Sarcopenia Diagnosis and Management in Hematological Malignancies and Differences with Cachexia and Frailty
Abstract
:Simple Summary
Abstract
1. Introduction
2. Definitions and Features: Sarcopenia, Cachexia, Frailty
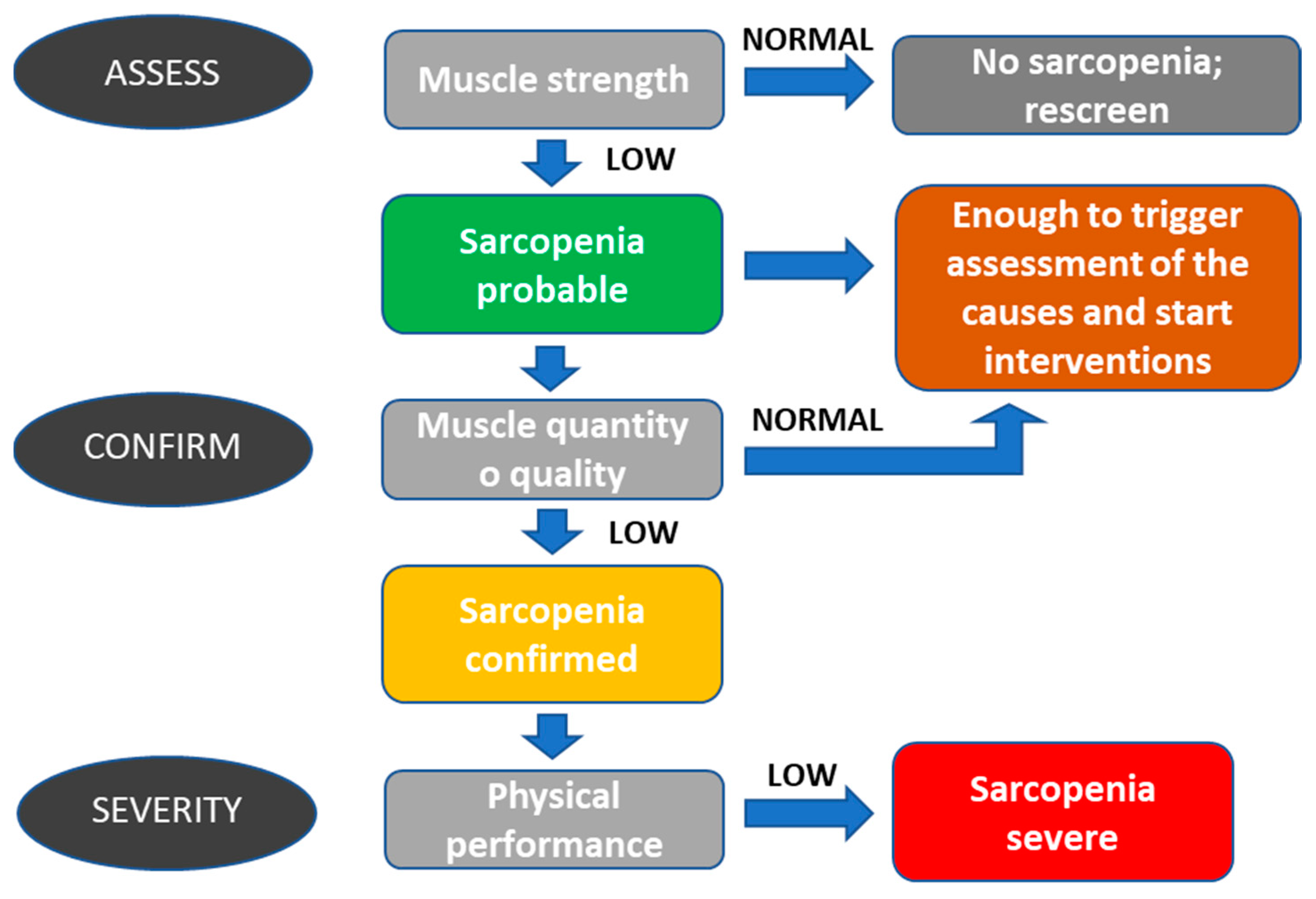
2.1. Sarcopenia
2.2. Sarcopenic Obesity
2.3. Frailty
2.4. Cachexia
3. Causes of Sarcopenia
3.1. Inflammation
3.2. Malnutrition and Weight Loss
4. Management of Sarcopenia
5. Cancer Treatments and Sarcopenia
6. Sarcopenia in Hematological Patients
7. Conclusions
- Identification of sarcopenia. This process must be quick and easy to use. The tests chosen for a screening or diagnostic evaluation should be easily feasible. Therefore, the one that can be reasonably and quickly performed on all patients in the context of the reference structure must be chosen among the available tests.
