The Multikinase Inhibitor AD80 Induces Mitotic Catastrophe and Autophagy in Pancreatic Cancer Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagent Chemicals
2.2. Cell Viability Assay
2.3. Colony Formation Assay
2.4. Cell Cycle Analysis
2.5. Morphology Analysis via Immunofluorescence
2.6. Cell Death Analysis
2.7. Western Blotting
2.8. Acidic Vesicular Organelle Analysis via Fluorescence Microscopy
2.9. PCR Array Analysis
2.10. Quantitative PCR
2.11. Soft Agar Assay
2.12. Cell Viability Analysis in the Spheroid Model
2.13. Statistical Analysis
3. Results
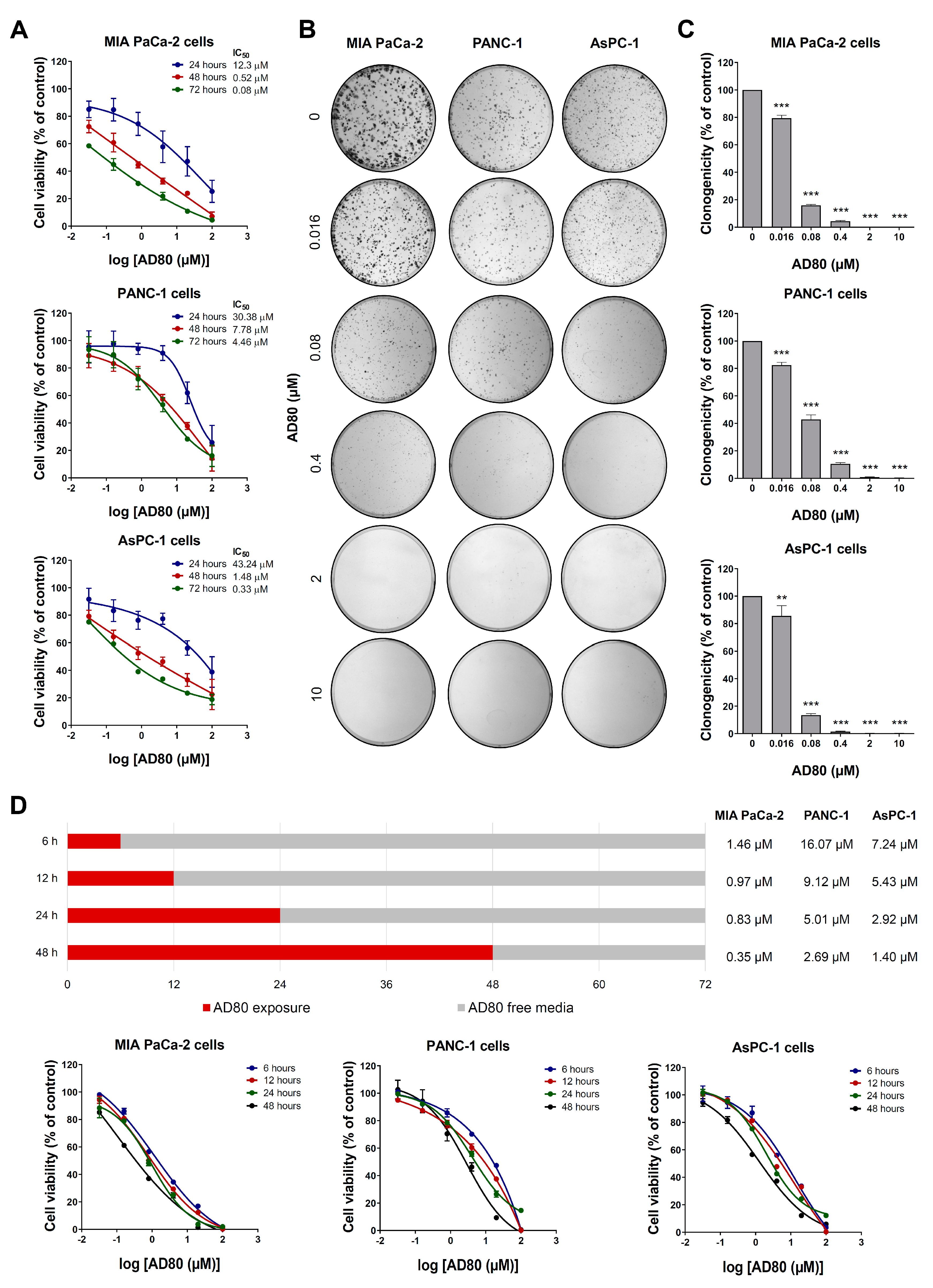
3.1. AD80 Exhibits Antineoplastic Activity in Pancreatic Cancer Cells
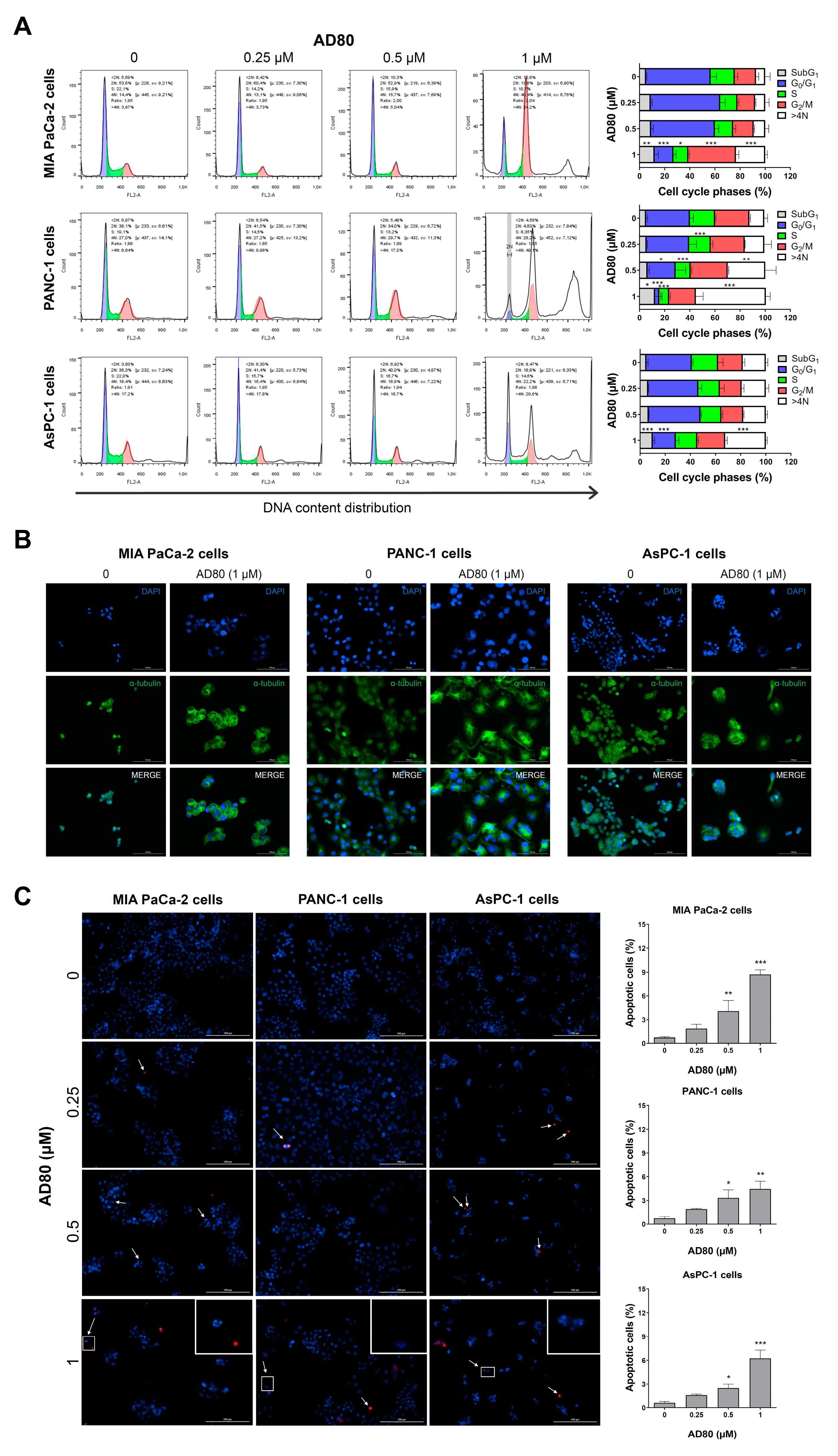
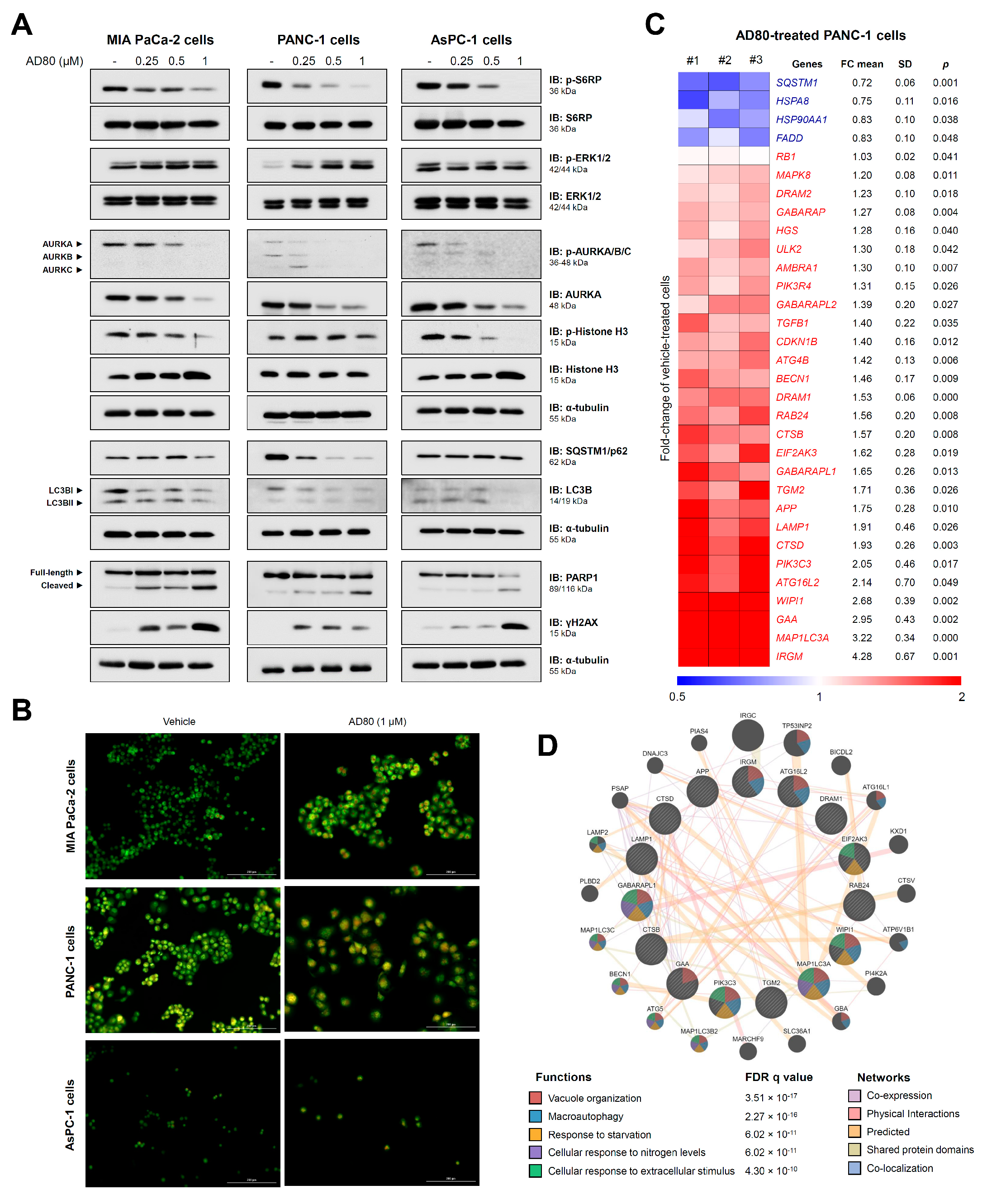
3.2. AD80 Induces Mitotic Catastrophe and Autophagy Molecular Marker Expression in Pancreatic Cancer Cells
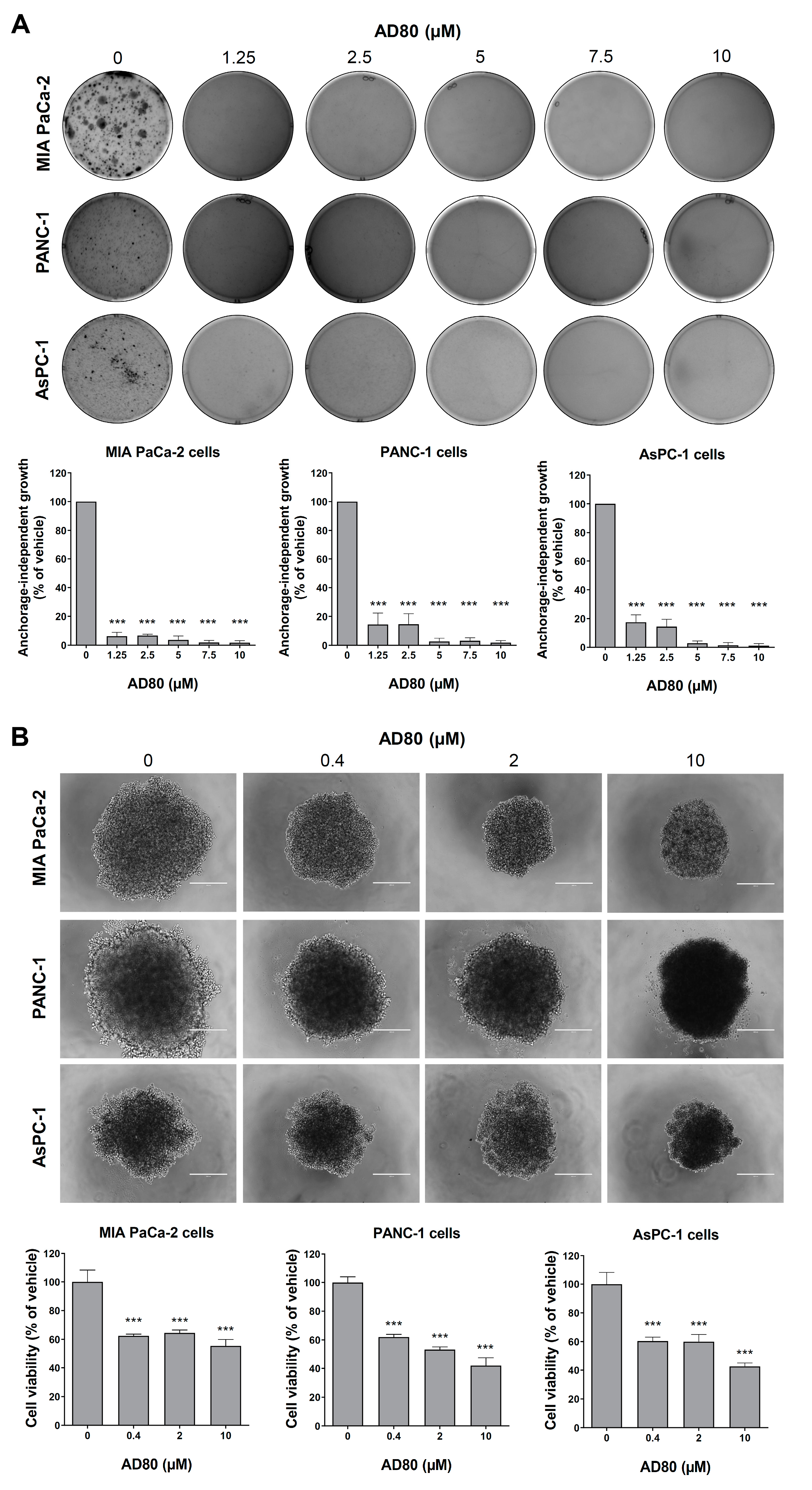
3.3. AD80 Reduces Anchorage-Independent Growth and Spheroid Cell Viability in Pancreatic Cancer
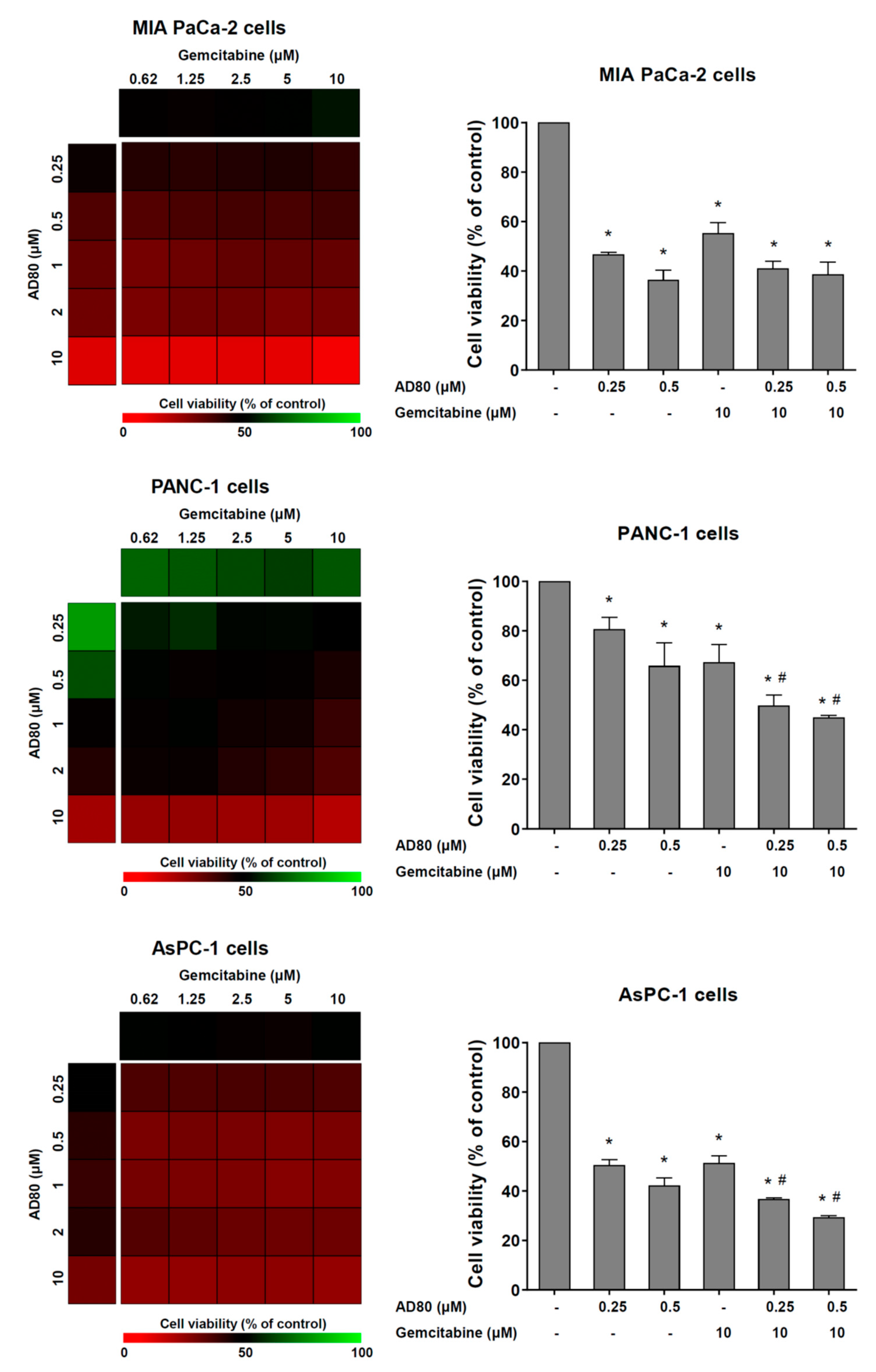
