Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnosis of HCC
2.3. Lenvatinib and Atezo/Bev Treatment and Assessment of Adverse Events
2.4. Definition of the Subsequent Treatment Procedure
2.5. Treatment Protocol for Subsequent TACE and TAI during Lenvatinib Treatment
2.6. Evaluation of Treatment Response
2.7. Definition of TACE Failure
2.8. Decision Process Regarding the Timing and Method of Subsequent Treatment
2.9. Assessment of Hepatic Functional Reserve
2.10. Follow-Up Protocol
2.11. Statistical Analysis
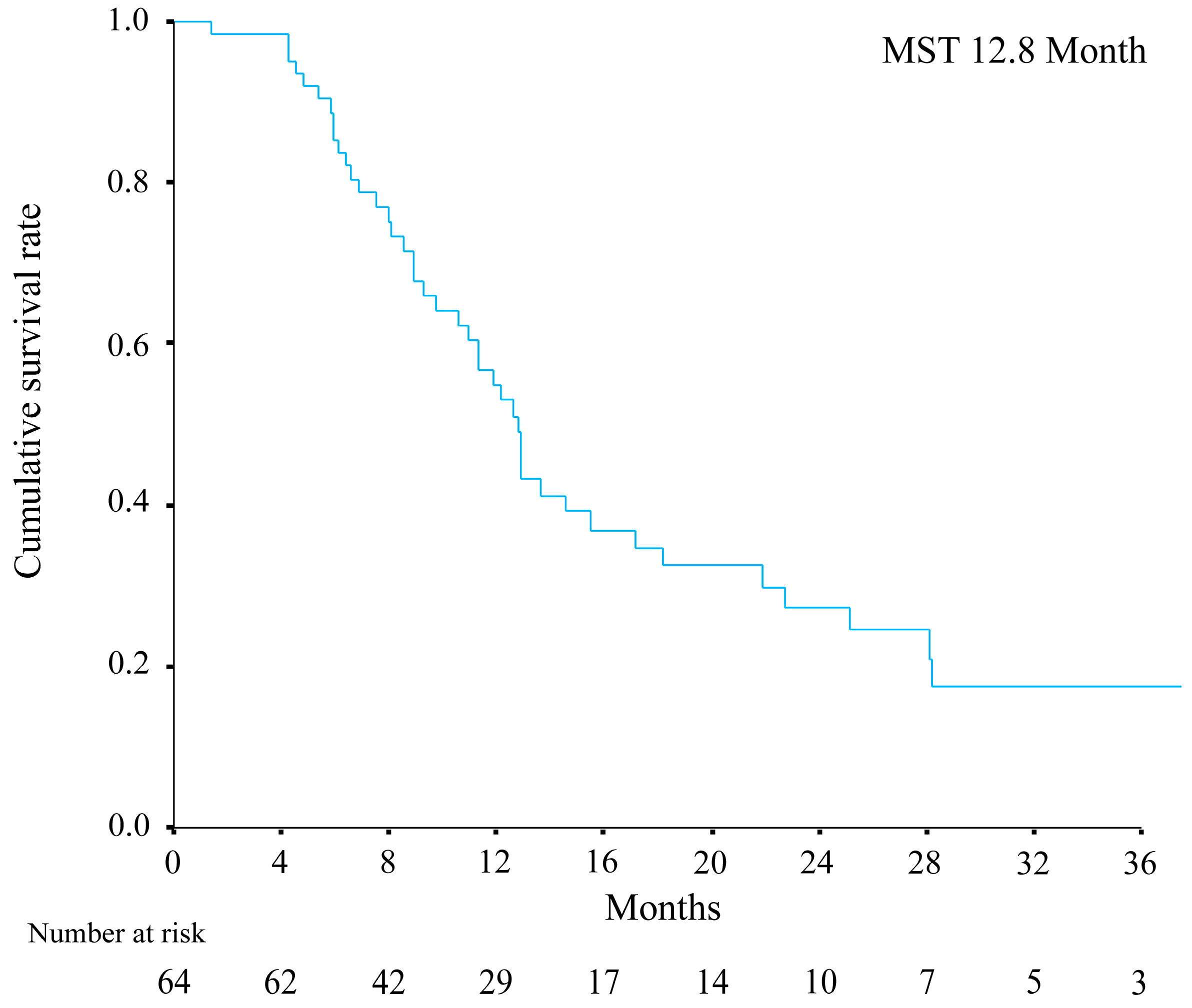
3. Results
3.1. Overview
3.2. Treatment Response after Initiation of Initial Systemic Therapy
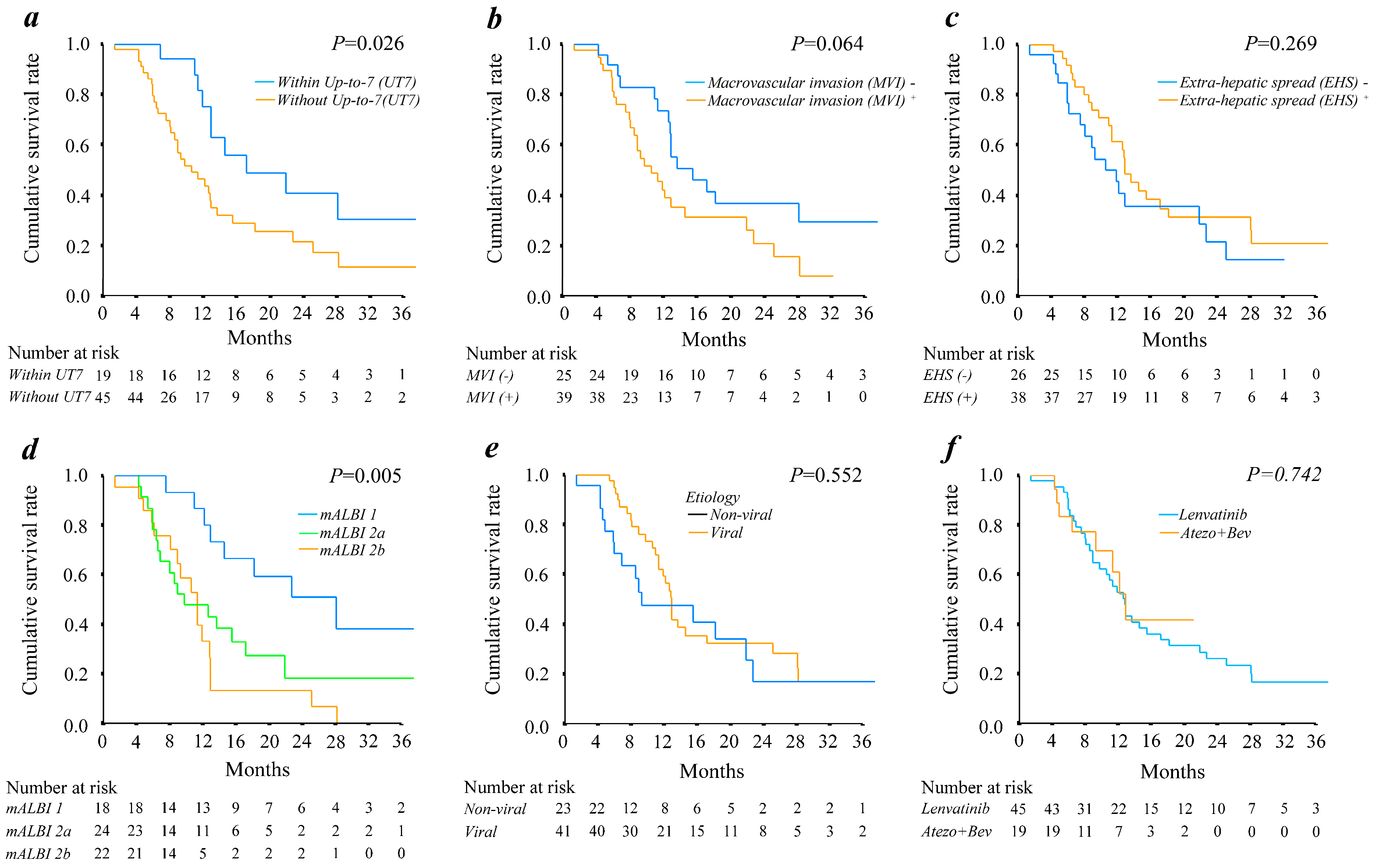
3.3. Impact of General Landmark Predictive Factors for Overall Survival of Patients with BCLC Stage C HCC
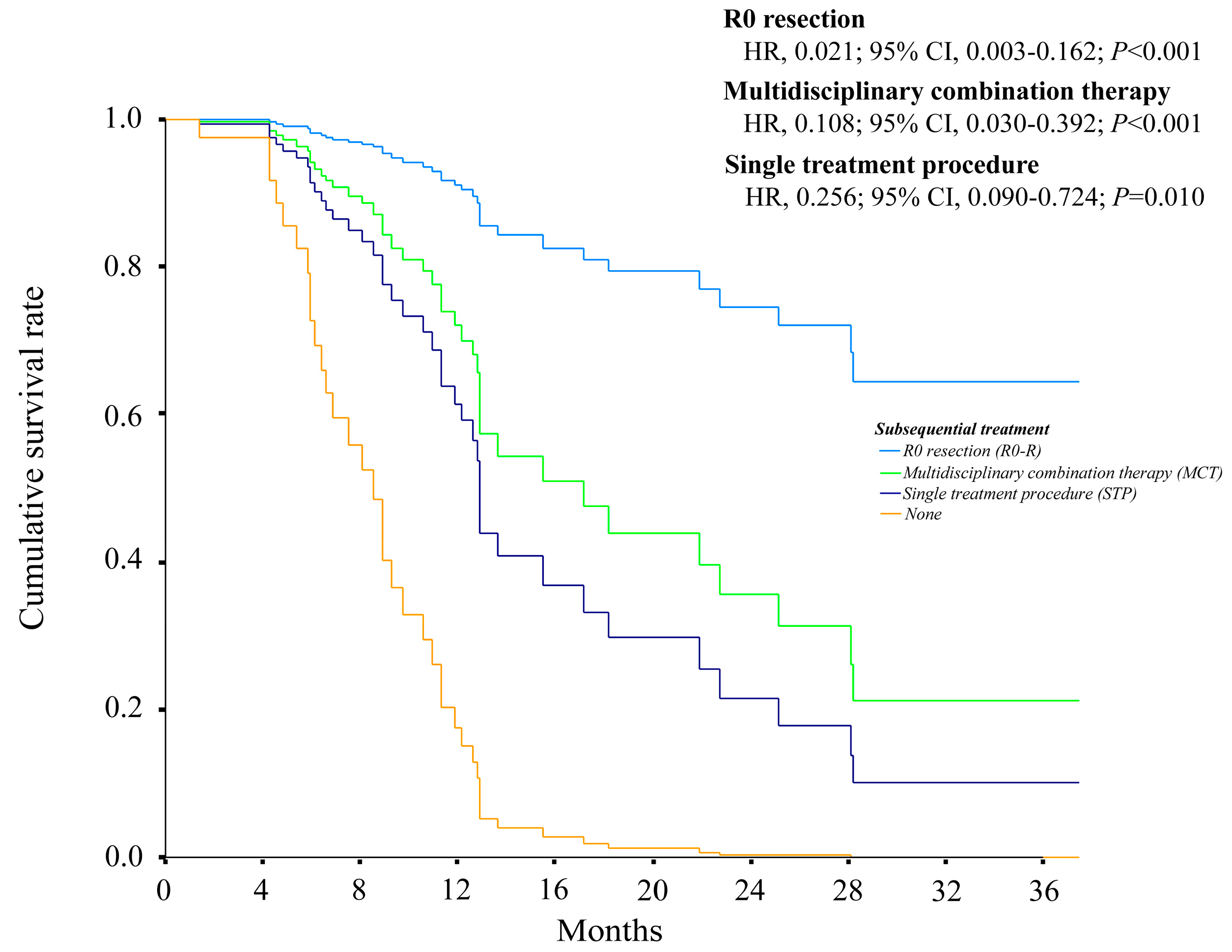
3.4. Predictors of Overall Survival after Initiation of Systemic Therapy in Patients with BCLC Stage C HCC
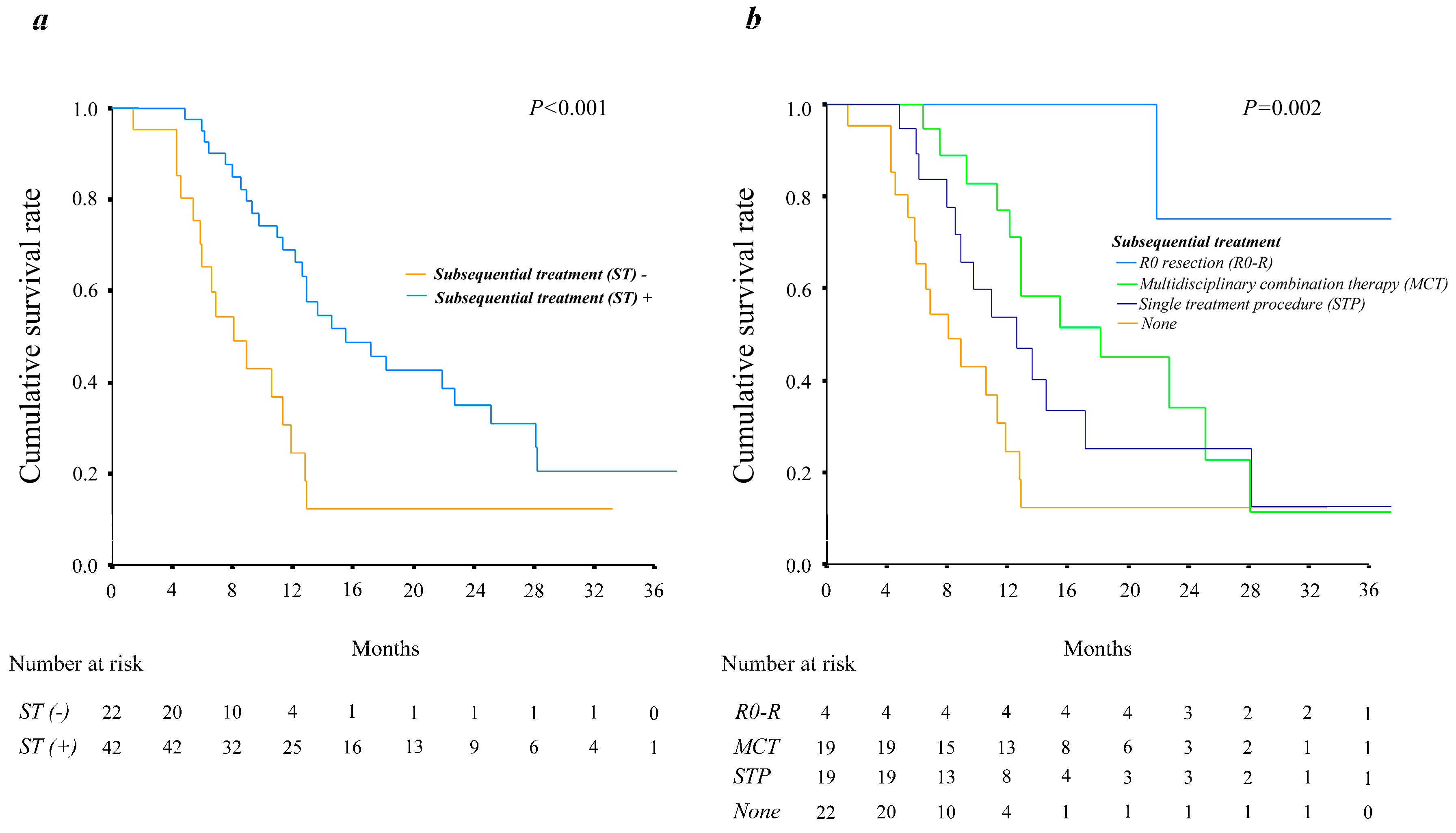
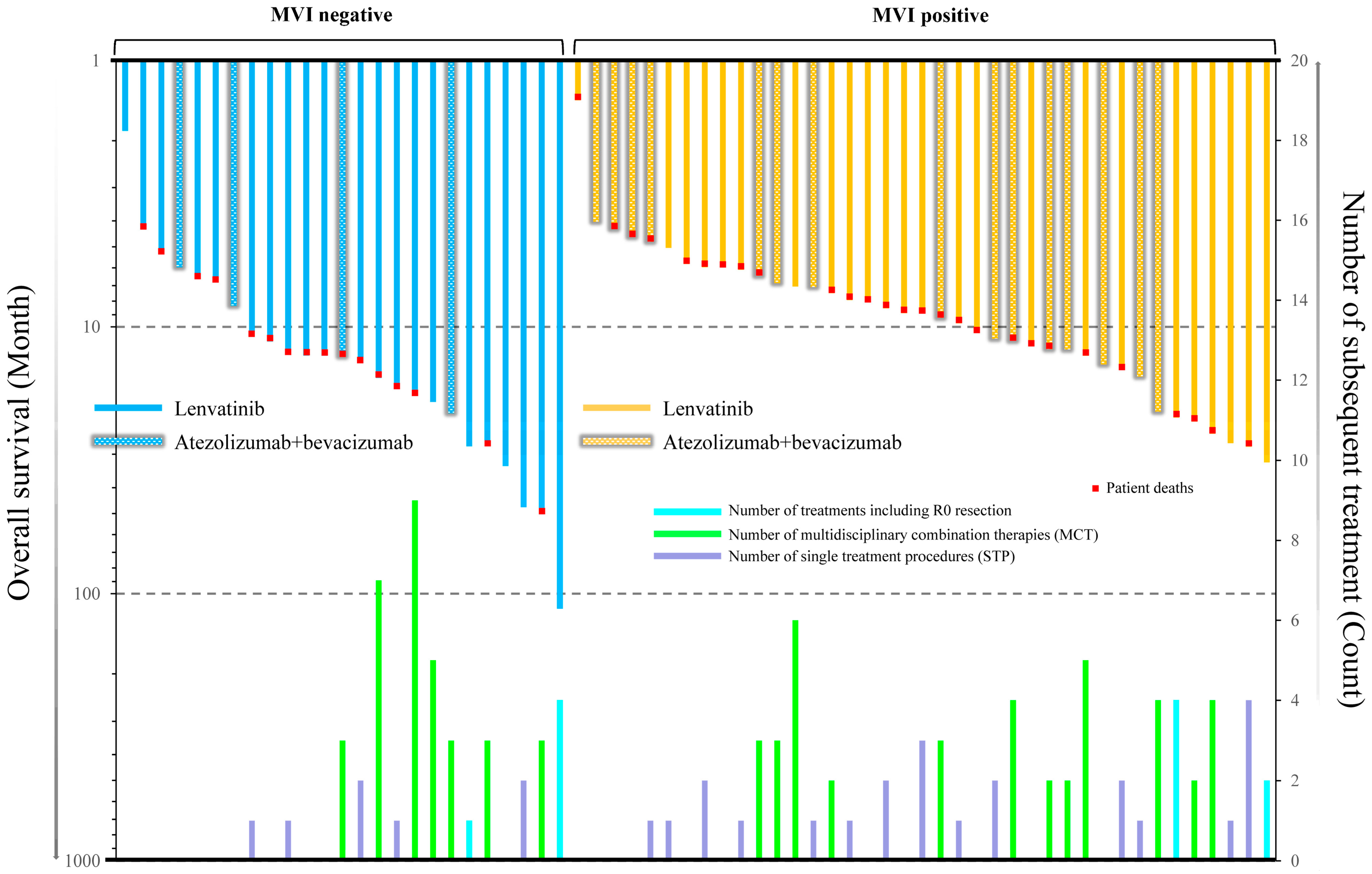
3.5. The Relationship between Overall Survival and Various Types of Subsequent Treatments with or without Macrovascular Invasion
3.6. Clinical Features of Patients with HCC Treated with Systemic Therapy Who Received or Did Not Receive Subsequent Treatment
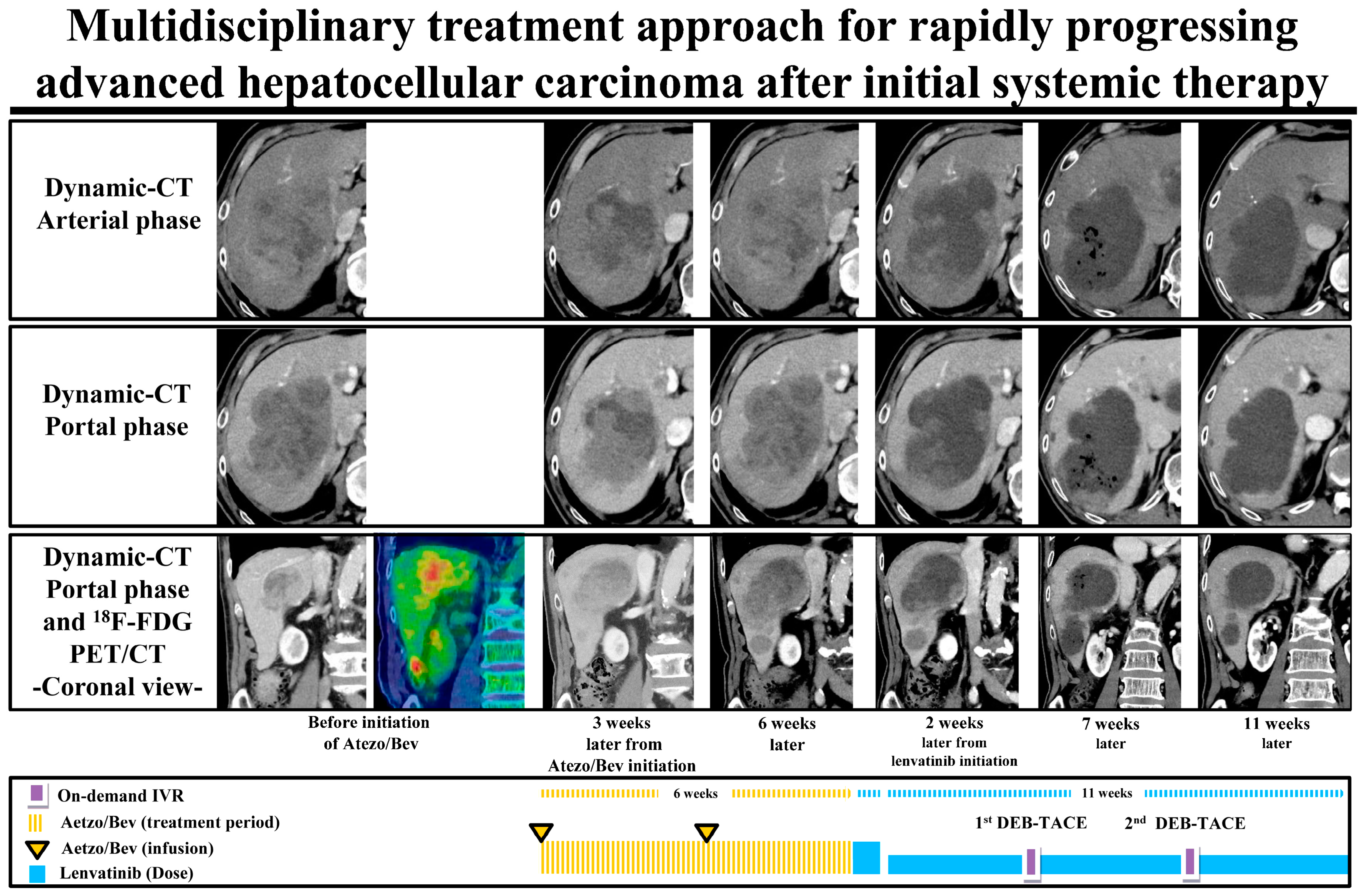
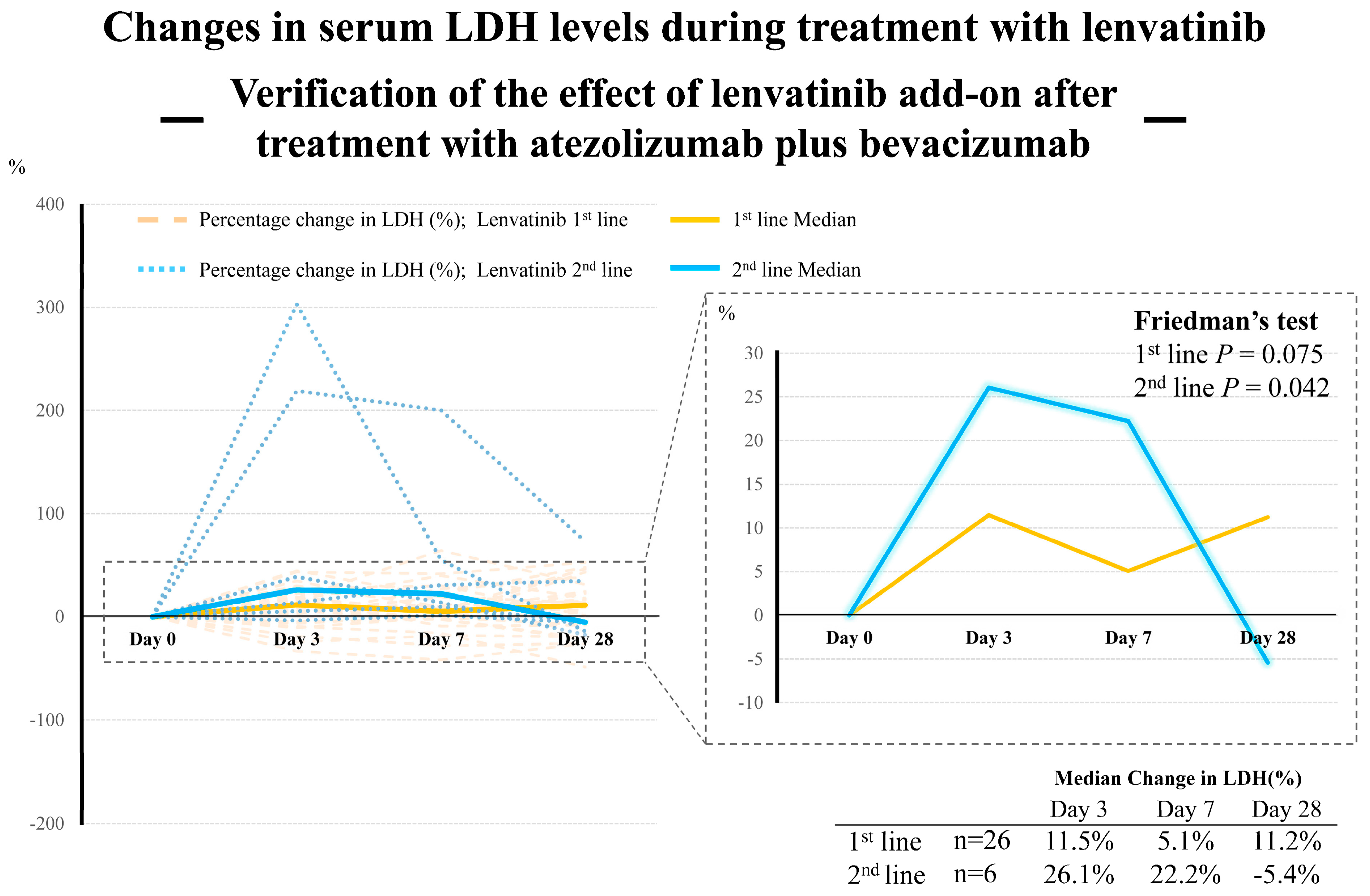
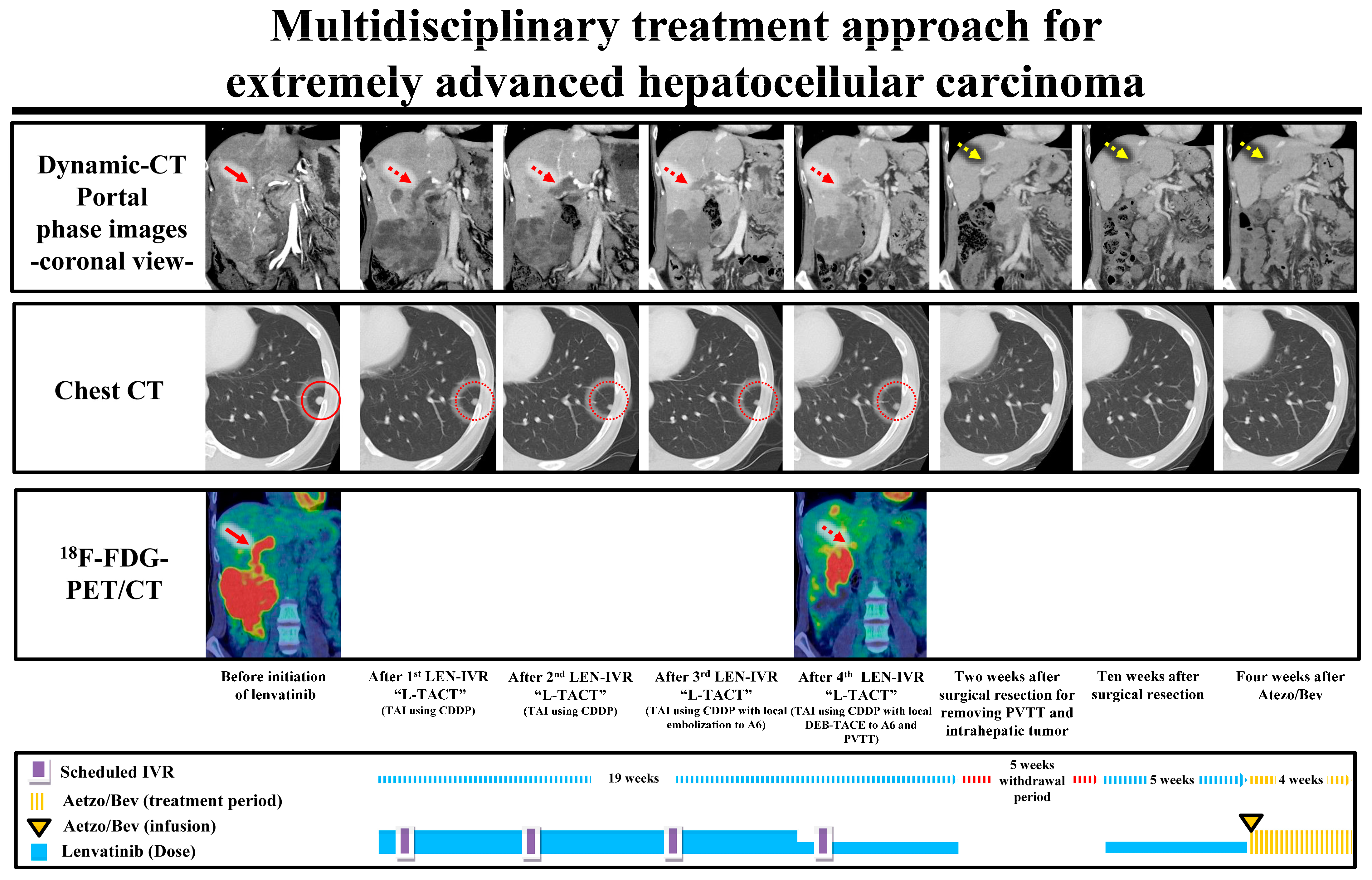