- Evaluation of sarcopenia as an influencing factor on toxicity and response to treatment. It is essential to discuss treatment options to reduce the risk of toxicity to the patient and start treating sarcopenia or, in any case, reduce its impact on the patient.
- Treatment of sarcopenia. All sarcopenia treatments are directed towards an improvement in the quality level of the muscle. This is obtained by increasing protein intake, which is associated with increased aerobic and counter-resistance physical exercise. It is essential to remember that muscle mass is a complex parameter to monitor as much as the quality of the muscle, which is highlighted above all in an alteration of performance.
- Sarcopenia is a geriatric syndrome characterized by a progressive loss of systemic muscle mass plus a decrease in muscle strength or physical function;
- Sarcopenia has a multifactorial pathogenesis: nutritional deficits, physical inactivity, and chronic diseases are the leading causes of sarcopenia;
- Sarcopenia is associated with adverse outcomes such as falls, functional decline, frailty, disability, multiple hospitalizations, and mortality;
- When low muscle mass, low muscle strength, and low physical performance are simultaneously present in a patient, we refer to it as severe sarcopenia;
- When sarcopenia is diagnosed as an obese condition, we have a far more complex syndrome than the sum of the two, called sarcopenic obesity;
- Although sarcopenia and cachexia share standard features, they are two distinct conditions for which a differential diagnosis is needed to best manage our patients;
- In oncology, sarcopenia is associated with an increased risk of treatment toxicity, postoperative complications, sensitivity to anti-blastic treatments, and a higher mortality rate;
- The most effective intervention for reversing sarcopenia is physical exercise combined with nutrition;
- In patients with hematologic malignancy, low muscle mass is associated with adverse outcomes and is a predictor of overall survival and non-relapse mortality.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Demling, R.H. Nutrition, Anabolism, and the Wound Healing Process: An Overview. Eplasty 2009, 9, e9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2642618/ (accessed on 28 August 2023).
- Gallagher, D.; Ruts, E.; Visser, M.; Heshka, S.; Baumgartner, R.N.; Wang, J.; Pierson, R.N.; Pi-Sunyer, F.X.; Heymsfield, S.B.; Yim, J.-E.; et al. Weight stability masks sarcopenia in elderly men and women. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E366–E375. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; Amog, K.; Phillips, S.; Parise, G.; McNicholas, P.D.; de Souza, R.J.; Thabane, L.; Raina, P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing 2019, 48, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil–based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Coriat, R.; Blanchet, B.; Durand, J.-P.; Boudou-Rouquette, P.; Michels, J.; Ropert, S.; Vidal, M.; Pol, S.; Chaussade, S.; et al. Sarcopenia Predicts Early Dose-Limiting Toxicities and Pharmacokinetics of Sorafenib in Patients with Hepatocellular Carcinoma. PLoS ONE 2012, 7, e37563. [Google Scholar] [CrossRef]
