First-Line Treatment of Older Patients with CLL: A New Approach in the Chemo-Free Era
Abstract
:Simple Summary
Abstract
1. Introduction
2. Literature Search
3. Older Patients
3.1. Impact of Comorbidities
3.2. Impact of CGA
4. Data from Trials
4.1. Bruton Tyrosine Kinase Inhibitors
4.2. The BCL-2 Inhibitor Venetoclax
4.3. Combination Therapy
5. Real-World Evidence
6. Cost-Effectiveness
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- National Cancer Institute. Chronic Lymphocytic Leukemia—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 15 May 2023).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Haematological Malignancy Research Network. Available online: https://hmrn.org/statistics/prevalence (accessed on 15 May 2023).
- van der Straten, L.; Maas, C.C.H.M.; Levin, M.-D.; Visser, O.; Posthuma, E.F.M.; Doorduijn, J.K.; Langerak, A.W.; Kater, A.P.; Dinmohamed, A.G. Long-Term Trends in the Loss in Expectation of Life after a Diagnosis of Chronic Lymphocytic Leukemia: A Population-Based Study in the Netherlands, 1989–2018. Blood Cancer J. 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jain, N.; Ayer, T.; Wierda, W.G.; Flowers, C.R.; O’Brien, S.M.; Keating, M.J.; Kantarjian, H.M.; Chhatwal, J. Economic Burden of Chronic Lymphocytic Leukemia in the Era of Oral Targeted Therapies in the United States. J. Clin. Oncol. 2017, 35, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Cuneo, A.; Cavazzini, F.; Ciccone, M.; Daghia, G.; Sofritti, O.; Saccenti, E.; Negrini, M.; Rigolin, G.M. Modern Treatment in Chronic Lymphocytic Leukemia: Impact on Survival and Efficacy in High-Risk Subgroups. Cancer Med. 2014, 3, 555–564. [Google Scholar] [CrossRef]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of Rituximab to Fludarabine and Cyclophosphamide in Patients with Chronic Lymphocytic Leukaemia: A Randomised, Open-Label, Phase 3 Trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Thompson, P.A.; Tam, C.S.; OBrien, S.M.; Wierda, W.G.; Stingo, F.; Plunkett, W.; Smith, S.C.; Kantarjian, H.M.; Freireich, E.J.; Keating, M.J. Fludarabine, Cyclophosphamide, and Rituximab Treatment Achieves Long-Term Disease-Free Survival in IGHV-Mutated Chronic Lymphocytic Leukemia. Blood 2015, 127, 303–309. [Google Scholar] [CrossRef]
- Rossi, D.; Terzi-di-Bergamo, L.; De Paoli, L.; Cerri, M.; Ghilardi, G.; Chiarenza, A.; Bulian, P.; Visco, C.; Mauro, F.R.; Morabito, F.; et al. Molecular Prediction of Durable Remission after First-Line Fludarabine-Cyclophosphamide-Rituximab in Chronic Lymphocytic Leukemia. Blood 2015, 126, 1921–1924. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.-S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, K.; Cramer, P.; Busch, R.; Böttcher, S.; Bahlo, J.; Schubert, J.; Pflüger, K.H.; Schott, S.; Goede, V.; Isfort, S.; et al. Bendamustine in Combination with Rituximab for Previously Untreated Patients with Chronic Lymphocytic Leukemia: A Multicenter Phase II Trial of the German Chronic Lymphocytic Leukemia Study Group. J. Clin. Oncol. 2012, 30, 3209–3216. [Google Scholar] [CrossRef]
- Cuneo, A.; Marchetti, M.; Barosi, G.; Billio, A.; Brugiatelli, M.; Ciolli, S.; Laurenti, L.; Mauro, F.R.; Molica, S.; Montillo, M.; et al. Appropriate Use of Bendamustine in First-Line Therapy of Chronic Lymphocytic Leukemia. Recommendations from SIE, SIES, GITMO Group. Leuk. Res. 2014, 38, 1269–1277. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.C.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.; et al. Long-Term Outcomes for Ibrutinib-Rituximab and Chemoimmunotherapy in CLL: Updated Results of the E1912 Trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and Safety in a 4-Year Follow-up of the ELEVATE-TN Study Comparing Acalabrutinib with or without Obinutuzumab versus Obinutuzumab plus Chlorambucil in Treatment-Naïve Chronic Lymphocytic Leukemia. Leukemia 2022, 36, 1171–1175. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; et al. Zanubrutinib versus Bendamustine and Rituximab in Untreated Chronic Lymphocytic Leukaemia and Small Lymphocytic Lymphoma (SEQUOIA): A Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Zhang, C.; Lu, T.; Liao, M.Z.; Panchal, A.; Robrecht, S.; Ching, T.; Tandon, M.; Fink, A.-M.; Tausch, E.; et al. Minimal Residual Disease Dynamics after Venetoclax-Obinutuzumab Treatment: Extended Off-Treatment Follow-up from the Randomized CLL14 Study. J. Clin. Oncol. 2021, 39, 4049–4060. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanafelt, T. Treatment of Older Patients with Chronic Lymphocytic Leukemia: Key Questions and Current Answers. Hematology 2013, 2013, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linn, B.S.; Linn, M.W.; Gurel, L. CUMULATIVE ILLNESS RATING SCALE. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Novak, J.; Strugov, V.; Gill, D.; et al. First-Line Treatment of Chronic Lymphocytic Leukemia with Ibrutinib plus Obinutuzumab versus Chlorambucil plus Obinutuzumab: Final Analysis of the Randomized, Phase 3 ILLUMINATE Trial. Haematologica 2022, 107, 2108–2120. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.-D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without Obinutuzumab versus Chlorambucil and Obinutuzumab for Treatment-Naive Chronic Lymphocytic Leukaemia (ELEVATE-TN): A Randomised, Controlled, Phase 3 Trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Strati, P.; Parikh, S.A.; Chaffee, K.G.; Kay, N.E.; Call, T.G.; Achenbach, S.J.; Cerhan, J.R.; Slager, S.L.; Shanafelt, T.D. Relationship between Co-Morbidities at Diagnosis, Survival and Ultimate Cause of Death in Patients with Chronic Lymphocytic Leukaemia (CLL): A Prospective Cohort Study. Br. J. Haematol. 2017, 178, 394–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancik, R. Cancer Burden in the Aged: An Epidemiologic and Demographic Overview. Cancer 1997, 80, 1273–1283. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yancik, R.; Wesley, M.N.; Ries, L.A.G.; Havlik, R.J.; Long, S.; Edwards, B.K.; Yates, J.W. Comorbidity and Age as Predictors of Risk for Early Mortality of Male and Female Colon Carcinoma Patients. Cancer 1998, 82, 2123–2134. [Google Scholar] [CrossRef]
- Rigolin, G.M.; Cavallari, M.; Quaglia, F.M.; Formigaro, L.; Lista, E.; Urso, A.; Guardalben, E.; Liberatore, C.; Faraci, D.; Saccenti, E.; et al. In CLL, Comorbidities and the Complex Karyotype Are Associated with an Inferior Outcome Independently of CLL-IPI. Blood 2017, 129, 3495–3498. [Google Scholar] [CrossRef]
- Rotbain, E.C.; Niemann, C.U.; Rostgaard, K.; da Cunha-Bang, C.; Hjalgrim, H.; Frederiksen, H. Mapping Comorbidity in Chronic Lymphocytic Leukemia: Impact of Individual Comorbidities on Treatment, Mortality, and Causes of Death. Leukemia 2021, 35, 2570–2580. [Google Scholar] [CrossRef]
- Manda, S.; James, S.; Wang, R.; Krishnan, R.; Danilov, A.V. Impact of Comorbidities on Treatment Outcomes in Chronic Lymphocytic Leukemia: A Retrospective Analysis. Blood 2014, 124. [Google Scholar] [CrossRef]
- Gordon, M.J.; Churnetski, M.; Alqahtani, H.; Rivera, X.; Kittai, A.; Amrock, S.M.; James, S.; Hoff, S.; Manda, S.; Spurgeon, S.E.; et al. Comorbidities Predict Inferior Outcomes in Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cancer 2018, 124, 3192–3200. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, A.; Frustaci, A.M.; Mauro, F.R.; Chiarenza, A.; Coscia, M.; Ciolli, S.; Reda, G.; Laurenti, L.; Varettoni, M.; Murru, R.; et al. Do Age, Fitness and Concomitant Medications Influence Management and Outcomes of CLL Patients Treated with Ibrutinib? Blood 2020, 136 (Suppl. 1), 54–55. [Google Scholar] [CrossRef]
- Gordon, M.J.; Kaempf, A.; Sitlinger, A.; Shouse, G.; Mei, M.; Brander, D.M.; Salous, T.; Hill, B.T.; Alqahtani, H.; Choi, M.; et al. The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model. Clin. Cancer Res. 2021, 27, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Rotbain, E.C.; Gordon, M.J.; Vainer, N.; Frederiksen, H.; Hjalgrim, H.; Danilov, A.V.; Niemann, C.U. The CLL Comorbidity Index in a Population-Based Cohort: A Tool for Clinical Care and Research. Blood Adv. 2022, 6, 2701–2706. [Google Scholar] [CrossRef]
- Klepin, H.D. Ready for Prime Time: Role for Geriatric Assessment to Improve Quality of Care in Hematology Practice. Blood 2019, 134, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Repetto, L.; Fratino, L.; Audisio, R.A.; Venturino, A.; Gianni, W.; Vercelli, M.; Parodi, S.; Dal Lago, D.; Gioia, F.; Monfardini, S.; et al. Comprehensive Geriatric Assessment Adds Information to Eastern Cooperative Oncology Group Performance Status in Elderly Cancer Patients: An Italian Group for Geriatric Oncology Study. J. Clin. Oncol. 2002, 20, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Bahlo, J.; Chataline, V.; Eichhorst, B.; Dürig, J.; Stilgenbauer, S.; Kolb, G.; Honecker, F.; Wedding, U.; Hallek, M. Evaluation of Geriatric Assessment in Patients with Chronic Lymphocytic Leu-kemia: Results of the CLL9 Trial of the German CLL Study Group. Leuk. Lymphoma 2015, 57, 789–796. [Google Scholar] [CrossRef]
- Bonanad, S.; De la Rubia, J.; Gironella, M.; Pérez Persona, E.; González, B.; Fernández Lago, C.; Arnan, M.; Zudaire, M.; Hernández Rivas, J.A.; Soler, A.; et al. Development and Psychometric Validation of a Brief Comprehensive Health Status Assessment Scale in Older Patients with Hematological Malignancies: The GAH Scale. J. Geriatr. Oncol. 2015, 6, 353–361. [Google Scholar] [CrossRef]