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.E.; de Lope, C.R.; Bruix, J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, D.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; Chung, Y.H.; Lee, Y.S.; Park, S.R.; Ryu, M.H.; Ryoo, B.Y.; et al. Role of transarterial chemoembolization in relation with sorafenib for patients with advanced hepatocellular carcinoma. Oncotarget 2016, 7, 74303–74313. [Google Scholar] [CrossRef][Green Version]
- Uka, K.; Aikata, H.; Takaki, S.; Shirakawa, H.; Jeong, S.C.; Yamashina, K.; Hiramatsu, A.; Kodama, H.; Takahashi, S.; Chayama, K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J. Gastroenterol. 2007, 13, 414–420. [Google Scholar] [CrossRef]
- Uchino, K.; Tateishi, R.; Shiina, S.; Kanda, M.; Masuzaki, R.; Kondo, Y.; Goto, T.; Omata, M.; Yoshida, H.; Koike, K. Hepatocellular carcinoma with extrahepatic metastasis: Clinical features and prognostic factors. Cancer 2011, 117, 4475–4483. [Google Scholar] [CrossRef]
- Yoo, D.J.; Kim, K.M.; Jin, Y.J.; Shim, J.H.; Ko, G.Y.; Yoon, H.K.; Sung, K.B.; Lee, J.L.; Kang, Y.K.; Lim, Y.S.; et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: Does transarterial chemoembolization improve survival in these patients? J. Gastroenterol. Hepatol. 2011, 26, 145–154. [Google Scholar] [CrossRef]
- Jung, S.M.; Jang, J.W.; You, C.R.; Yoo, S.H.; Kwon, J.H.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Chung, K.W.; Kay, C.S.; et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J. Gastroenterol. Hepatol. 2012, 27, 684–689. [Google Scholar] [CrossRef]
- Wu, F.X.; Chen, J.; Bai, T.; Zhu, S.L.; Yang, T.B.; Qi, L.N.; Zou, L.; Li, Z.H.; Ye, J.Z.; Li, L.Q. The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer 2017, 17, 645. [Google Scholar] [CrossRef]