- Parsons, H.A.; Baracos, V.E.; Dhillon, N.; Hong, D.S.; Kurzrock, R. Body Composition, Symptoms, and Survival in Advanced Cancer Patients Referred to a Phase I Service. PLoS ONE 2012, 7, e29330. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, M.-H.; Borget, I.; Broutin, S.; Baracos, V.E.; Leboulleux, S.; Baudin, E.; Paci, A.; Deroussent, A.; Schlumberger, M.; Antoun, S. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: Results from a placebo-controlled study. J. Clin. Endocrinol. Metab. 2013, 98, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Au, P.C.-M.; Lee, G.K.-Y.; Li, G.H.-Y.; Chan, M.; Cheung, B.M.-Y.; Wong, I.C.-K.; Lee, V.H.-F.; Mok, J.; Yip, B.H.-K.; et al. Different definitions of sarcopenia and mortality in cancer: A meta-analysis. Osteoporos. Sarcopenia 2021, 7 (Suppl. S1), S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argiles, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Zilioli, V.R.; Albano, D.; Arcari, A.; Merli, F.; Coppola, A.; Besutti, G.; Marcheselli, L.; Gramegna, D.; Muzi, C.; Manicone, M.; et al. Clinical and prognostic role of sarcopenia in elderly patients with classical Hodgkin lymphoma: A multicentre experience. J. Cachexia Sarcopenia Muscle 2021, 12, 1042–1055. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic obesity: Time to meet the challenge. Clin. Nutr. 2018, 37, 1787–1793. [Google Scholar] [CrossRef]
- Pahor, M.; Manini, T.; Cesari, M. Sarcopenia: Clinical evaluation, biological markers and other evaluation tools. J. Nutr. Health Aging 2009, 13, 724–728. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19657557 (accessed on 16 September 2019). [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. S5), 990S–991S. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bayens, J.P.; Bauer, J.M. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Tsuchida, K.; Fujihara, Y.; Hiroki, J.; Hakamata, T.; Sakai, R.; Nishida, K.; Sudo, K.; Tanaka, K.; Hosaka, Y.; Takahashi, K.; et al. Significance of Sarcopenia Evaluation in Acute Decompensated Heart Failure. Int. Heart J. 2018, 59, 143–148. [Google Scholar] [CrossRef]
- Dudgeon, D.; Baracos, V.E. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: A debilitating intersection of sarcopenia, cachexia and breathlessness. Curr. Opin. Support. Palliat. Care 2016, 10, 236–241. [Google Scholar] [CrossRef]
- Androga, L.; Sharma, D.; Amodu, A.; Abramowitz, M.K. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int. Rep. 2017, 2, 201–211. [Google Scholar] [CrossRef]
- Prado, C.M.; Bell, J.J.; Gonzalez, M.C.; Prado, C.M.; Bell, J.J.; Gonzalez, M.C. Untangling Malnutrition, Physical Dysfunction, Sarcopenia, Frailty and Cachexia in Ageing. Springer: Cham, Switzerland, 2021; pp. 99–113. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef]
- Davison, K.K.; Ford, E.S.; Cogswell, M.E.; Dietz, W.H.; DrPH, M.E.C. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J. Am. Geriatr. Soc. 2002, 50, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Di Francesco, V.; Guralnik, J.M.; Mazzali, G.; Bortolani, A.; Guariento, S.; Sergi, G.; Bosello, O.; Zamboni, M. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.R.; Ricca, M.; Ferrucci, L. True osteoporosis and frailty-related osteopenia: Two different clinical entities. J. Am. Geriatr. Soc. 2000, 48, 1738–1739. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; Technical Report Series 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Larsson, L.; Li, X.; Frontera, W.R. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am. J. Physiol. Physiol. 1997, 272, C638–C649. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Carlson, C.L.; Visser, M.; Kelley, D.E.; Scherzinger, A.; Harris, T.B.; Stamm, E.; Newman, A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 2001, 90, 2157–2165. [Google Scholar] [CrossRef]
- Delbono, O. Neural control of aging skeletal muscle. Aging Cell 2003, 2, 21–29. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Colloca, G.; Di Capua, B.; Bellieni, A.; Cesari, M.; Marzetti, E.; Valentini, V.; Calvani, R. Muscoloskeletal aging, sarcopenia and cancer. J. Geriatr. Oncol. 2018, 10, 504–509. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Pimentel, G.D.; O Costa, F.; Carvalheira, J.B.C. Molecular and neuroendocrine mechanisms of cancer cachexia. J. Endocrinol. 2015, 226, R29–R43. [Google Scholar] [CrossRef]
