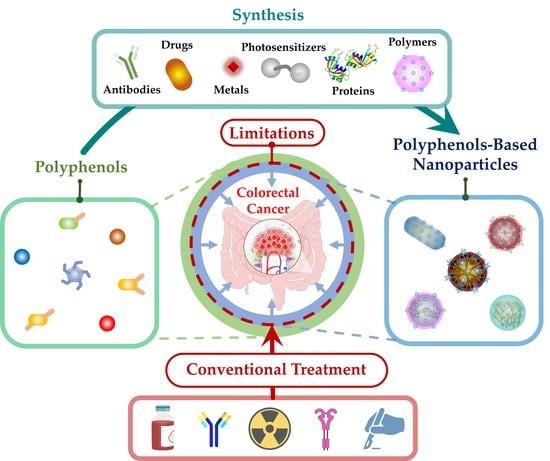
Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Polyphenols-Based NPs: Synthesis and Mechanisms of Action
2.1. Synthesis
2.1.1. Dynamic Covalent Bonding
2.1.2. Non-Covalent Bonding
2.1.3. Polymerization
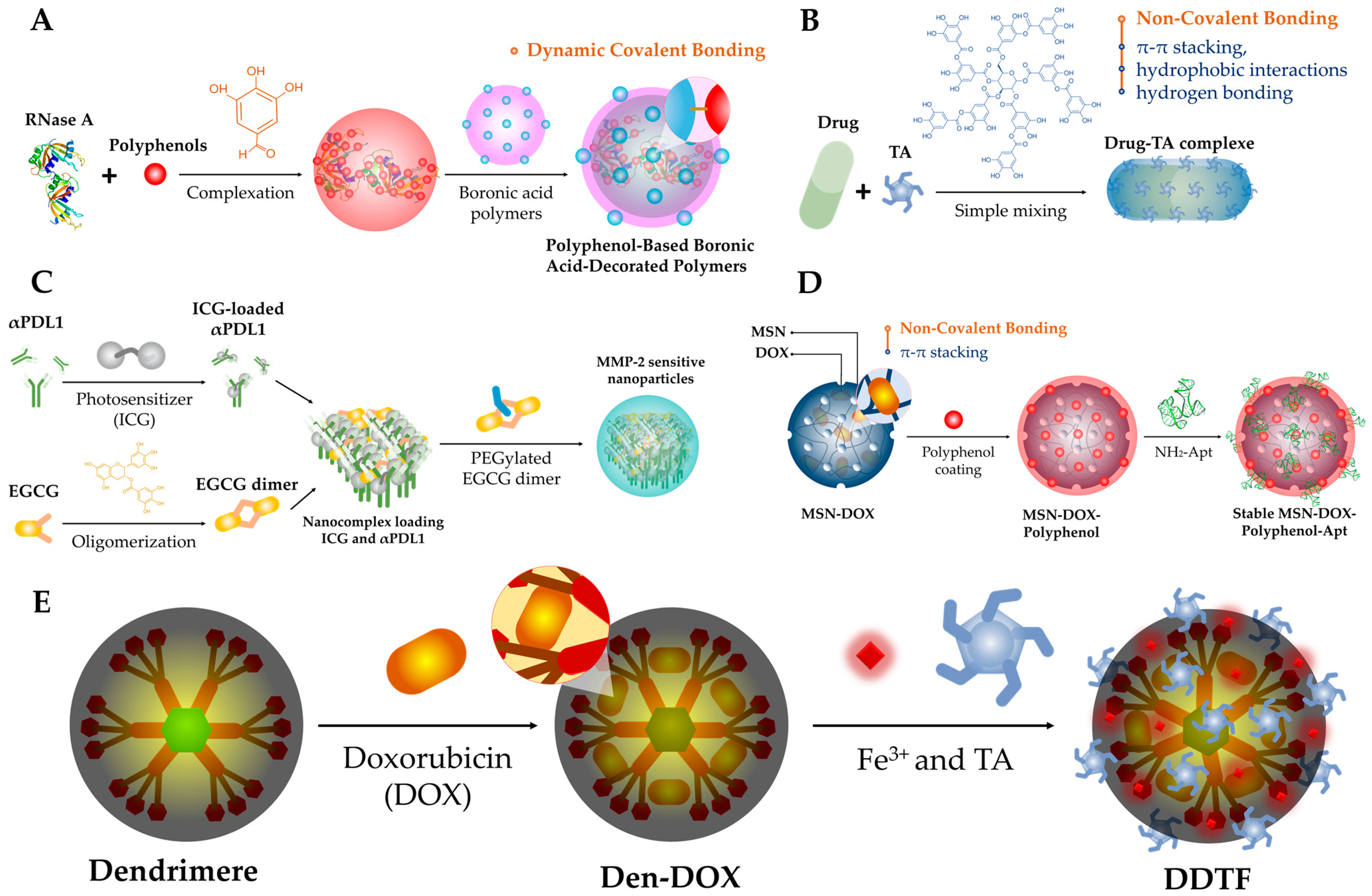
2.1.4. Chemical Conjugation
2.1.5. Reduction
2.1.6. Metal–Polyphenol Networks (MPNs)
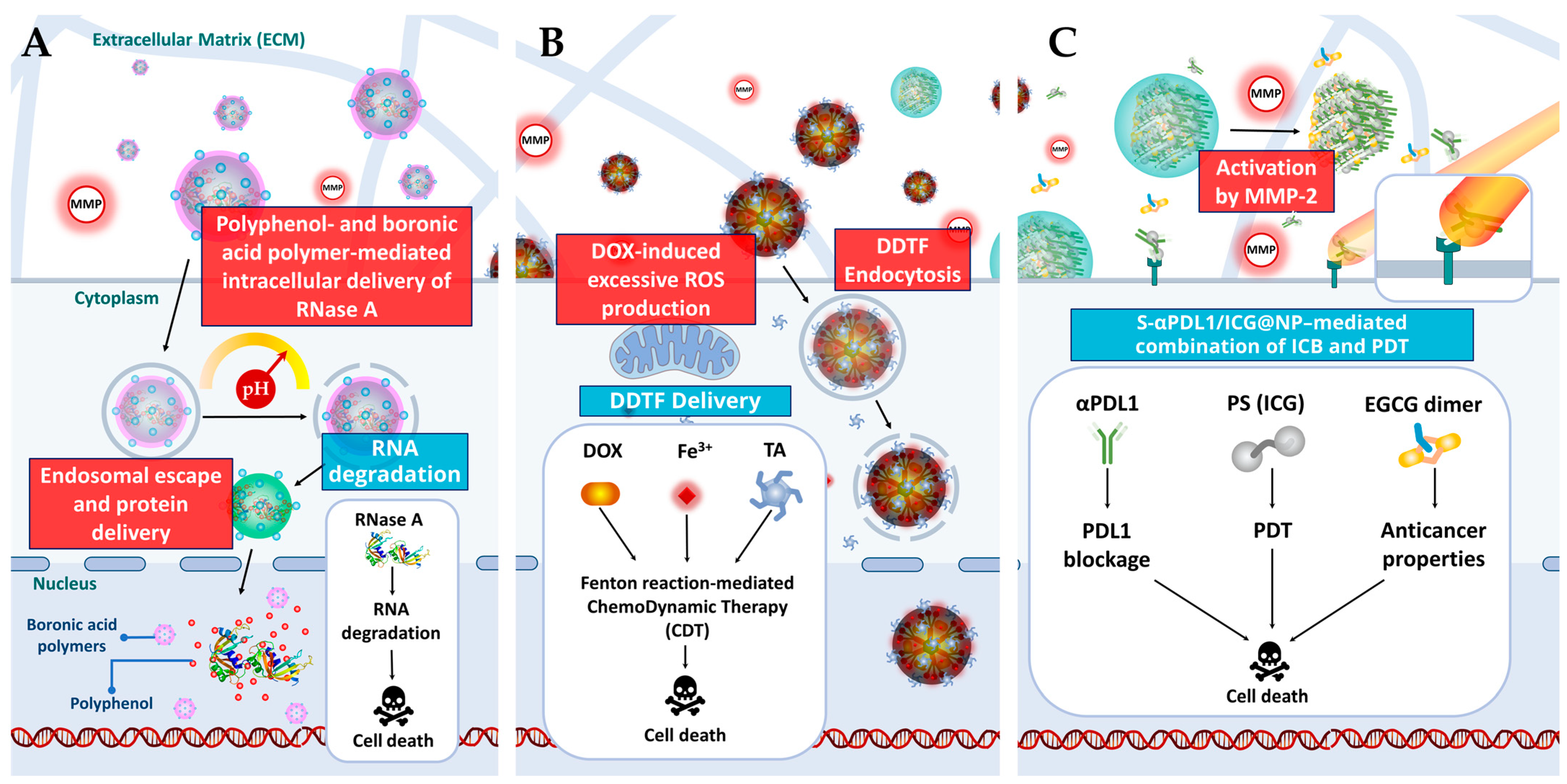
2.2. Mechanisms of Action
3. Polyphenol-Based NPs: Intrinsic Anticancer Activity and Enhancement
3.1. Polyphenols-Intrinsic Anticancer Properties
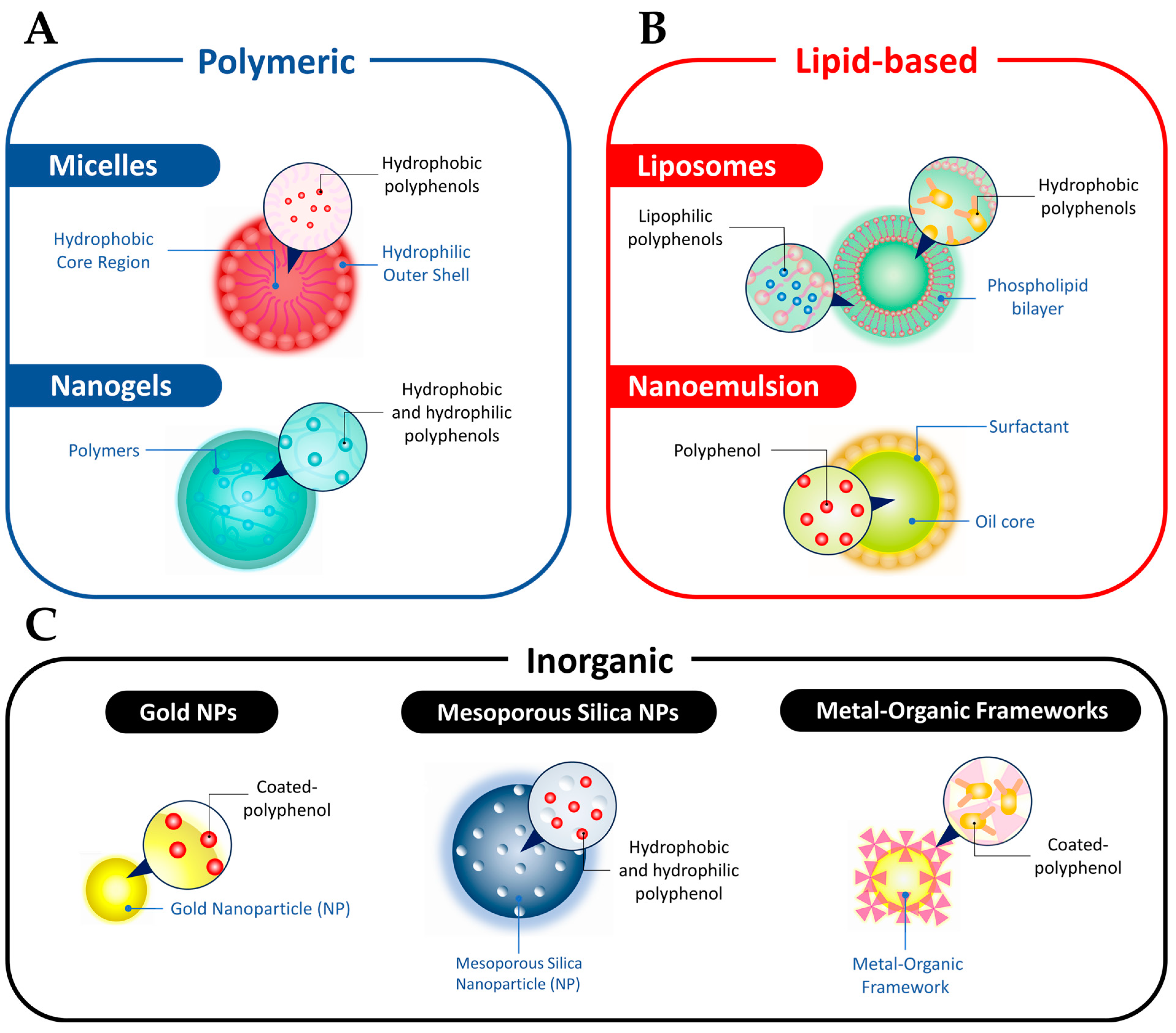
3.2. Polyphenols Properties Enhancement via Nano-Based Delivery Systems
3.2.1. Micelles
3.2.2. Nanogels
3.2.3. Liposomes
3.2.4. Nanoemulsions
3.2.5. AuNPs
3.2.6. MSNs
3.2.7. MOFs
| Polyphenols-Intrinsic Anticancer Properties | |||||
| Polyphenol | Source | Mechanisms | Type of Studies | Refs. | |
| EGCG | Green tea |
| In vitro and in vivo | [14,15,16,17,18,19,20] | |
| Quercetin | Green tea, onion, etc. |
| In vitro | [21,22] | |
| Tannic acid | Nutgalls |
| In vitro | [23] | |
| Resveratrol | Grapes, red wine, and peanuts, etc. |
| In vitro | [24,25,26] | |
| Nano-Based Drug Delivery Systems of Polyphenols | |||||
| Polyphenol | Nanocarriers/Nanosystem | Therapy Strategies | Mechanisms | Type of Studies | Ref. |
| EGCG, (+)-catechin hydrate, procyanidin, or ellagic acid | BSA and boronic acid decorated polymer (Figure 2A and Figure 3A) | Targeted gene delivery |
| In vitro | [27] |
| dOEGCG | MMP-2 sensitive NPs (Figure 2C and Figure 3C) | Immunotherapy/PDT |
| In vitro and in vivo | [28] |
| Quercetin | Cyclodextrin-based nanoformulation | Chemotherapy/Immunotherapy |
| In vitro and in vivo | [29] |
| Tannic acid | Metal–phenolic network (Figure 2E and Figure 3B) | Chemotherapy/Immunotherapy |
| In vitro | [30] |
| Quercetin | Nanoemulsion | Chemo-therapy |
| In vitro | [101] |
3.3. Challenges Related to Nano-Based Delivery Systems
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Aquina, C.T.; Mohile, S.G.; Tejani, M.A.; Becerra, A.Z.; Xu, Z.; Hensley, B.J.; Arsalani-Zadeh, R.; Boscoe, F.P.; Schymura, M.J.; Noyes, K.; et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. Br. J. Cancer 2017, 116, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barazzuol, L.; Coppes, R.P.; van Luijk, P. Prevention and treatment of radiotherapy-induced side effects. Mol. Oncol. 