- de la Rubia, J.; González, B.; Cruz-Jentoft, A.J.; Iglesias, L.; Jarque, I.; Persona, E.P.; Lluch, R.; Marrero, C.; Zudaire, M.; Gironella, M.; et al. Geriatric Assessment in Hematology Scale Predicts Treatment Tolerability in Older Patients Diagnosed with Hematological Malignancies: The RETROGAH Study. J. Geriatr. Oncol. 2022, 14, 101401. [Google Scholar] [CrossRef]
- Connor Johnson, P.; Woyach, J.A.; Ulrich, A.; Marcotte, V.; Nipp, R.D.; Lage, D.E.; Nelson, A.M.; Newcomb, R.A.; Rice, J.; Lavoie, M.W.; et al. Geriatric Assessment Measures Are Predictive of Outcomes in Chronic Lymphocytic Leukemia. J. Geriatr. Oncol. 2023, 14, 101538. [Google Scholar] [CrossRef]
- Woyach, J.A. Making Clinical Trials Work for Older Patients with Chronic Lymphocytic Leukemia. J. Geriatr. Oncol. 2020, 11, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.F.; Busch, R.; Stilgenbauer, S.; Stauch, M.; Bergmann, M.A.; Ritgen, M.; Kranzhöfer, N.; Rohrberg, R.; Söling, U.; Burkhard, O.; et al. First-Line Therapy with Fludarabine Compared with Chlorambucil Does Not Result in a Major Benefit for Elderly Patients with Advanced Chronic Lymphocytic Leukemia. Blood 2009, 114, 3382–3391. [Google Scholar] [CrossRef] [Green Version]
- Hallek, M.; Al-Sawaf, O. Chronic Lymphocytic Leukemia: 2022 Update on Diagnostic and Therapeutic Procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Jones, J.A.; Coutre, S.E.; Mato, A.R.; Hillmen, P.; Tam, C.; Österborg, A.; Siddiqi, T.; Thirman, M.J.; Furman, R.R.; et al. Ibrutinib for Patients with Relapsed or Refractory Chronic Lymphocytic Leukaemia with 17p Deletion (RESONATE-17): A Phase 2, Open-Label, Multicentre Study. Lancet Oncol. 2016, 17, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients with Chronic Lymphocytic Leukemia with 17p Deletion: Results from the Full Population of a Phase II Pivotal Trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus Obinutuzumab versus Chlorambucil plus Obinutuzumab in First-Line Treatment of Chronic Lymphocytic Leukaemia (ILLUMINATE): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-Year Follow-up from RESONATE-2: First-Line Ibrutinib Treatment for Patients with Chronic Lymphocytic Leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef]
- Barr, P.M.; Robak, T.; Owen, C.; Tedeschi, A.; Bairey, O.; Bartlett, N.L.; Burger, J.A.; Hillmen, P.; Coutre, S.; Devereux, S.; et al. Sustained Efficacy and Detailed Clinical Follow-up of First-Line Ibrutinib Treatment in Older Patients with Chronic Lymphocytic Leukemia: Extended Phase 3 Results from RESONATE-2. Haematologica 2018, 103, 1502–1510. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Long-Term Results of Alliance A041202 Show Continued Advantage of Ibrutinib-Based Regimens Compared with Bendamustine plus Rituximab (BR) Chemoimmunotherapy. Blood 2021, 138 (Suppl. 1), 639. [Google Scholar] [CrossRef]
- Ruppert, A.S.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Coutre, S.; Brown, J.R.; Nattam, S.; Larson, R.A.; Erba, H.P.; et al. Adverse Event Burden in Older Patients with CLL Receiving Bendamustine plus Rituximab or Ibrutinib Regimens: Alliance A041202. Leukemia 2021, 35, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Liu, D. Acalabrutinib (ACP-196): A Selective Second-Generation BTK Inhibitor. J. Hematol. Oncol. 2016, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Munir, T.; Shadman, M.; Robak, T.; Brown, J.; Kahl, B.; Ghia, P.; Giannopoulos, K.; Simkovic, M.; Österberg, A.; Laurenti, L.; et al. Zanubrutinib (ZANU) vs. Bendamustine + Rituximab (BR) in Patients (PTS) with Treatment-Naïve Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): Extended Follow-up of the Sequoia Study. In Proceedings of the EHA2023 Hybrid Congress, Frankfurt, Germany, 8–11 June 2023. [Google Scholar]
- Tam, C.S.; Robak, T.; Ghia, P.; Kahl, B.S.; Walker, P.; Janowski, W.; Simpson, D.; Shadman, M.; Ganly, P.S.; Laurenti, L.; et al. Zanubrutinib Monotherapy for Patients with Treatment-Naïve Chronic Lymphocytic Leukemia and 17p Deletion. Haematologica 2020, 106, 2354–2363. [Google Scholar] [CrossRef]
- Kapoor, I.; Bodo, J.; Hill, B.T.; Hsi, E.D.; Almasan, A. Targeting BCL-2 in B-Cell Malignancies and Overcoming Therapeutic Resistance. Cell Death Dis. 2020, 11, 941. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in Relapsed or Refractory Chronic Lymphocytic Leukaemia with 17p Deletion: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Jin, H.Y.; Robrecht, S.; Choi, Y.; Balasubramanian, S.; Kotak, A.; Chang, Y.M.; Fink, A.M.; Tausch, E.; et al. Transcriptomic Profiles and 5-Year Results from the Randomized CLL14 Study of Venetoclax plus Obinutuzumab versus Chlorambucil plus Obinutuzumab in Chronic Lymphocytic Leukemia. Nat. Commun. 2023, 14, 2147. [Google Scholar] [CrossRef]