- Argilés, J.M.; Anker, S.D.; Evans, W.J.; Morley, J.E.; Fearon, K.C.; Strasser, F.; Muscaritoli, M.; Baracos, V.E. Consensus on Cachexia Definitions. J. Am. Med. Dir. Assoc. 2010, 11, 229–230. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.J.; Mozer, M. Differentiating Sarcopenia and Cachexia Among Patients With Cancer. Nutr. Clin. Pract. 2017, 32, 30–39. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Cesari, M.; Tosato, M.; Marini, F.; Manes-Gravina, E.; Bernabei, R.; Landi, F.; Marzetti, E. Biomarkers for Sarcopenia: Reductionism vs. Complexity. Curr. Protein Pept. Sci. 2018, 19, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Bossola, M.; Allocca, E.; Menghi, A.; Pesce, V.; Lezza, A.M.S.; Bernabei, R.; Landi, F.; Marzetti, E. Update on mitochondria and muscle aging: All wrong roads lead to sarcopenia. Biol. Chem. 2018, 399, 421–436. [Google Scholar] [CrossRef]
- Frontera, W.R.; Zayas, A.R.; Rodriguez, N. Aging of human muscle: Understanding sarcopenia at the single muscle cell level. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 201–207. [Google Scholar] [CrossRef]
- Marzetti, E.; Lees, H.A.; Wohlgemuth, S.E.; Leeuwenburgh, C. Sarcopenia of aging: Underlying cellular mechanisms and protection by calorie restriction. BioFactors 2009, 35, 28–35. [Google Scholar] [CrossRef]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical Pathways of Sarcopenia and Their Modulation by Physical Exercise: A Narrative Review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, obesity, and inflammation—Results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Abbatecola, A.M.; Ferrucci, L.; Ceda, G.; Russo, C.R.; Lauretani, F.; Bandinelli, S.; Barbieri, M.; Valenti, G.; Paolisso, G. Insulin resistance and muscle strength in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1278–1282. [Google Scholar] [CrossRef]
- Morley, J. Anorexia of aging: Physiologic and pathologic. Am. J. Clin. Nutr. 1997, 66, 760–773. [Google Scholar] [CrossRef]
- Marcell, T.J. Review Article: Sarcopenia: Causes, Consequences, and Preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M911–M916. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Guo, Z.; Jiang, B.; Zhang, K.; Zhu, W.; Lian, X.; Xu, Y.; Zhao, Z.; Liu, L. Factors affecting sarcopenia in older patients with chronic diseases. Ann. Palliat. Med. 2022, 11, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Montoya, I.; Correa-Pérez, A.; Abraha, I.; Soiza, R.L.; Cherubini, A.; O’Mahony, D.; Cruz-Jentoft, A.J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview—The SENATOR Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721–740. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Bueno-Notivol, J.; Martínez-Amat, A.; Cruz-Díaz, D.; Hernandez, A.V.; Pérez-López, F.R. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: A meta-analysis of randomized controlled trials. Maturitas 2018, 116, 24–35. [Google Scholar] [CrossRef]
- Calvani, R.; Miccheli, A.; Landi, F.; Bossola, M.; Cesari, M.; Leeuwenburgh, C.; Sieber, C.; Bernabei, R.; Marzetti, E. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J. Frailty Aging 2013, 2, 38. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’angelo, E.; Sisto, A.; Marzetti, E. Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Cesari, M.; Uchida, M.C.; Calvani, R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients 2020, 12, 2915. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Calvani, R.; Azzolino, D.; Picca, A.; Tosato, M.; Landi, F.; Cesari, M.; Marzetti, E. Protein Intake and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8718. [Google Scholar] [CrossRef]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef]