3.4. AD80 Potentiates Gemcitabine-Reduced Cell Viability in PANC-1 and AsPC-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, S.; Yang, M. Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options. World J. Gastroenterol. 2022, 28, 6827–6845. [Google Scholar] [CrossRef]
- TCGA. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e113. [Google Scholar] [CrossRef] [Green Version]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, A.; Giordano, S. From single-to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr. Med. Chem. 2008, 15, 422–432. [Google Scholar]
- Dar, A.C.; Das, T.K.; Shokat, K.M.; Cagan, R.L. Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature 2012, 486, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Feng, X.; Ennis, K.N.; Behrmann, C.A.; Sarma, P.; Jiang, T.T.; Kofuji, S.; Niu, L.; Stratton, Y.; Thomas, H.E.; et al. Pharmacologic Targeting of S6K1 in PTEN-Deficient Neoplasia. Cell Rep. 2017, 18, 2088–2095. [Google Scholar] [CrossRef]
- Carlos, J.; Lima, K.; Costa-Lotufo, L.V.; Leitao, A.; Machado-Neto, J.A. AD80, a multikinase inhibitor, exhibits antineoplastic effects in acute leukemia cellular models targeting the PI3K/STMN1 axis. Investig. New Drugs 2021, 39, 1139–1149. [Google Scholar] [CrossRef]
- Yu, J.X.; Craig, A.J.; Duffy, M.E.; Villacorta-Martin, C.; Miguela, V.; Ruiz de Galarreta, M.; Scopton, A.P.; Silber, L.; Maldonado, A.Y.; Rialdi, A.; et al. Phenotype-Based Screens with Conformation-Specific Inhibitors Reveal p38 Gamma and Delta as Targets for HCC Polypharmacology. Mol. Cancer Ther. 2019, 18, 1506–1519. [Google Scholar] [CrossRef] [Green Version]
- Plenker, D.; Riedel, M.; Bragelmann, J.; Dammert, M.A.; Chauhan, R.; Knowles, P.P.; Lorenz, C.; Keul, M.; Buhrmann, M.; Pagel, O.; et al. Drugging the catalytically inactive state of RET kinase in RET-rearranged tumors. Sci. Transl. Med. 2017, 9, eaah6144. [Google Scholar] [CrossRef] [Green Version]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Wang, L.; Cybula, M.; Drumond-Bock, A.L.; Moxley, K.M.; Bieniasz, M. Multi-kinase targeted therapy as a promising treatment strategy for ovarian tumors expressing sfRon receptor. Genes Cancer 2020, 11, 106–121. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- do Amaral, J.B.; Rezende-Teixeira, P.; Freitas, V.M.; Machado-Santelli, G.M. MCF-7 cells as a three-dimensional model for the study of human breast cancer. Tissue Eng. Part C Methods 2011, 17, 1097–1107. [Google Scholar] [CrossRef]
- Khan, S.U.; Fatima, K.; Malik, F. Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis. Clin. Exp. Metastasis 2022, 39, 715–726. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Muccioli, G.; Terreno, E.; Serpe, L. Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids. Life Sci. 2020, 254, 117784. [Google Scholar] [CrossRef]
- VanDeusen, H.R.; Ramroop, J.R.; Morel, K.L.; Bae, S.Y.; Sheahan, A.V.; Sychev, Z.; Lau, N.A.; Cheng, L.C.; Tan, V.M.; Li, Z.; et al. Targeting RET Kinase in Neuroendocrine Prostate Cancer. Mol. Cancer Res. 2020, 18, 1176–1188. [Google Scholar] [CrossRef]
- de Almeida, L.C.; Carlos, J.; Rezende-Teixeira, P.; Machado-Neto, J.A.; Costa-Lotufo, L.V. AD80, a multikinase inhibitor, as a potential drug candidate for colorectal cancer therapy. Life Sci. 2022, 308, 120911. [Google Scholar] [CrossRef]
- Gomes-Filho, S.M.; Dos Santos, E.O.; Bertoldi, E.R.M.; Scalabrini, L.C.; Heidrich, V.; Dazzani, B.; Levantini, E.; Reis, E.M.; Basseres, D.S. Aurora A kinase and its activator TPX2 are potential therapeutic targets in KRAS-induced pancreatic cancer. Cell Oncol. 2020, 43, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Wang, Y.; Mukhopadhyay, D.; Bi, Y.; Ji, B. Aurora kinase a inhibitor MLN8237 suppresses pancreatic cancer growth. Pancreatology 2022, 22, 619–625. [Google Scholar] [CrossRef]
- Nigg, E.A. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2001, 2, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Poon, R.Y.C. Aurora kinases and DNA damage response. Mutat. Res. 2020, 821, 111716. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, K.; de Miranda, L.B.L.; Del Milagro Bernabe Garnique, A.; de Almeida, B.O.; do Nascimento, M.C.; Alcântara, G.A.S.; Machado-Santelli, G.M.; Rego, E.M.; Machado-Neto, J.A. The Multikinase Inhibitor AD80 Induces Mitotic Catastrophe and Autophagy in Pancreatic Cancer Cells. Cancers 2023, 15, 3866. https://doi.org/10.3390/cancers15153866
Lima K, de Miranda LBL, Del Milagro Bernabe Garnique A, de Almeida BO, do Nascimento MC, Alcântara GAS, Machado-Santelli GM, Rego EM, Machado-Neto JA. The Multikinase Inhibitor AD80 Induces Mitotic Catastrophe and Autophagy in Pancreatic Cancer Cells. Cancers. 2023; 15(15):3866. https://doi.org/10.3390/cancers15153866
Chicago/Turabian StyleLima, Keli, Lívia Bassani Lins de Miranda, Anali Del Milagro Bernabe Garnique, Bruna Oliveira de Almeida, Mariane Cristina do Nascimento, Guilherme Augusto Sousa Alcântara, Glaucia Maria Machado-Santelli, Eduardo Magalhães Rego, and João Agostinho Machado-Neto. 2023. "The Multikinase Inhibitor AD80 Induces Mitotic Catastrophe and Autophagy in Pancreatic Cancer Cells" Cancers 15, no. 15: 3866. https://doi.org/10.3390/cancers15153866