2020, 14, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Lirosi, M.C.; D’Ugo, D.; Fico, V.; Ricci, R.; Santullo, F.; Rizzuto, A.; Cananzi, F.C.; Persiani, R. Neo-adjuvant chemo(radio)therapy in gastric cancer: Current status and future perspectives. World J. Gastrointest. Oncol. 2015, 7, 389–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiri, P.; Ramezanpour, S.; Amani, A.M.; Dehaen, W. A patent review on efficient strategies for the total synthesis of pazopanib, regorafenib and lenvatinib as novel anti-angiogenesis receptor tyrosine kinase inhibitors for cancer therapy. Mol. Divers. 2022, 26, 2981–3002. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; Youlyouz-Marfak, I.; Liagre, B.; Sol, V.; Oudghiri, M.; Duval, R.E.; Limami, Y. Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches. Pharmaceutics 2023, 15, 1767. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Jisha Pillai, U.; Ray, A.; Maan, M.; Dutta, M. Repurposing drugs targeting metabolic diseases for cancer therapeutics. Drug Discov. Today 2023, 28, 103684. [Google Scholar] [CrossRef]
- Gencel-Augusto, J.; Wu, W.; Bivona, T.G. Long Non-Coding RNAs as Emerging Targets in Lung Cancer. Cancers 2023, 15, 3135. [Google Scholar] [CrossRef]
- Dissanayake, R.; Towner, R.; Ahmed, M. Metastatic Breast Cancer: Review of Emerging Nanotherapeutics. Cancers 2023, 15, 2906. [Google Scholar] [CrossRef]
- Chuang, Y.T.; Shiau, J.P.; Tang, J.Y.; Farooqi, A.A.; Chang, F.R.; Tsai, Y.H.; Yen, C.Y.; Chang, H.W. Connection of Cancer Exosomal LncRNAs, Sponging miRNAs, and Exosomal Processing and Their Potential Modulation by Natural Products. Cancers 2023, 15, 2215. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, M.; Silva, S.G.; Evangelista, J.; do Vale, M.L.C.; Farooqi, A.A.; Pinheiro, M. Nanomedicine for the Delivery of RNA in Cancer. Cancers 2022, 14, 2677. [Google Scholar] [CrossRef]
- Hudita, A.; Radu, I.C.; Galateanu, B.; Ginghina, O.; Herman, H.; Balta, C.; Rosu, M.; Zaharia, C.; Costache, M.; Tanasa, E.; et al. Bioinspired silk fibroin nano-delivery systems protect against 5-FU induced gastrointestinal mucositis in a mouse model and display antitumor effects on HT-29 colorectal cancer cells in vitro. Nanotoxicology 2021, 15, 973–994. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018, 33, 463–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Jiang, Q. Roles of the polyphenol–gut microbiota interaction in alleviating colitis and preventing colitis-associated colorectal cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Joe, A.K.; McKoy, J.F.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of HER3 and expression of cyclooxygenase-2 in human colon cancer cells. J. Exp. Ther. Oncol. 2005, 5, 69–78. [Google Scholar]
- Shimizu, M.; Deguchi, A.; Lim, J.T.E.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Hong, J.; Zhou, J.-n.; Pan, Z.; Bose, M.; Liao, J.; Yang, G.-y.; Liu, Y.Y.; Hou, Z.; Lin, Y. Inhibition of intestinal tumorigenesis in Apc min/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005, 65, 10623–10631. [Google Scholar] [CrossRef] [Green Version]
- Porath, D.; Riegger, C.; Drewe, J.; Schwager, J. Epigallocatechin-3-gallate impairs chemokine production in human colon epithelial cell lines. J. Pharmacol. Exp. Ther. 2005, 315, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.D.; Kim, M.S.; Shin, B.A.; Chay, K.O.; Ahn, B.W.; Liu, W.; Bucana, C.D.; Gallick, G.E.; Ellis, L.M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, T.; Chen, D.; Ma, Q.; Zheng, Y.; Liao, S.; Wang, Y.; Zhang, J. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol. Int. 2019, 43, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR-and hypoxia-induced factor 1α-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef] [Green Version]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X.; et al. Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef] [Green Version]
- Miki, H.; Uehara, N.; Kimura, A.; Sasaki, T.; Yuri, T.; Yoshizawa, K.; Tsubura, A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 2012, 40, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, R.; Fan, Q.; Li, Y.; Cheng, Y. Natural polyphenol assisted delivery of single-strand oligonucleotides by cationic polymers. Gene Ther. 2020, 27, 383–391. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, eaau6584. [Google Scholar] [CrossRef]