- Palmer, D.H.; Malagari, K.; Kulik, L.M. Role of locoregional therapies in the wake of systemic therapy. J. Hepatol. 2020, 72, 277–287. [Google Scholar] [CrossRef]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Kawamura, Y.; Kobayashi, M.; Shindoh, J.; Kobayashi, Y.; Okubo, S.; Tominaga, L.; Kajiwara, A.; Kasuya, K.; Iritani, S.; Fujiyama, S.; et al. Lenvatinib-Transarterial Chemoembolization Sequential Therapy as an Effective Treatment at Progression during Lenvatinib Therapy for Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 756–770. [Google Scholar] [CrossRef]
- Ando, Y.; Kawaoka, T.; Amioka, K.; Naruto, K.; Ogawa, Y.; Yoshikawa, Y.; Kikukawa, C.; Kosaka, Y.; Uchikawa, S.; Morio, K.; et al. Efficacy and Safety of Lenvatinib-Transcatheter Arterial Chemoembolization Sequential Therapy for Patients with Intermediate-Stage Hepatocellular Carcinoma. Oncology 2021, 99, 507–517. [Google Scholar] [CrossRef]
- Fu, Z.; Li, X.; Zhong, J.; Chen, X.; Cao, K.; Ding, N.; Liu, L.; Zhang, X.; Zhai, J.; Qu, Z. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): A retrospective controlled study. Hepatol. Int. 2021, 15, 663–675. [Google Scholar] [CrossRef]
- Muraishi, N.; Kawamura, Y.; Akuta, N.; Shindoh, J.; Matsumura, M.; Okubo, S.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; Sezaki, H.; et al. The impact of lenvatinib on tumor blood vessel shrinkage of hepatocellular carcinoma during treatment: An imaging-based analysis. Oncology 2022, 101, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef]