- Thompson, M.; Brander, D.; Nabhan, C.; Mato, A. Minimal Residual Disease in Chronic Lymphocytic Leukemia in the Era of Novel Agents. JAMA Oncol. 2018, 4, 394. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawaf, O.; Gentile, B.; Devine, J.; Zhang, C.; Sail, K.; Tandon, M.; Fink, A.; Kutsch, N.; Wendtner, C.; Eichhorst, B.; et al. Health-Related Quality of Life with Fixed-Duration Venetoclax-Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukemia: Results from the Randomized, Phase 3 CLL14 Trial. Am. J. Hematol. 2021, 96, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, S.; Franzen, C.A.; Venkataraman, G.; McClure, R.; Li, L.; Wu, W.; Niu, N.; Sukhanova, M.; Pei, J.; et al. Ibrutinib and Venetoclax Target Distinct Subpopulations of CLL Cells: Implication for Resid-ual Disease Eradication. Blood Cancer J. 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Isik, E.; Fernandes, S.M.; Brown, J.R.; Letai, A.; Davids, M.S. Bruton’s Tyrosine Kinase Inhibition Increases BCL-2 Dependence and Enhances Sensitivity to Venetoclax in Chronic Lymphocytic Leukemia. Leukemia 2017, 31, 2075–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Fowler, N.; Kadia, T.; et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N. Engl. J. Med. 2019, 380, 2095–2103. [Google Scholar] [CrossRef]
- Wierda, W.G.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; Moreno, C.; et al. Ibrutinib plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results from the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. J. Clin. Oncol. 2021, 39, 3853–3865. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-Duration Ibrutinib plus Venetoclax for First-Line Treatment of CLL: Primary Analysis of the CAPTIVATE FD Cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Marchetti, M.; Vitale, C.; Rigolin, G.M.; Vasile, A.; Visentin, A.; Scarfò, L.; Coscia, M.; Cuneo, A. Old and New Drugs for Chronic Lymphocytic Leukemia: Lights and Shadows of Real-World Evidence. J. Clin. Med. 2022, 11, 2076. [Google Scholar] [CrossRef]
- El-Galaly, T.C.; Cheah, C.Y.; Villa, D. Real World Data as a Key Element in Precision Medicine for Lymphoid Malignancies: Potentials and Pitfalls. Br. J. Haematol. 2019, 186, 409–419. [Google Scholar] [CrossRef]
- Forum, U.C. Ibrutinib for Relapsed/Refractory Chronic Lymphocytic Leukemia: A UK and Ireland Analysis of Outcomes in 315 Patients. Haematologica 2016, 101, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Karim, S.; Booth, C.M. Effectiveness in the Absence of Efficacy: Cautionary Tales from Real-World Evidence. J. Clin. Oncol. 2019, 37, 1047–1050. [Google Scholar] [CrossRef]
- Islam, P.; Mato, A.R. Utilizing Real-World Evidence (RWE) to Improve Care in Chronic Lymphocytic Leukemia: Challenges and Opportunities. Curr. Hematol. Malig. Rep. 2020, 15, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Cuneo, A. Ibrutinib in the Real World Patient: Many Lights and Some Shades. Haematologica 2016, 101, 1448–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, R.K.; Nagar, S.P.; Kabadi, S.M.; Le, H.; Davis, K.L.; Kaye, J.A. Overall Survival, Adverse Events, and Economic Burden in Patients with Chronic Lymphocytic Leukemia Receiving Systemic Therapy: Real-World Evidence from the Medicare Population. Cancer Med. 2021, 10, 2690–2702. [Google Scholar] [CrossRef]
- Narezkina, A.; Akhter, N.; Lu, X.; Emond, B.; Panjabi, S.; Forbes, S.P.; Hilts, A.; Liu, S.; Lafeuille, M.-H.; Lefebvre, P.; et al. Real-World Persistence and Time to next Treatment with Ibrutinib in Patients with Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Including Patients at High Risk for Atrial Fibrillation or Stroke. Clin. Lymphoma Myeloma Leuk. 2022, 22, e959–e971. [Google Scholar] [CrossRef]
- Mato, A.R.; Tang, B.; Azmi, S.; Yang, K.; Zhang, X.; Stern, J.C.; Hedrick, E.; Huang, J.-S.; Sharman, J.P. A Clinical Practice Comparison of Patients with Chronic Lymphocytic Leukemia with and without Deletion 17p Receiving First-Line Treatment with Ibrutinib. Haematologica 2022, 107, 2630–2640. [Google Scholar] [CrossRef]
- Rigolin, G.M.; Olimpieri, P.P.; Summa, V.; Celant, S.; Scarfò, L.; Tognolo, L.; Ballardini, M.P.; Urso, A.; Sessa, M.; Gambara, S.; et al. Outcomes in Patients with Chronic Lymphocytic Leukemia and TP53 Aberration Who Received First Line Ibrutinib: A Nationwide Registry Study from the Italian Medicines Agency. Blood Cancer J. 2023, 13, 99. [Google Scholar] [CrossRef]
- Visentin, A.; Mauro, F.R.; Cibien, F.; Vitale, C.; Reda, G.; Fresa, A.; Ciolli, S.; Pietrasanta, D.; Marchetti, M.; Murru, R.; et al. Continuous Treatment with Ibrutinib in 100 Untreated Patients with TP 53 Disrupted Chronic Lymphocytic Leukemia: A Real-Life Campus CLL Study. Am. J. Hematol. 2021, 97, E95–E99. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Mato, A.R.; Rigolin, G.M.; Piciocchi, A.; Gentile, M.; Laurenti, L.; Allan, J.N.; Pagel, J.M.; Brander, D.M.; Hill, B.T.; et al. Efficacy of Bendamustine and Rituximab in Unfit Patients with Previously Untreated Chronic Lymphocytic Leukemia. Indirect Comparison with Ibrutinib in a Real-World Setting. A GIMEMA-ERIC and US Study. Cancer Med. 2020, 9, 8468–8479. [Google Scholar] [CrossRef]