- Sun, D.; Zou, Y.; Song, L.; Han, S.; Yang, H.; Chu, D.; Dai, Y.; Ma, J.; O’Driscoll, C.M.; Yu, Z.; et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm. Sin. B 2022, 12, 378–393. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.-G.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- Date, A.A.; Hanes, J.; Ensign, L.M. Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J. Control. Release 2016, 240, 504–526. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.; Nunes, R.; Sarmento, B.; das Neves, J. Rectal administration of nanosystems: From drug delivery to diagnostics. Mater. Today Chem. 2018, 10, 128–141. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [Green Version]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef]
- Huang, Z.; Delparastan, P.; Burch, P.; Cheng, J.; Cao, Y.; Messersmith, P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 2018, 6, 2487–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.J.; Xu, F.J.; Pehkonen, S.O.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Grafting of antibacterial polymers on stainless steel via surface-initiated atom transfer radical polymerization for inhibiting biocorrosion by Desulfovibrio desulfuricans. Biotechnol. Bioeng. 2009, 103, 268–281. [Google Scholar] [CrossRef]
- Shin, M.; Lee, H.-A.; Lee, M.; Shin, Y.; Song, J.-J.; Kang, S.-W.; Nam, D.-H.; Jeon, E.J.; Cho, M.; Do, M.; et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, J.P.; Robinson, W.B. Formation of complexes between protein and tannic acid. J. Agric. Food Chem. 1969, 17, 772–777. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Sei, Y.; Yamaguchi, K.; Suganuma, M.; Fujiki, H. DNA and RNA as new binding targets of green tea catechins. J. Biol. Chem. 2006, 281, 17446–17456. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907–912. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine--a nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- Yi, Z.; Sun, Z.; Chen, G.; Zhang, H.; Ma, X.; Su, W.; Cui, X.; Li, X. Size-controlled, colloidally stable and functional nanoparticles based on the molecular assembly of green tea polyphenols and keratins for cancer therapy. J. Mater. Chem. B 2018, 6, 1373–1386. [Google Scholar] [CrossRef]
- Teng, Z.; Su, X.; Zheng, Y.; Zhang, J.; Liu, Y.; Wang, S.; Wu, J.; Chen, G.; Wang, J.; Zhao, D.; et al. A Facile Multi-interface Transformation Approach to Monodisperse Multiple-Shelled Periodic Mesoporous Organosilica Hollow Spheres. J. Am. Chem. Soc. 2015, 137, 7935–7944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, Y.; Yan, J.; Yang, M. Engineering polyphenol-based polymeric nanoparticles for drug delivery and bioimaging. Chem. Eng. J. 2022, 439, 135661. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Sun, W.; Jia, H.-R.; Zhu, Y.-X.; Zhang, X.; Zhou, N.; Wu, F.-G. Metal–phenolic network-based nanocomplexes that evoke ferroptosis by apoptosis: Promoted nuclear drug influx and reversed drug resistance of cancer. Chem. Mater. 2019, 31, 10071–10084. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, S.; Zhang, F.; Yang, K.; Ma, Q.; Zhu, L. Activatable hyaluronic acid nanoparticle as a theranostic agent for optical/photoacoustic image-guided photothermal therapy. ACS Nano 2014, 8, 12250–12258. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Choi, J.H.; Deng, Z.J.; Cho, N.J.; Hammond, P.T. Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano 2014, 8, 8374–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.-R.; Zhu, Y.-X.; Liu, X.; Pan, G.-Y.; Gao, G.; Sun, W.; Zhang, X.; Jiang, Y.-W.; Wu, F.-G. Construction of Dually Responsive Nanotransformers with Nanosphere-Nanofiber-Nanosphere Transition for Overcoming the Size Paradox of Anticancer Nanodrugs. ACS Nano 2019, 13, 11781–11792. [Google Scholar] [CrossRef]
- Akhavan, O.; Kalaee, M.; Alavi, Z.S.; Ghiasi, S.M.A.; Esfandiar, A. Increasing the antioxidant activity of green tea polyphenols in the presence of iron for the reduction of graphene oxide. Carbon 2012, 50, 3015–3025. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Fei, J.; Zhao, J.; Du, C.; Wang, A.; Zhang, H.; Dai, L.; Li, J. One-pot ultrafast self-assembly of autofluorescent polyphenol-based core@shell nanostructures and their selective antibacterial applications. ACS Nano 2014, 8, 8529–8536. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Zakaria, R.; Zein, S.H.S. Green tea polyphenol–reduced graphene oxide: Derivatisation, reduction efficiency, reduction mechanism and cytotoxicity. RSC Adv. 2014, 4, 34510–34518. [Google Scholar] [CrossRef]
- Hashemi, H.; Namazi, H. Sonochemically synthesized blue fluorescent functionalized graphene oxide as a drug delivery system. Ultrason. Sonochem. 2018, 42, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wan, S.S.; Li, C.X.; Xu, L.; Cheng, H.; Zhang, X.Z. An Adenosine Triphosphate-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation with Self-Supplied H2O2 and Acceleration of Fe(III)/Fe(II) Conversion. Nano Lett. 2018, 18, 7609–7618. [Google Scholar] [CrossRef]