- Kawamura, Y.; Kobayashi, M.; Shindoh, J.; Kobayashi, Y.; Kasuya, K.; Sano, T.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; Sezaki, H.; et al. (18)F-Fluorodeoxyglucose Uptake in Hepatocellular Carcinoma as a Useful Predictor of an Extremely Rapid Response to Lenvatinib. Liver Cancer 2020, 9, 84–92. [Google Scholar] [CrossRef]
- Kawamura, Y.; Kobayashi, M.; Shindoh, J.; Kobayashi, Y.; Kasuya, K.; Sano, T.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; Sezaki, H.; et al. Pretreatment Heterogeneous Enhancement Pattern of Hepatocellular Carcinoma May Be a Useful New Predictor of Early Response to Lenvatinib and Overall Prognosis. Liver Cancer 2020, 9, 275–292. [Google Scholar] [CrossRef]
- Kawamura, Y.; Akuta, N.; Shindoh, J.; Matsumura, M.; Okubo, S.; Tominaga, L.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; Sezaki, H.; et al. Well-preserved liver function enhances the clinical impact of curative-intent subsequent treatment during lenvatinib treatment for unresectable hepatocellular carcinoma. Clin. J. Gastroenterol. 2022, 16, 1–12. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2019, 69, 1492–1501. [Google Scholar] [CrossRef]
- Kuroda, H.; Oikawa, T.; Ninomiya, M.; Fujita, M.; Abe, K.; Okumoto, K.; Katsumi, T.; Sato, W.; Igarashi, G.; Iino, C.; et al. Objective Response by mRECIST to Initial Lenvatinib Therapy Is an Independent Factor Contributing to Deep Response in Hepatocellular Carcinoma Treated with Lenvatinib-Transcatheter Arterial Chemoembolization Sequential Therapy. Liver Cancer 2022, 11, 383–396. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, W.; Zhu, B.; Wang, G.; Sun, J.; Xiao, C.; Huang, F.; Tang, R.; Cheng, Y.; Huang, Z.; et al. Lenvatinib Combined with Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J. Clin. Oncol. 2022, 41, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021, 73 (Suppl. S1), 158–191. [Google Scholar] [CrossRef]
- National Cancer Institute. Division of Cancer Treatment and Diagnosis. Cancer Therapy Evaluation Program. Adverse Events/CTCAE. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 23 November 2022).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014, 87 (Suppl. S1), 22–31. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kudo, M.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Tsurusaki, M.; et al. Exploratory Analysis of Lenvatinib Therapy in Patients with Unresectable Hepatocellular Carcinoma Who Have Failed Prior PD-1/PD-L1 Checkpoint Blockade. Cancers 2020, 12, 3048. [Google Scholar] [CrossRef]
- Kudo, M. Sequential Therapy for Hepatocellular Carcinoma after Failure of Atezolizumab plus Bevacizumab Combination Therapy. Liver Cancer 2021, 10, 85–93. [Google Scholar] [CrossRef]
- Ikeda, M.; Yamashita, T.; Ogasawara, S.; Kudo, M.; Inaba, Y.; Morimoto, M.; Tsuchiya, K.; Shimizu, S.; Kojima, Y.; Hiraoka, A.; et al. 937P Multicenter phase II trial of lenvatinib plus hepatic intra-arterial infusion chemotherapy with cisplatin for advanced hepatocellular carcinoma: LEOPARD. Ann. Oncol. 2021, 32, S821–S822. [Google Scholar] [CrossRef]
| Patient Characteristics and Laboratory Data | |