- De Spiegeleer, A.; Beckwee, D.; Bautmans, I.; Petrovic, M.; on behalf of the Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Drugs Aging 2018, 35, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, K.; Tsuruoka, H.; Morikawa, K.; Furuya, N.; Inoue, T.; Miyazawa, T.; Mineshita, M. Differences in skeletal muscle loss caused by cytotoxic chemotherapy and molecular targeted therapy in patients with advanced non-small cell lung cancer. Thorac. Cancer 2018, 9, 99–104. [Google Scholar] [CrossRef]
- Prado, C.M.; Sawyer, M.B.; Ghosh, S.; Lieffers, J.R.; Esfandiari, N.; Antoun, S.; Baracos, V.E. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential? Am. J. Clin. Nutr. 2013, 98, 1012–1019. [Google Scholar] [CrossRef]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.; Goh, V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef]
- Surov, A.; Strobel, A.; Borggrefe, J.; Wienke, A. Low skeletal muscle mass predicts treatment response in oncology: A meta-analysis. Eur. Radiol. 2023, 33, 6426–6437. [Google Scholar] [CrossRef]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body composition and sarcopenia: The next-generation of personalized oncology and pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Surov, A.; Wienke, A. Sarcopenia predicts overall survival in patients with malignant hematological diseases: A meta-analysis. Clin. Nutr. 2021, 40, 1155–1160. [Google Scholar] [CrossRef]
- Prado, C.M.; Siervo, M.; Mire, E.; Heymsfield, S.B.; Stephan, B.C.; Broyles, S.; Smith, S.R.; Wells, J.C.; Katzmarzyk, P.T. A population-based approach to define body-composition phenotypes. Am. J. Clin. Nutr. 2014, 99, 1369–1377. [Google Scholar] [CrossRef]
- Sucak, G.T.; Suyanı, E.; Baysal, N.A.; Altındal, Ş.; Çakar, M.K.; Akı, Ş.Z.; Yeğin, Z.A.; Şanlıer, N. The role of body mass index and other body composition parameters in early post-transplant complications in patients undergoing allogeneic stem cell transplantation with busulfan–cyclophosphamide conditioning. Int. J. Hematol. 2012, 95, 95–101. [Google Scholar] [CrossRef]
- Ballet, F.; Vrignaud, P.; Robert, J.; Rey, C.; Poupon, R. Hepatic extraction, metabolism and biliary excretion of doxorubicin in the isolated perfused rat liver. Cancer Chemother. Pharmacol. 1987, 19, 240–245. [Google Scholar] [CrossRef]
- Neuendorff, N.R.; Loh, K.P.; Mims, A.S.; Christofyllakis, K.; Soo, W.-K.; Bölükbasi, B.; Oñoro-Algar, C.; Hundley, W.G.; Klepin, H.D. Anthracycline-related cardiotoxicity in older patients with acute myeloid leukemia: A Young SIOG review paper. Blood Adv. 2020, 4, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Katheria, V.; Hurria, A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA A Cancer J. Clin. 2010, 60, 120–132. [Google Scholar] [CrossRef]
- García-Baztán, A.; Viguria-Alegria, M.C.; Ramón-Espinoza, M.F.; Tamayo-Rodríguez, I.; Gonzales-Montejo, N.J.; Martínez-Velilla, N.; Oteiza-Olaso, J. Hand grip strength, short physical performance battery, and gait speed: Key tools for function in Non-Hodgkin Lymphoma. Ann. Hematol. 2023, 102, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
| Strength Measurement | ||||
|---|---|---|---|---|
| Tool | Cut Off | PROs | Cons | |
| Grip strength (kg) | Assessment of hand grip strength using a dynamometer. | <27 men; <16 women |
|
|
| Chair stand test (5 rises) (s) | Amount of time needed for a patient to rise five times from a seated position without using their arms | >15 s |
|
|
| Performance measurement | ||||
| Tool | Cut Off | PROs | Cons | |
| Gait speed (m/s) 4-m usual walking speed test | The time needed for a patient to walk, at his or her usual speed, a linear path of 4 m | ≤0.8 |