- Park, C.; Yang, B.J.; Jeong, K.B.; Kim, C.B.; Lee, S.; Ku, B.-C. Signal-Induced Release of Guests from a Photolatent Metal-Phenolic Supramolecular Cage and Its Hybrid Assemblies. Angew. Chem. Int. Ed. 2017, 56, 5485–5489. [Google Scholar] [CrossRef]
- Ringwald, C.; Ball, V. Layer-by-layer deposition of tannic acid and Fe3+ cations is of electrostatic nature but almost ionic strength independent at pH 5. J. Colloid Interface Sci. 2015, 450, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, G.; Chen, F.; Bai, M.; Liang, Y.; Wang, H.; Zhao, D.; Zhao, Y. Sol-Gel Synthesis of Metal-Phenolic Coordination Spheres and Their Derived Carbon Composites. Angew. Chem. Int. Ed. 2018, 57, 9838–9843. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Lim, E.-K.; Chung, H.B.; Chung, J.S. Recent Advances in pH-Sensitive Polymeric Nanoparticles for Smart Drug Delivery in Cancer Therapy. Curr. Drug Targets 2018, 19, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of p53-targeting microRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sefton, P. Testing for BRCA1/2 mutations. JAMA 2017, 318, 2054. [Google Scholar] [CrossRef]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Liu, J.; Xu, Y. The inhibitory effect of (−)-epigallocatechin-3-gallate on breast cancer progression via reducing SCUBE2 methylation and DNMT activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef] [Green Version]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and other polyphenols act as epigenetic modifiers in breast cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.C.; Yang, J.S.; Lu, H.F.; Ip, S.W.; Lo, C.; Wu, C.C.; Lin, J.P.; Tang, N.Y.; Chung, J.G.; Chou, M.J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharmacal Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression-independent manner in HPV-positive human cervical cancer-derived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Messaoudene, M.; Pidgeon, R.; Richard, C.; Ponce, M.; Diop, K.; Benlaifaoui, M.; Nolin-Lapalme, A.; Cauchois, F.; Malo, J.; Belkaid, W. A Natural Polyphenol Exerts Antitumor Activity and Circumvents Anti–PD-1 Resistance through Effects on the Gut MicrobiotaCastalagin Prebiotic Potentiates Antitumor and PD-1 Efficacy. Cancer Discov. 2022, 12, OF1–OF18. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://clinicaltrials.gov/ (accessed on 12 July 2023).