|---|---|
| Number of patients | 64 |
| Sex, males:females, n (%) | 51 (80%):13 (20%) |
| Age, yr (range) † | 73.5 (41–93) |
| Body mass index, kg/m2 (range) | 22.6 (16.4–34.8) |
| HCV: HBV: nonB, nonC, n (%) | 28 (44%):13 (20%):23 (36%) |
| Performance status 0:1, n (%) | 60 (94%):4 (6%) |
| Platelet count, ×103/μL (range) † | 171 (52–371) |
| Albumin, g/dL (range) † | 3.7 (2.9–4.6) |
| Total bilirubin, mg/dL (range) † | 1.0 (0.3–2.1) |
| Prothrombin activity, % (range) † | 86.3 (63.3–109.2) |
| AST, IU/L (range) † | 41.5(15–351) |
| Neutrophil-to-lymphocyte ratio, % (range) | 3.13 (1.35–8.94) |
| AFP, μg/L (range) † | 89.0 (1.5–61,040.7) |
| DCP, AU/L (range) † | 691.0 (12.0–194,798.0) |
| Child-Pugh score 5:6, n (%) | 41 (64%):23 (36%) |
| mALBI grade (1:2a:2b:3), n (%) | 18 (28%):24 (38%):22 (34%):0 (0%) |
| Lenvatinib: Atezolizumab plus Bevacizumab [n (%)] | 45 (70%):19 (30%) |
| Initial dose of lenvatinib, 4 mg:8 mg:12 mg [n (%)] | 4 (9%):18 (40%):23 (51%) |
| Reduced starting dose of lenvatinib [n (%)] | 5 (11%) |
| Tumor characteristics | |
| Largest tumor diameter, mm (range) † | 46.0 (11.0–175.0) |
| Exceeding the Up-to-7 criteria, n (%) | 45 (70%) |
| Macrovascular invasion, n (%) | 39 (61%) |
| Extrahepatic metastasis, n (%) | 38 (59%) |
| TACE failure/refractoriness, n (%) | 29 (45%) |
| p * | Coefficients † | SE | Wald χ2 | HR | 95% CI | |
|---|---|---|---|---|---|---|
| Exceeding the Up-to-7 criteria | 0.011 | 1.112 | 0.437 | 6.480 | 3.040 | 1.291–7.155 |
| AFP +100 µg/L | 0.023 | 0.003 | 0.001 | 5.198 | 1.003 | 1.000–1.006 |