|
|
| SPPB (point/12) | a test consisting of 3 instruments, each assigned a maximum score of 4: (1) gait speed, (2) chair stand test, and (3) balance test. The balance test consists of measuring how long the patient can maintain specific positions in standing | ≤8 |
|
|
| Timed Up and Go test (s) TUG | The tool consists of getting up from a chair, walking for 3 m, turning around, walking back, and sitting down again | ≥20 s |
|
|
| 400 m walking test | Participants are asked to complete 20 laps of 20 m, each lap as fast as possible, and are allowed up to two rest stops during the test | Non-completion or ≥6 min for completion |
|
|
| Muscle mass measurement: appendicular skeletal muscle index more than two SDs below that typical of healthy adults (i.e., 5·45 kg/m2 for women and 7·26 kg/m2 for men) | ||||
| Tool | Cut Off | PROs | Cons | |
| Dual-energy X-ray absorptiometry (DXA) | Whole-body skeletal muscle mass (SMM) or appendicular skeletal muscle mass (ASM), both adjusted for height2, weight or BMI | Skeletal Muscle Mass Index (Appendicular skeletal muscle mass/height2) <7 men kg/m2; <5.5 women kg/m2 |
|
|
| Bioelectrical impedance analysis (BIA) | Whole-body skeletal muscle mass (SMM) BIA equation of Janssen et al.: skeletal muscle mass (kg) [(height2/BIA-resistance 0.401) (gender 3.825) (age 0.071)] 5.102 | SM/height2: Men: 8.87 kg/m2 Women: 6.42 kg/m2 |
|
|
| CT or MRI | Lumbar muscle cross-sectional L3 area Mid-thigh muscle area psoas muscle |
|
| |
| Sarcopenia | Cachexia | |
|---|---|---|
| Weight | ↓ or = or ↑↑ | ↓↓ |
| Lean Tissue | ↓↓ | ↓↓ |
| Fat Tissue | = or ↑ | ↓ |
| Appetite | = | ↓ |
| Cortisol | = | ↑ |
| Inflammation | = or ↑ | ↑↑↑ |
| Pathway | It does not lead to cachexia | May lead to sarcopenia |
| Nutritional |
| Low protein intake Low energy intake Micronutrient deficiency Malabsorption and other gastrointestinal conditions Anorexia |
| Inactivity |
| Bed rest, immobility, deconditioning Low activity, sedentary lifestyle |
| Disease |
| Bone and joint diseases Cardiorespiratory disorders, including chronic heart failure and chronic obstructive pulmonary disease Metabolic disorders (particularly diabetes) Endocrine diseases (particularly androgen deprivation) Neurological disorders Cancer Liver and kidney disorders |
| Iatrogenic |
| Hospital admission Drug-related |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colloca, G.F.; Bellieni, A.; Di Capua, B.; Iervolino, M.; Bracci, S.; Fusco, D.; Tagliaferri, L.; Landi, F.; Valentini, V. Sarcopenia Diagnosis and Management in Hematological Malignancies and Differences with Cachexia and Frailty. Cancers 2023, 15, 4600. https://doi.org/10.3390/cancers15184600
Colloca GF, Bellieni A, Di Capua B, Iervolino M, Bracci S, Fusco D, Tagliaferri L, Landi F, Valentini V. Sarcopenia Diagnosis and Management in Hematological Malignancies and Differences with Cachexia and Frailty. Cancers. 2023; 15(18):4600. https://doi.org/10.3390/cancers15184600
Chicago/Turabian StyleColloca, Giuseppe Ferdinando, Andrea Bellieni, Beatrice Di Capua, Marialuisa Iervolino, Serena Bracci, Domenico Fusco, Luca Tagliaferri, Francesco Landi, and Vincenzo Valentini. 2023. "Sarcopenia Diagnosis and Management in Hematological Malignancies and Differences with Cachexia and Frailty" Cancers 15, no. 18: 4600. https://doi.org/10.3390/cancers15184600
APA StyleColloca, G. F., Bellieni, A., Di Capua, B., Iervolino, M., Bracci, S., Fusco, D., Tagliaferri, L., Landi, F., & Valentini, V. (2023). Sarcopenia Diagnosis and Management in Hematological Malignancies and Differences with Cachexia and Frailty. Cancers, 15(18), 4600. https://doi.org/10.3390/cancers15184600