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L.; Zeng, X.-A. Dihydromyricetin: A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Liu, F.; Antoniou, J.; Li, Y.; Majeed, H.; Liang, R.; Ma, Y.; Ma, J.; Zhong, F. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for tea polyphenol encapsulation. Food Hydrocoll. 2016, 57, 291–300. [Google Scholar] [CrossRef]
- Le, Z.; Chen, Y.; Han, H.; Tian, H.; Zhao, P.; Yang, C.; He, Z.; Liu, L.; Leong, K.W.; Mao, H.-Q.; et al. Hydrogen-Bonded Tannic Acid-Based Anticancer Nanoparticle for Enhancement of Oral Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 42186–42197. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Liu, D.; Ge, Y.; Zhao, M.; Zhu, X.; Li, W.; Wang, L.; Zheng, T.; Li, J. Oral Curcumin via Hydrophobic Porous Silicon Carrier: Preparation, Characterization, and Toxicological Evaluation In Vivo. ACS Appl. Mater. Interfaces 2019, 11, 31661–31670. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Y.; Dong, Z.; Chen, X.; Wang, Y.; Li, T.; Li, J.; Zang, S.; He, X.; Chen, D. Cellular Hypoxia Mitigation by Dandelion-like Nanoparticles for Synergistic Photodynamic Therapy of Oral Squamous Cell Carcinoma. ACS Appl. Mater. Interfaces 2022, 14, 44039–44053. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; Raza, F.; Zafar, H.; Huang, G.; Yang, Y.; Shi, F.; Wang, D.; He, X. Design of GSH-Responsive Curcumin Nanomicelles for Oesophageal Cancer Therapy. Pharmaceutics 2022, 14, 1802. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, L.; Fan, T.; Zhang, C.; Zhang, B.; Al-Hartomy, O.A.; Al-Ghamdi, A.; Wageh, S.; Qiu, M.; Zhang, H. Strategic Design of Intelligent-Responsive Nanogel Carriers for Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 54621–54647. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, G.; Wang, B.; Cao, G.; Li, D.; Wang, Y.; Zhang, Y.; Geng, J.; Li, H.; Li, Y. Reinforcing the Combinational Immuno-Oncotherapy of Switching “Cold” Tumor to “Hot” by Responsive Penetrating Nanogels. ACS Appl. Mater. Interfaces 2021, 13, 36824–36838. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, S.; Wang, Z.; Huang, P.; Wang, W.; Xing, J. Nanogels loading curcumin in situ through microemulsion photopolymerization for enhancement of antitumor effects. J. Mater. Chem. B 2022, 10, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Wu, Y.; Song, Z.; Li, S.; Du, M.; Deng, J.; Xu, Q.; Deng, L.; Bahlol, H.S.; Han, H. Tea Polyphenol Liposomes Overcome Gastric Mucus to Treat Helicobacter Pylori Infection and Enhance the Intestinal Microenvironment. ACS Appl. Mater. Interfaces 2022, 14, 13001–13012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Z.; Chen, Y.; Gao, D.; Wang, P.; Lin, Y.; Wang, Y.; Wang, F.; Han, Y.; Yuan, H. Co-delivery of Docetaxel and Resveratrol by liposomes synergistically boosts antitumor efficiency against prostate cancer. Eur. J. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Kucinska, M.; Tomczak, S.; Mlynarczyk, D.T.; Piskorz, J.; Goslinski, T.; Murias, M.; Jelinska, A. Liposomal Nanoformulation as a Carrier for Curcumin and pEGCG—Study on Stability and Anticancer Potential. Nanomaterials 2022, 12, 1274. [Google Scholar]
- Caddeo, C.; Gabriele, M.; Nácher, A.; Fernàndez-Busquets, X.; Valenti, D.; Maria Fadda, A.; Pucci, L.; Manconi, M. Resveratrol and artemisinin eudragit-coated liposomes: A strategy to tackle intestinal tumors. Int. J. Pharm. 2021, 592, 120083. [Google Scholar] [CrossRef]
