open 2022, 12, e058879. [Google Scholar] [CrossRef] [PubMed]
- Croes, K.D.; Jones, N.R.; DuBenske, L.L.; Schrager, S.B.; Mahoney, J.E.; Little, T.A.; Burnside, E.S. Core Elements of Shared Decision-making for Women Considering Breast Cancer Screening: Results of a Modified Delphi Survey. J. Gen. Intern. Med. 2020, 35, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- DuBenske, L.; Ovsepyan, V.; Little, T.; Schrager, S.; Burnside, E. Preliminary Evaluation of a Breast Cancer Screening Shared Decision-Making Aid Utilized Within the Primary Care Clinical Encounter. J. Patient Exp. 2021, 8, 23743735211034039. [Google Scholar] [CrossRef] [PubMed]
- Engelen, A.; Vanderhaegen, J.; Van Poppel, H.; Van Audenhove, C. The use of decision aids on early detection of prostate cancer: Views of men and general practitioners. Health Expect. 2017, 20, 221–231. [Google Scholar] [CrossRef]
- Kuss, K.; Adarkwah, C.C.; Becker, M.; Donner-Banzhoff, N.; Schloessler, K. Delivering the unexpected—Information needs for PSA screening from Men’s perspective: A qualitative study. Health Expect. 2021, 24, 1403–1412. [Google Scholar] [CrossRef]
- Maschke, A.; Paasche-Orlow, M.K.; Kressin, N.R.; Schonberg, M.A.; Battaglia, T.A.; Gunn, C.M. Discussions of Potential Mammography Benefits and Harms among Patients with Limited Health Literacy and Providers: “Oh, There are Harms”? J. Health Commun. 2020, 25, 951–961. [Google Scholar] [CrossRef]
- Pannebakker, M.M.; Mills, K.; Johnson, M.; Emery, J.D.; Walter, F.M. Understanding implementation and usefulness of electronic clinical decision support (eCDS) for melanoma in English primary care: A qualitative investigation. BJGP Open 2019, 3, bjgpopen18X101635. [Google Scholar] [CrossRef]
- Toledo-Chávarri, A.; Rué, M.; Codern-Bové, N.; Carles-Lavila, M.; Perestelo-Pérez, L.; Pérez-Lacasta, M.; Feijoo-Cid, M.; the InforMa Study Group. A qualitative study on a decision aid for breast cancer screening: Views from women and health professionals. Eur. J. Cancer Care 2017, 26, e12660. [Google Scholar] [CrossRef]
- Wiener, R.S.; Koppelman, E.; Bolton, R.; Lasser, K.E.; Borrelli, B.; Au, D.H.; Slatore, C.G.; Clark, J.A.; Kathuria, H. Patient and Clinician Perspectives on Shared Decision-making in Early Adopting Lung Cancer Screening Programs: A Qualitative Study. J. Gen. Intern. Med. 2018, 33, 1035–1042. [Google Scholar] [CrossRef]
- Baptista, S.; Heleno, B.; Pinto, M.; Guimarães, B.; China, D.; Ramos, J.P.; Teixeira, A.; Taylor, K.L.; Martins, C. Translation and cultural adaptation of a prostate cancer screening decision aid: A qualitative study in Portugal. BMJ Open 2020, 10, e034384. [Google Scholar] [CrossRef] [PubMed]
- Crothers, K.; Kross, E.K.; Reisch, L.M.; Shahrir, S.; Slatore, C.; Zeliadt, S.B.; Triplette, M.; Meza, R.; Elmore, J.G. Patients’ Attitudes Regarding Lung Cancer Screening and Decision Aids: A survey and focus group study. Ann. Am. Thorac. Soc. 2016, 13, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.B.; Thomas, T.L.; Owens, O.L.; Hébert, J.R. It Takes Two to Talk About Prostate Cancer: A Qualitative Assessment of African American Men’s and Women’s Cancer Communication Practices and Recommendations. Am. J. Men’s Health 2012, 6, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Schapira, M.M.; Aggarwal, C.; Akers, S.; Aysola, J.; Imbert, D.; Langer, C.; Simone, C.B.; Strittmatter, E.; Vachani, A.; Fraenkel, L. How Patients View Lung Cancer Screening: The Role of Uncertainty in Medical Decision Making. Ann. Am. Thorac. Soc. 2016, 13, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.H.; O’doherty, K.C.; Bentley, C.; Schmidt, K.K.; Burgess, M.M. Layperson Views about the Design and Evaluation of Decision Aids: A Public Deliberation. Med. Decis. Mak. 2021, 41, 527–539. [Google Scholar] [CrossRef]
- Tatari, C.R.; Andersen, B.; Brogaard, T.; Badre-Esfahani, S.; Jaafar, N.; Kirkegaard, P. The SWIM study: Ethnic minority women’s ideas and preferences for a tailored intervention to promote national cancer screening programmes—A qualitative interview study. Health Expect. 2021, 24, 1692–1700. [Google Scholar] [CrossRef]
- Vahabi, M. Breast cancer and screening information needs and preferred communication medium among Iranian immigrant women in Toronto. Health Soc. Care Community 2011, 19, 626–635. [Google Scholar] [CrossRef]
- Schonberg, M.A.; Jacobson, A.R.; Aliberti, G.M.; Hayes, M.; Hackman, A.; Karamourtopolous, M.; Kistler, C. Primary Care–Based Staff Ideas for Implementing a Mammography Decision Aid for Women 75+: A Qualitative Study. J. Gen. Intern. Med. 2019, 34, 2414–2420. [Google Scholar] [CrossRef]
- Reese, T.J.; Schlechter, C.R.; Kramer, H.; Kukhareva, P.; Weir, C.R.; Del Fiol, G.; Caverly, T.; Hess, R.; Flynn, M.C.; Taft, T.; et al. Implementing lung cancer screening in primary care: Needs assessment and implementation strategy design. Transl. Behav. Med. 2022, 12, 187–197. [Google Scholar] [CrossRef]
- Hernández-Leal, M.J.; Codern-Bové, N.; Pérez-Lacasta, M.J.; Cardona, A.; Vidal-Lancis, C.; Carles-Lavila, M. Development of support material for health professionals who are implementing Shared Decision-making in breast cancer screening: Validation using the Delphi technique. BMJ Open 2022, 12, e052566. [Google Scholar] [CrossRef]
- Miller, D.P.; Spangler, J.G.; Case, L.D.; Goff, D.C.; Singh, S.; Pignone, M.P. Effectiveness of a web-based colorectal cancer screening patient decision aid: A randomized controlled trial in a mixed-literacy population. Am. J. Prev. Med. 2011, 40, 608–615. [Google Scholar] [CrossRef]
- Durand, M.-A.; Carpenter, L.; Dolan, H.; Bravo, P.; Mann, M.; Bunn, F.; Elwyn, G. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS ONE 2014, 9, e94670. [Google Scholar] [CrossRef]


| Study ID, Country | Study Design | SDM Tool/s Evaluated | Target Group | Comparator | Knowledge | Decision Conflict | Readiness to Decide | Risk Perception | Satisfaction with DA | Screening Uptake/Test Ordered | Intention to Screen | Decisional Regret | SDM Process | Self-Efficacy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allen 2010 [46], United States | RCT | Computer-tailored decision aid with personalized risk assessment tool | V | Usual care | ⇡ MA | ⇡ MA | ⇡ | . | ⇡ | . | . | . | . | ⇡ |
| Barry 2015 [47], UnitedStates | Before–after | “The PSA test: Is it right for you?” DA | NV | NC | . | . | ⇡ | . | . | . | ⇣ MA | . | ⇡ | . |
| Brenner 2016 [48], United States | RCT | Spanish (OPCIONES) and English CHOICE | V | Food safety video | ⇞ MA | . | . | . | . | ⇞ | ⇞ MA | . | ⇞ | . |