| DCP +100 AU/L | 0.006 | 0.001 | 0.000 | 7.587 | 1.001 | 1.000–1.002 |
| Subsequent treatment during treatment period | ||||||
| No subsequent treatment | ||||||
| Single treatment procedure | 0.003 | −1.276 | 0.429 | 8.862 | 0.279 | 0.120–0.647 |
| Multidisciplinary combination therapy | <0.001 | −1.667 | 0.448 | 13.838 | 0.189 | 0.078–0.454 |
| R0 resection | 0.006 | −2.932 | 1.077 | 7.412 | 0.053 | 0.006–0.440 |
| None | STP | MCT | R0 | p-Value | |
|---|---|---|---|---|---|
| n = 22 | n = 19 | n = 19 | n = 4 | ||
| Pretreatment patient characteristics | |||||
| Age, yr (range) † | 77.5 (45–93) | 79.0 (55–88) | 70.0 (41–86) | 68.5 (64–70) | 0.039 |
| Viral:Non-viral, n (%) | 14 (64%):8 (36%) | 12 (63%):7 (37%) | 14 (74%):5 (26%) | 1 (25%):3 (75%) | 0.371 |
| mALBI grade (1:2a:2b), n (%) | 2 (9%):10 (45%):10 (45%) | 5 (26%):8 (42%):6 (32%) | 8 (42%):5 (26%):6 (32%) | 3 (75%):1 (25%):0 (0%) | 0.091 |
| AFP, µg/L (range) † | 144.0 (1.5–61,040.7) | 121.1 (3.0–55,372.0) | 28.3 (3.0–18,861.6) | 10.7 (4.9–87.0) | 0.143 |
| DCP, AU/L (range) † | 1628.5 (16.0–63,347.0) | 294.0 (12.0–116,157.0) | 483.0 (18.0–194,798.0) | 1671.0 (175.0–3608.0) | 0.502 |
| NLR, % (range) † | 3.48 (1.35–6.92) | 2.76 (1.36–6.08) | 3.27 (1.89–8.94) | 2.18 (1.56–2.62) | 0.101 |
| Lenvatinib:Atezo plus Bev [n (%)] | 16 (73%):6 (27%) | 15 (79%):4 (21%) | 10 (53%):9 (47%) | 4 (100%):0 (0%) | 0.188 |
| Pretreatment tumor characteristics | |||||
| Largest tumor diameter, mm (range) † | 50.9 (11–175) | 46.0 (11–116) | 46.0 (17–108) | 36.5 (22–54) | 0.774 |