- Visentin, A.; Mauro, F.R.; Catania, G.; Fresa, A.; Vitale, C.; Sanna, A.; Mattiello, V.; Cibien, F.; Sporto-letti, P.; Gentile, M.; et al. Obinutuzumab plus Chlorambucil versus Ibrutinib in Previously Untreated Chronic Lymphocytic Leukemia Patients without TP53 Disruptions: A Real-Life CLL Campus Study. Front. Oncol. 2022, 12, 1033413. [Google Scholar] [CrossRef]
- Green, T.; Bron, D.; Chomienne, C.; de Wit, T.D.; de Haas, F.; Engert, A.; Hagenbeek, A.; Jäger, U.; MacIntyre, E.; Muckenthaler, M.U.; et al. Costs of Haematological Disease High and Rising. Lancet Haematol. 2016, 3, e353–e354. [Google Scholar] [CrossRef]
- Scheffer Cliff, E.R.; Kesselheim, A.S.; Rome, B.N.; Feldman, W.B. Trends in Medicare Spending on Oral Drugs for Chronic Lymphocytic Leukemia from 2014 to 2020. JAMA Netw. Open 2023, 6, e237467. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Borah, B.J.; Finnes, H.D.; Chaffee, K.G.; Ding, W.; Leis, J.F.; Chanan-Khan, A.A.; Parikh, S.A.; Slager, S.L.; Kay, N.E.; et al. Impact of Ibrutinib and Idelalisib on the Pharmaceutical Cost of Treating Chronic Lymphocytic Leukemia at the Individual and Societal Levels. J. Oncol. Pract. 2015, 11, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, C.; Masaquel, A.; Sutphin, J.; Weiss, E.; Gutierrez, M.; Wilson, J.; Boeri, M.; Li, J.; Reyes, C. Patients’ Priorities in Selecting Chronic Lymphocytic Leukemia Treatments. Blood Adv. 2017, 1, 2176–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuneo, A.; Cavazzini, F.; Cavallari, M.; Foà, R.; Rigolin, G.M. Optimal Management of Chronic Lymphocytic Leukemia and Economic Constraints. Cancer J. 2021, 27, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.M.; Ramsey, S.; Shankaran, V. Financial Toxicity in Cancer Care: Implications for Clinical Care and Potential Practice Solutions. J. Clin. Oncol. 2023, 41, 3051–3058. [Google Scholar] [CrossRef]
- Chatterjee, A.; van de Wetering, G.; Goeree, R.; Owen, C.; Desbois, A.M.; Barakat, S.; Manzoor, B.S.; Sail, K. A Probabilistic Cost-Effectiveness Analysis of Venetoclax and Obinutuzumab as a First-Line Therapy in Chronic Lymphocytic Leukemia in Canada. Pharmacoecon. Open 2023, 7, 199–216. [Google Scholar] [CrossRef]
- Munir, T.; Genovez, V.; Genestier, V.; Ryan, K.; Liljas, B.; Gaitonde, P. Cost-Effectiveness of Acalabrutinib Regimens in Treatment-Naïve Chronic Lymphocytic Leukemia in the United States. Expert. Rev. Pharmacoecon. Outcomes Res. 2023, 23, 579–589. [Google Scholar] [CrossRef]
- Slot, M.; Niemann, C.U.; Ehlers, L.H.; Rotbain, E.C. Cost-effectiveness of targeted treatment vs chemoimmunotherapy in treatment-naïve unfit CLL without TP53 aberrations. Blood Adv. 2023; ahead of print. [Google Scholar]
- Vokinger, K.N.; Hwang, T.J.; Grischott, T.; Reichert, S.; Tibau, A.; Rosemann, T.; Kesselheim, A.S. Prices and Clinical Benefit of Cancer Drugs in the USA and Europe: A Cost–Benefit Analysis. Lancet Oncol. 2020, 21, 664–670. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects. 2022. Available online: https://population.un.org/wpp/Graphs/Probabilistic/POP/65plus/1829 (accessed on 15 May 2023).
- Eurostat Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/SEPDF/cache/80393.pdf (accessed on 15 May 2023).
- Wierda, W.G.; Brown, J.; Abramson, J.S.; Awan, F.; Bilgrami, S.F.; Bociek, G.; Brander, D.; Chanan-Khan, A.A.; Coutre, S.E.; Davis, R.S.; et al. NCCN Guidelines® Insights: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 622–634. [Google Scholar] [CrossRef]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic Lymphocytic Leukaemia: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef]
- Ahn, I.E.; Brown, J.R. Selecting Initial Therapy in CLL. Hematol. Am. Soc. Hematol. Educ. Program. 2022, 2022, 323–328. [Google Scholar] [CrossRef]
- Kim, M.S.; Prasad, V. Front-Line Chronic Lymphocytic Leukemia: The Role of Chemoimmunotherapy. Am. J. Hematol. 2023, 98, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R. Relapsed CLL: Sequencing, Combinations, and Novel Agents. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittai, A.S.; Miller, C.; Goldstein, D.; Huang, Y.; Abruzzo, L.V.; Beckwith, K.; Bhat, S.A.; Bond, D.A.; Grever, M.R.; Heerema, N.A.; et al. The impact of increasing karyotypic complexity and evolution on survival in patients with CLL treated with ibrutinib. Blood 2021, 138, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Paoloni, F.; Molica, S.; Reda, G.; Trentin, L.; Sportoletti, P.; Marchetti, M.; Pietrasanta, D.; Marasca, R.; Gaidano, G.; et al. Efficacy of Front-Line Ibrutinib and Rituximab Combination and the Impact of Treatment Discontinuation in Unfit Patients with Chronic Lymphocytic Leukemia: Results of the Gimema LLC1114 Study. Cancers 2021, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Rigolin, G.M.; Del Giudice, I.; Bardi, A.; Melandri, A.; García-Jacobo, R.E.; Cura, F.; Raponi, S.; Ilari, C.; Cafforio, L.; Piciocchi, A.; et al. Complex Karyotype in Unfit Patients with CLL Treated with Ibrutinib and Rituximab: The GIMEMA LLC1114 Phase 2 Study. Blood 2021, 138, 2727–2730. [Google Scholar] [CrossRef]