| Cadet 2021 [49], United States | Before–after | “Should I continue having mammograms? For women aged 75–84 years,” DA | V | NC | ⇡ | ↮ MA | . | . | ⇡ | . | . | . | . | . |
| Eden 2015 [50], United States | Before–after | Mobile application “Mammopad” DA | NV | NC | . | ⇡ | . | . | . | . | ↮ | . | . | ⇡ |
| Gokce 2017 [51], United States | Before–after | ACS (American Cancer Society) DA | NV | NC | ⇡ | ⇡ | . | . | . | . | . | . | . | . |
| Halley 2015 [45], United States | RCT | Web-based DESI (the decision support intervention) | NV | DVD-first DESI | ⇡ MA | . | . | . | . | . | . | . | . | . |
| Hoffman 2017 [52], United States | RCT | Video-based PtDA | V | Attention control video | ↑ MA | ↑ | . | . | . | ↮ | ↮ | . | . | . |
| Hoffman 2018 [53], United States | Before–after | “Lung Cancer Screening: Is it Right for Me?” Web -based DA | V | NC | ⇡ | ⇡ | . | . | . | . | ⇡ MA | . | ⇡ | . |
| Housten 2020 [54], United States | RCT (3-arm) | Animated video DA and status-video DA | V | Audio–booklet DA | ↮ MA | . | . | . | . | . | . | . | . | . |
| Lau 2015 [55], United States | Before–after | “Lung Cancer Screening: Should I get screened?” web- based DA | NV | NC | ⇡ | ⇡ | . | . | ⇡ | . | ⇡ MA | . | . | . |
| Lau 2021, United States [56] | Before–after | Modified “Lung Cancer Screening: Should I get screened?” web-based DA | V | NC | ⇡ | ⇡ | . | . | ⇡ ** | . | . | . | . | . |
| Lepore 2012, United States [57] | RCT | “Prostate Cancer: Your Life-You Decide” pamphlet with tailored telephone education | V | Attention control | ↑ MA | ↑ MA | . | . | . | ↮ * | ↮ MA | . | ↑ | . |
| Lewis 2015, United States [58] | RCT | PSA (prostate-specific antigen)-based DESI + SMA | NV | SMA invitation | ⇟ MA | . | . | . | . | . | . | . | . | . |
| Lewis 2018, United States [59] | RCT | ‘‘Making a Decision about Colon Cancer Screening” paper-based DA | NV | Attention control | ↑ MA | . | ↑ | . | . | . | ↑ MA | . | ↑ | . |
| Manners 2020, Australia [60] | Before–after (quasi) | PtDA + PLCOm2012 risk estimates | V | NC | . | ↮ MA | . | . | . | . | ↮ MA | . | . | . |
| Miller 2018 [61], United States | RCT | mPATH-CRC DA | V | Control program | . | . | . | . | . | ↑ | ↑ MA | . | ↑ | . |
| Perestelo-Perez 2019 [62], Spain | RCT | Web-based DA | V | Usual care | ↑ MA | ↑ | . | . | . | . | ↑ MA | . | . | . |
| Reuland 2018 [63], United States | Before–after | Video-based DA | NV | NC | ⇡ | . | . | . | ⇡ ** | . | . | . | . | . |
| Ruparel 2019 [64], United Kingdom | RCT | Information film + information booklet DA | V | Booklet alone | ⇡ | ⇡ MA | . | . | ⇡ | . | . | . | . | . |
| Rubel 2010 [65], United States | Solomon four- group | CDC-developed PCa screening DA | V | Usual care | ↑ | ↮ | . | . | . | . | . | . | . | . |
| Salkeld 2016 [66], Australia | RCT | Annalisa software-personalized decision support tool | V | Annalisa fixed attribute | . | ↑ | . | . | . | . | ↑ | . | ↑ | . |
| Schapira 2019 [67], United States | RCT | BCS (breast cancer screening)–PtDA | V | Usual care | ↑ | ↮ MA | . | ↮ | . | ↮ * | ↮ | ↮ | . | . |
| Schroy 2011 [68], United States | RCT (3-arm) | Web-based DA + “Your Disease Risk (YDR)” | V | Generic lifestyle website | ⇟ MA | . | . | . | . | ⇟ | ⇟ | . | ⇟ | . |