| Exceeding the Up-to-7 criteria, n (%) | 13 (59%) | 15 (79%) | 16 (84%) | 1 (25%) | 0.055 |
| Macrovascular invasion, n (%) | 11 (50%) | 14 (74%) | 12 (63%) | 2 (50%) | 0.453 |
| Extrahepatic metastasis, n (%) | 15 (68%) | 11 (58%) | 11 (58%) | 2 (50%) | 0.723 |
| TACE failure/refractoriness, n (%) | 12 (55%) | 9 (47%) | 7 (37%) | 1 (25%) | 0.641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, Y.; Akuta, N.; Shindoh, J.; Matsumura, M.; Okubo, S.; Tominaga, L.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; Sezaki, H.; et al. Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma. Cancers 2023, 15, 3789. https://doi.org/10.3390/cancers15153789
Kawamura Y, Akuta N, Shindoh J, Matsumura M, Okubo S, Tominaga L, Fujiyama S, Hosaka T, Saitoh S, Sezaki H, et al. Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma. Cancers. 2023; 15(15):3789. https://doi.org/10.3390/cancers15153789
Chicago/Turabian StyleKawamura, Yusuke, Norio Akuta, Junichi Shindoh, Masaru Matsumura, Satoshi Okubo, Licht Tominaga, Shunichiro Fujiyama, Tetsuya Hosaka, Satoshi Saitoh, Hitomi Sezaki, and et al. 2023. "Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma" Cancers 15, no. 15: 3789. https://doi.org/10.3390/cancers15153789
APA StyleKawamura, Y., Akuta, N., Shindoh, J., Matsumura, M., Okubo, S., Tominaga, L., Fujiyama, S., Hosaka, T., Saitoh, S., Sezaki, H., Suzuki, F., Suzuki, Y., Ikeda, K., Arase, Y., Hashimoto, M., Kozuka, T., & Kumada, H. (2023). Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma. Cancers, 15(15), 3789. https://doi.org/10.3390/cancers15153789