- Fürstenau, M.; Thus, Y.J.; Robrecht, S.; Mellink, C.H.M.; van der Kevie-Kersemaekers, A.F.; Dubois, J.; von Tresckow, J.; Patz, M.; Gregor, M.; Thornton, P.; et al. High karyotypic complexity is an independent prognostic factor in patients with CLL treated with venetoclax combinations. Blood, 2023; ahead of print. [Google Scholar]
- Cuneo, A.; Scarfò, L.; Reda, G.; Varettoni, M.; Quaglia, F.M.; Marchetti, M.; De Paoli, L.; Re, F.; Pietrasanta, D.; Rigolin, G.M.; et al. Chronic Lymphocytic Leukemia Management in Italy during the COVID-19 Pandemic: A Campus CLL Report. Blood 2020, 136, 763–766. [Google Scholar] [CrossRef]
- Chatzikonstantinou, T.; Kapetanakis, A.; Scarfò, L.; Karakatsoulis, G.; Allsup, D.; Cabrero, A.A.; Andres, M.; Antic, D.; Baile, M.; Baliakas, P.; et al. COVID-19 Severity and Mortality in Patients with CLL: An Update of the International ERIC and Campus CLL Study. Leukemia 2021, 35, 3444–3454. [Google Scholar] [CrossRef]
- Herishanu, Y.; Rahav, G.; Levi, S.; Braester, A.; Itchaki, G.; Bairey, O.; Dally, N.; Shvidel, L.; Ziv-Baran, T.; Polliack, A.; et al. Efficacy of a Third BNT162b2 MRNA COVID-19 Vaccine Dose in Patients with CLL Who Failed Standard Two-Dose Vaccination. Blood 2022, 139, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Vardell, V.A.; Ermann, D.A.; Fitzgerald, L.A.; Shah, H.; Hu, B.; Stephens, D.M. Influence of Racial and Ethnic Identity on Overall Survival in Patients with Chronic Lymphocytic Leukemia. Am. J. Hematol. 2023, 98, E172–E174. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Barrientos, J.C.; Rai, K.R. How Have Targeted Agents Changed the Treatment Landscape for Elderly Patients with CLL? Curr. Oncol. Rep. 2022, 24, 1705–1713. [Google Scholar] [CrossRef]
- Van Der Straten, L.; Stege, C.A.M.; Kersting, S.; Nasserinejad, K.; Dubois, J.; Dobber, J.A.; Mellink, C.H.M.; van der Kevie-Kersemaekers, A.F.; Evers, L.M.; de Boer, F.; et al. Fixed-Duration Venetoclax plus Obinutuzumab Improves Quality of Life and Geriatric Impairments in FCR-Unfit CLL Patients. Blood, 2023; ahead of print. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, S.; Efficace, F.; Kieffer, J.M.; Kyriakou, C.; Xochelli, A.; Levedahl, K.; Petranovic, D.; Borges, F.C.; Bredart, A.; Shamieh, O.; et al. International Validation of the EORTC QLQ-CLL17 Questionnaire for Assessment of Health-Related Quality of Life for Patients with Chronic Lymphocytic Leukaemia. Br. J. Haematol. 2022, 197, 431–441. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Health and Care Excellence. Evidence-Based Recommendations on Venetoclax (Venclyxto) with Obinutuzumab for Untreated Chronic Lymphocytic Leukaemia in Adults. Available online: https://www.nice.org.uk/guidance/ta663 (accessed on 15 May 2023).
- Zorginstituut Nederland. Available online: https://english.zorginstituutnederland.nl/publications/reports/2020/11/16/venetoclax-venclyxto (accessed on 15 May 2023).
- Do, N.; Thielen, F. Cost-Effectiveness of Venetoclax plus Obinutuzumab versus Chlorambucil plus Obinutuzumab for the First-Line Treatment of Adult Patients with Chronic Lymphocytic Leukemia—An Extended Societal View. Value Health 2022, 26, 477–486. [Google Scholar] [CrossRef]
- Barnes, J.I.; Divi, V.; Begaye, A.; Wong, R.; Coutre, S.; Owens, D.K.; Goldhaber-Fiebert, J.D. Cost-Effectiveness of Ibrutinib as First-Line Therapy for Chronic Lymphocytic Leukemia in Older Adults without Deletion 17p. Blood Adv. 2018, 2, 1946–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, R.; Redekop, W.K. Cost-Effectiveness of Ibrutinib Compared with Obinutuzumab with Chlorambucil in Untreated Chronic Lymphocytic Leukemia Patients with Comorbidities in the United Kingdom. Clin. Lymphoma Myeloma Leuk. 2018, 18, e131–e142. [Google Scholar] [CrossRef]
- The National Institute for Health and Care Excellence. Acalabrutinib for Treating Chronic Lymphocytic Leukaemia. Available online: https://www.nice.org.uk/guidance/TA689/chapter/1-Recommendations (accessed on 15 May 2023).
- The National Institute for Health and Care Excellence. Ibrutinib with Venetoclax for Untreated Chronic Lymphocytic Leukaemia. Available online: https://www.nice.org.uk/guidance/TA891/chapter/1-Recommendations (accessed on 15 May 2023).