| Schwartz 2019 [69], United States | RCT | Quantitative DA | V | Verbal DA | . | ↮ MA | . | ↑ | . | ↮ * | ↮ | . | . | . |
| Sepucha 2022 [70], United States | RCT | Decision worksheet + telephone session | V | Usual care | . | ↑ MA | . | . | . | ↑ * | . | . | ↑ | . |
| Sferra 2021 [71], United States | RCT | Option grid decision support tool | V | Shouldiscreen.com DA | ⇟ | . | . | . | . | . | . | ⇟ | ↮ | . |
| Sheridan 2012 [72], United States | RCT | Video-based DA + patient coaching + provider education | NV | Highway safety attention control | ⇟ | . | . | . | . | ⇞ | ⇞ MA | . | ↮ | . |
| Sheridan 2016 [73], United States | RCT (4-arm) | Framed decision support sheet | NV | Qualitative decision support sheet | ↮ MA | . | . | ↮ | . | . | ↮ | . | . | ↓ |
| Smith 2010 [74], Australia | RCT (3-arm) | Booklet + DVD-based DA | V | Standard booklet | ↑ MA | ↑ MA | . | . | . | ↑ | ↑ | . | ↑ | ↮ |
| Taylor 2013 [75], United States | RCT (3-arm) | PCa Web-based DA | NV | Usual care | ⇡ MA | ⇡ MA | . | . | . | ↮ | ⇡ | . | . | . |
| van Vugt 2010 [76], Netherlands | Before–after | Leaflet PRI (personalized risk indicator) | V | NC | ⇡ | . | . | . | . | . | ⇡ MA | . | ⇡ | . |
| Volk 2020 [77], United States | RCT | Video- or DVD-based patient decision aid | V | Standard material | ↑ MA | ↑ MA | ↑ | . | . | . | ↮ MA | . | ↑ | . |
| Williams 2013 [78], United States | RCT (4-arm) | Printed-based DA | NV | Usual care | ↑ MA | ↑ | . | . | . | . | . | . | . | . |
| Topic | Studies | Common Preferences (Presented in Codes) |
|---|---|---|
| Vulnerable People | ||
| Information-specific preferences | n = 13 [80,81,83,84,85,87,88,89,90,91,93,94,95] |
|
| Format-specific preferences | n = 13 [45,79,80,81,84,85,89,90,91,93,94,95,97] |
|
| Delivery-specific preferences | n = 11 [79,80,81,82,83,84,85,90,91,94,95] |
|
| Clinicians | ||
| Content-specific preferences | Studies: (n = 8) [79,80,82,83,85,86,97,98] |
|
| Format-specific preferences | n = 8 [79,80,83,85,86,88,97,98] |
|
| Delivery-specific preferences | n = 6 [79,80,83,86,96,97] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, D.J.; van de Veerdonk, W.; Berhe, N.M.; Talboom, S.; van Loo, M.; Alejos, A.R.; Ferrari, A.; Van Hal, G. Mixed-Method Systematic Review and Meta-Analysis of Shared Decision-Making Tools for Cancer Screening. Cancers 2023, 15, 3867. https://doi.org/10.3390/cancers15153867
Herrera DJ, van de Veerdonk W, Berhe NM, Talboom S, van Loo M, Alejos AR, Ferrari A, Van Hal G. Mixed-Method Systematic Review and Meta-Analysis of Shared Decision-Making Tools for Cancer Screening. Cancers. 2023; 15(15):3867. https://doi.org/10.3390/cancers15153867
Chicago/Turabian StyleHerrera, Deborah Jael, Wessel van de Veerdonk, Neamin M. Berhe, Sarah Talboom, Marlon van Loo, Andrea Ruiz Alejos, Allegra Ferrari, and Guido Van Hal. 2023. "Mixed-Method Systematic Review and Meta-Analysis of Shared Decision-Making Tools for Cancer Screening" Cancers 15, no. 15: 3867. https://doi.org/10.3390/cancers15153867
APA StyleHerrera, D. J., van de Veerdonk, W., Berhe, N. M., Talboom, S., van Loo, M., Alejos, A. R., Ferrari, A., & Van Hal, G. (2023). Mixed-Method Systematic Review and Meta-Analysis of Shared Decision-Making Tools for Cancer Screening. Cancers, 15(15), 3867. https://doi.org/10.3390/cancers15153867