- Enin, H.A.A.; Alquthami, A.F.; Alwagdani, A.M.; Yousef, L.M.; Albuqami, M.S.; Alharthi, M.A.; Alsaab, H.O. Utilizing TPGS for Optimizing Quercetin Nanoemulsion for Colon Cancer Cells Inhibition. Colloids Interfaces 2022, 6, 49. [Google Scholar] [CrossRef]
- Lotfi, M.; Kazemi, S.; Shirafkan, F.; Hosseinzadeh, R.; Ebrahimpour, A.; Barary, M.; Sio, T.T.; Hosseini, S.M.; Moghadamnia, A.A. The protective effects of quercetin nano-emulsion on intestinal mucositis induced by 5-fluorouracil in mice. Biochem. Biophys. Res. Commun. 2021, 585, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Costantini, P.E.; Di Giosia, M.; Ulfo, L.; Petrosino, A.; Saporetti, R.; Fimognari, C.; Pompa, P.P.; Danielli, A.; Turrini, E.; Boselli, L.; et al. Spiky Gold Nanoparticles for the Photothermal Eradication of Colon Cancer Cells. Nanomaterials 2021, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Cascione, M.; Rizzello, L.; Manno, D.E.; Di Guglielmo, C.; Rinaldi, R. Synergistic Effect Induced by Gold Nanoparticles with Polyphenols Shell during Thermal Therapy: Macrophage Inflammatory Response and Cancer Cell Death Assessment. Cancers 2021, 13, 3610. [Google Scholar] [CrossRef] [PubMed]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Barrett, D.G.; Sileika, T.S.; Messersmith, P.B. Molecular diversity in phenolic and polyphenolic precursors of tannin-inspired nanocoatings. Chem. Commun. 2014, 50, 7265–7268. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, T.; Wu, H.; Guo, L.; Ye, P.; Hao, Y.; Guo, Q.; Jiang, J.; Fu, F.; Chen, G. Mussel-inspired polydopamine coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Int. J. Pharm. 2014, 463, 22–26. [Google Scholar] [CrossRef]
- Shen, K.; Huang, Y.; Li, Q.; Chen, M.; Wu, L. Self-Assembled Polysaccharide-Diphenylalanine/Au Nanospheres for Photothermal Therapy and Photoacoustic Imaging. ACS Omega 2019, 4, 18118–18125. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering Phototheranostic Nanoscale Metal-Organic Frameworks for Multimodal Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Chen, Y.; Li, L.; Su, Z.; Wang, C. Precise synthesis of unique polydopamine/mesoporous calcium phosphate hollow Janus nanoparticles for imaging-guided chemo-photothermal synergistic therapy. Chem. Sci. 2017, 8, 8067–8077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, L.; Liu, L.; Lin, L.; Liu, F.; Xie, Z.; Tian, H.; Chen, X. Engineering Metal-Organic Frameworks for Photoacoustic Imaging-Guided Chemo-/Photothermal Combinational Tumor Therapy. ACS Appl. Mater. Interfaces 2018, 10, 41035–41045. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Liang, X.; Liang, J.; Zhang, C.; Yang, J.; Wang, C.; Kong, D.; Sun, H. ROS-responsive capsules engineered from green tea polyphenol–metal networks for anticancer drug delivery. J. Mater. Chem. B 2018, 6, 1000–1010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. https://doi.org/10.3390/cancers15153826
Wahnou H, Liagre B, Sol V, El Attar H, Attar R, Oudghiri M, Duval RE, Limami Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers. 2023; 15(15):3826. https://doi.org/10.3390/cancers15153826
Chicago/Turabian StyleWahnou, Hicham, Bertrand Liagre, Vincent Sol, Hicham El Attar, Rukset Attar, Mounia Oudghiri, Raphaël Emmanuel Duval, and Youness Limami. 2023. "Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment" Cancers 15, no. 15: 3826. https://doi.org/10.3390/cancers15153826
APA StyleWahnou, H., Liagre, B., Sol, V., El Attar, H., Attar, R., Oudghiri, M., Duval, R. E., & Limami, Y. (2023). Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers, 15(15), 3826. https://doi.org/10.3390/cancers15153826