- Lachaine, J.; Guinan, K.; Aw, A.; Banerji, V.; Fleury, I.; Owen, C. Impact of Fixed-Duration Oral Targeted Therapies on the Economic Burden of Chronic Lymphocytic Leukemia in Canada. Curr. Oncol. 2023, 30, 4483–4498. [Google Scholar] [CrossRef]
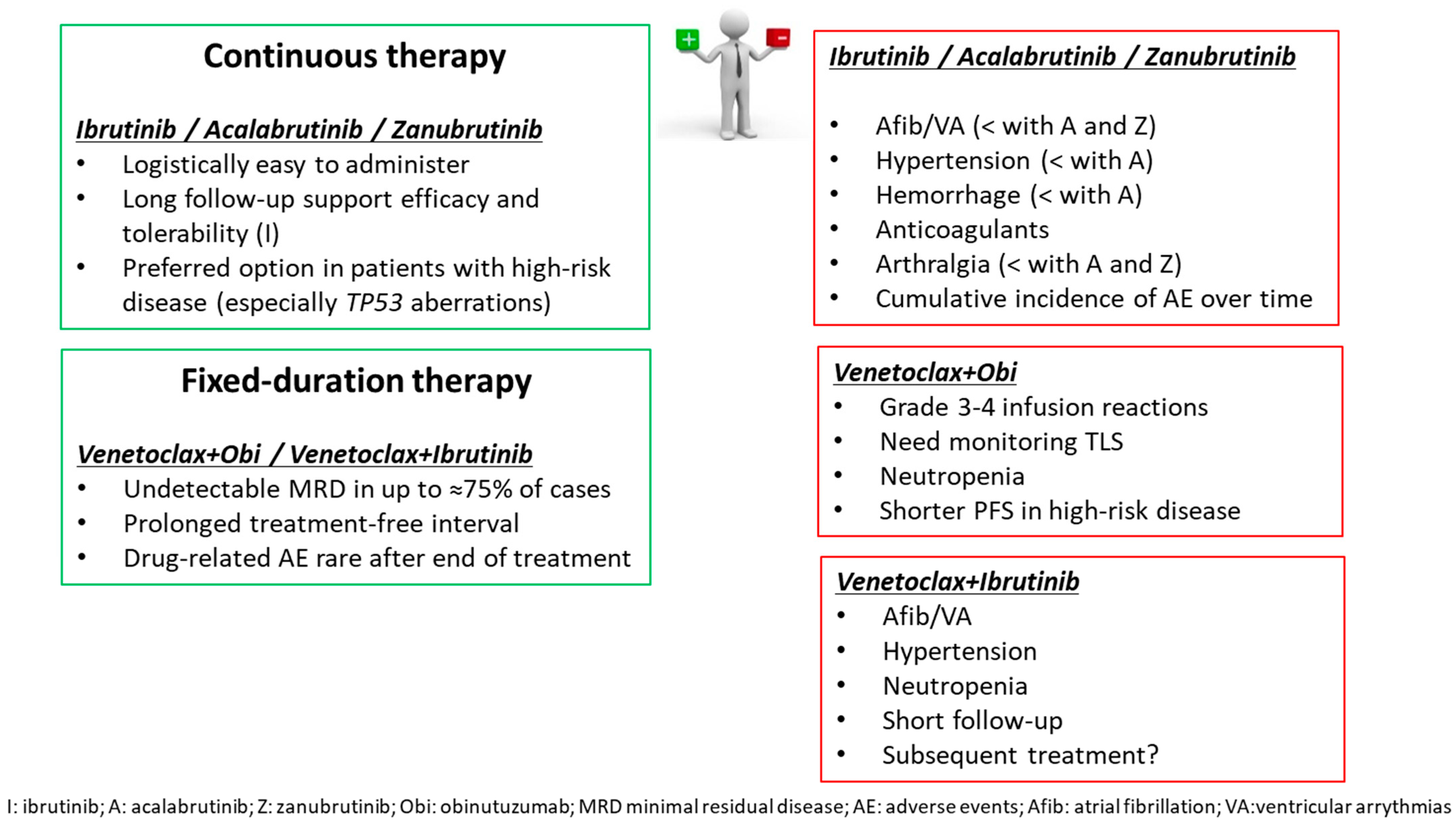
| N. of Patients | Median Age (Years) | Median Follow-Up (Months) | % TP53 Aberrations | PFS and HR (95% CI) | OS | CR/ORR (%) | Reference | |
|---|---|---|---|---|---|---|---|---|
| BTKi vs. comparator | ||||||||
| IBR vs. Chlor | 269 | 72–73 | 18.4 | 0% | 18-month PFS: IBR 90%; Chlor 52%; HR 0.16 (0.09–0.28) | 2-year OS: IBR 98%; Chlor 85% | 4/86 2/35 | [49] |
| IBR vs. IBR + R vs. BR | 547 | 71 | 38 | 10% | 2-year PFS: IBR 87%; IBR + R 88%; BR 74%; HR IBR 0.37 (0.25–0.56); HR IBR + R 0.40 (0.27–0.60) | 2-year OS: IBR 90%; IBR + R 94%; BR 95% | 7/93 12/94 26/81 | [21] |
| IBR + O vs. Chlor + O | 229 | 70–72 | 31.3 | 16%/20% | 30-month PFS: IBR + O 77%; CHLOR + O 16%; HR 0.23 (0.15–0.37) | 30-month OS: IBR + O 86%; CHLOR + O 85% | 19/88 8/73 | [50] |
| ACALA + O, ACALA, Chlor + O | 535 | 70 | 28.3 | 9% | 2-year PFS: ACALA 87%; ACALA + O 93%; CHLOR + O 47%; HR ACALA + O 0.10 (0.06–0.17) HR ACALA 0.20, (0.13–0.30) | 2-year OS: ACALA 95%; ACALA + O 95%; CHOLOR + O 92% | 1/85 24/94 5/79 | [24] |
| ZANU vs. BR | 479 | 70 years | 26.2 | 0% * | 24-month PFS: ZANU 85.5%; BR 69.5%; HR 0.42 (0.28–0.63) | 24-month OS: ZANU 94.3%; BR 94.6% | 7/95 15/85 | [15] |
| Venetoclax-containing regimen vs. comparator | ||||||||
| V + O vs. Chlor + O | 432 | 72–74 | 28.1 | 11.1% | 24-month PFS: VO 88.2%; CHLOR + O 64.1% HR 0.35 (0.23 to 0.53) | 24-month OS: VO 91.8%; CHLOR + O 93.3% | 49/85 33/71 | [20] |
| IBR + V vs. Chlor + O | 211 | 71 | 27.7 | 4.3% | 30-month PFS: IBRU + V 80.5%; CHLOR + O 35.8%; HR 0.216 (0.131 to 0.357) | NR | 39/87 11/85 | [23] |
| Trial | Median Follow-Up (Months) | AFib (*) | Hypertension | Bleeding | Infections ^ | Arthralgia | Reference |
|---|---|---|---|---|---|---|---|
| Resonate-2 | 18.4 | IBRU *6%/1.5%; CHLOR 0.7% | IBRU 4%; CHLOR 0% | IBRU 4% CHLOR 2% | 8% 4% | IBRU 16% **; CHLOR 7%; | [49] |
| Alliance | 38 | IBR *17%/9%; IBR + R *14%/6% BR 3%/3% | IBR 29%; IBR + R 34%; BR 15% | IBR 2% IBR + R 4% BR 0% | 20% 20% 15% | NR | [21] |
| iLLUMINATE | 31.3 | IBRU + O *12%/5%; CHLOR + O 0% | IBRU + O 4%; CHLOR + O 4% | NR | 11% 5% | IBRU + O 1% CHLOR + O 0% | [50] |
| ACAL + O, ACAL, Chlor + O | 28.3 | A *4%; A + O *3%; CHLOR + O: *1% | A 2%; A + O 3% | A 2%; A + O 2% | 11% 3.9% 2.4% | A 0.6%; A + O 1.1% | [24] |
| SEQUOIA | 26.2 | ZANU *3%; BR *3% | ZANU 6%; BR 5% | ZANU 3.5% BR 1.5% | 3% 5% | ZANU 1%; BR 0.5% | [15] |
| Trial | Median Follow-Up (Months) | Infusion Related Reactions | Tumor Lysis Syndrome | Neutropenia | Infections | AFib * | Reference |
|---|---|---|---|---|---|---|---|
| CLL14 | 28.1 | V + O 9%; Chlor + O 10.3% | V + O 0.5%; Chlor + O 1.9% | V + O 52.8%; Chlor + O 48.1% | V + O 17.5%; Chlor + O 15.0% | NA | [20] |
| GLOW | 27.7 | NA | Ibr + V 0%; Chlor + O 5.7% | Ibr + V 34.9%; Chlor + O 49.5% | Ibr + V 12.3%; Chlor + O 8.6% | Ibr + V ^ 14%/6%; Chlor + O 1.9%/0% | [23] |
| Source/Country/ Reference | WTP/QALY | Treatment | Comparator | Target Population | ICER | Comments | Cost- Effective |
|---|---|---|---|---|---|---|---|
| NICE/U.K./114 | GBP 20,000 to 30,000 | V + O | Ibrutinib | 17p | GBP 549,699 saved per QALY lost * | V + O results in cost saving of GBP 199,622 and QALY loss of 0.363 * | YES ^ |
| Chlor + O | Unsuitable for FCR/BR | NR | Dominant effect V + O vs. Chlor + O ° (more effective and less costly) | YES ^ | |||
| FR/BR | Suitable for FCR/BR | GBP 47,494 vs. FCR GBP 67,445 vs. BR per QALY gained | ICERs varied widely if the upper and lower bounds of the PFS and OS HR-CI were applied | NO | |||
| Dutch National Health Care Institute/ Holland/115 | EUR 50,000 | V + O | Chlor + O | Non-fit patients, uIGHV § | Incremental QALYs of 1.14 and cost saving EUR 159,276 | Dominant effect (more effective and less costly); negotiation of prices recommended | YES |
| Non-fit patients, mIGHV § | NR | V + O cost saving despite limited availability of data | YES | ||||
| Erasmus University Rotterdam/Holland/116 | EUR 20,000 | V + O | Chlor + O | All patients | 1.25 QALYs gained; EUR 62,316 saved | The sensitivity analyses demonstrated the robustness of these results | YES |
| Stanford University/ U.S.A./117 | USD 150,000 | Ibrutinib | Chlor + O | CLL without 17p | USD 189,000 per QALY gained | A reduction of USD 20,400 per year would be required to reach the WTP of USD 150,000 | NO # |
| Erasmus University Rotterdam/U.K./118 | GBP 20,000 to 30,000 | Ibrutinib | Chlor + O | CLL | GBP 75,648 per QALY gained | An adequate discount on ibrutinib is required to make it cost-effective as per the U.K. thresholds | NO # |
| NICE/U.K./119 | Acalabrutinib | Chlor + O | CLL unsuitable for FRC/BR, including 17p | GBP < 30,000 per QALY gained | Considering confidential discounts | YES | |
| NICE/U.K./120 | GBP 20,000 to 30,000 | Ibrutinib and venetoclax | FRC/BR | CLL suitable for FRC/BR, including 17p | GBP < 30,000 per QALY gained | Considering confidential discounts | YES |
| Chlor + O and V + O | Unsuitable for FRC/BR, including 17p | GBP <30,000 per QALY gained | Dominant effect vs. Chlo + O ° | YES | |||
| Acalabrutinib and ibrutinib | NR | Cost saving and a small QALY loss compared with acalabrutinib and ibrutinib | YES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, A.; Cavazzini, F.; Ballardini, M.P.; Gambara, S.; Consolo, S.; Rigolin, G.M.; Cuneo, A. First-Line Treatment of Older Patients with CLL: A New Approach in the Chemo-Free Era. Cancers 2023, 15, 3859. https://doi.org/10.3390/cancers15153859
Urso A, Cavazzini F, Ballardini MP, Gambara S, Consolo S, Rigolin GM, Cuneo A. First-Line Treatment of Older Patients with CLL: A New Approach in the Chemo-Free Era. Cancers. 2023; 15(15):3859. https://doi.org/10.3390/cancers15153859
Chicago/Turabian StyleUrso, Antonio, Francesco Cavazzini, Maria Pia Ballardini, Silvia Gambara, Sara Consolo, Gian Matteo Rigolin, and Antonio Cuneo. 2023. "First-Line Treatment of Older Patients with CLL: A New Approach in the Chemo-Free Era" Cancers 15, no. 15: 3859. https://doi.org/10.3390/cancers15153859
APA StyleUrso, A., Cavazzini, F., Ballardini, M. P., Gambara, S., Consolo, S., Rigolin, G. M., & Cuneo, A. (2023). First-Line Treatment of Older Patients with CLL: A New Approach in the Chemo-Free Era. Cancers, 15(15), 3859. https://doi.org/10.3390/cancers15153859







