Gender Differences in a Mouse Model of Hepatocellular Carcinoma Revealed Using Multi-Modal Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
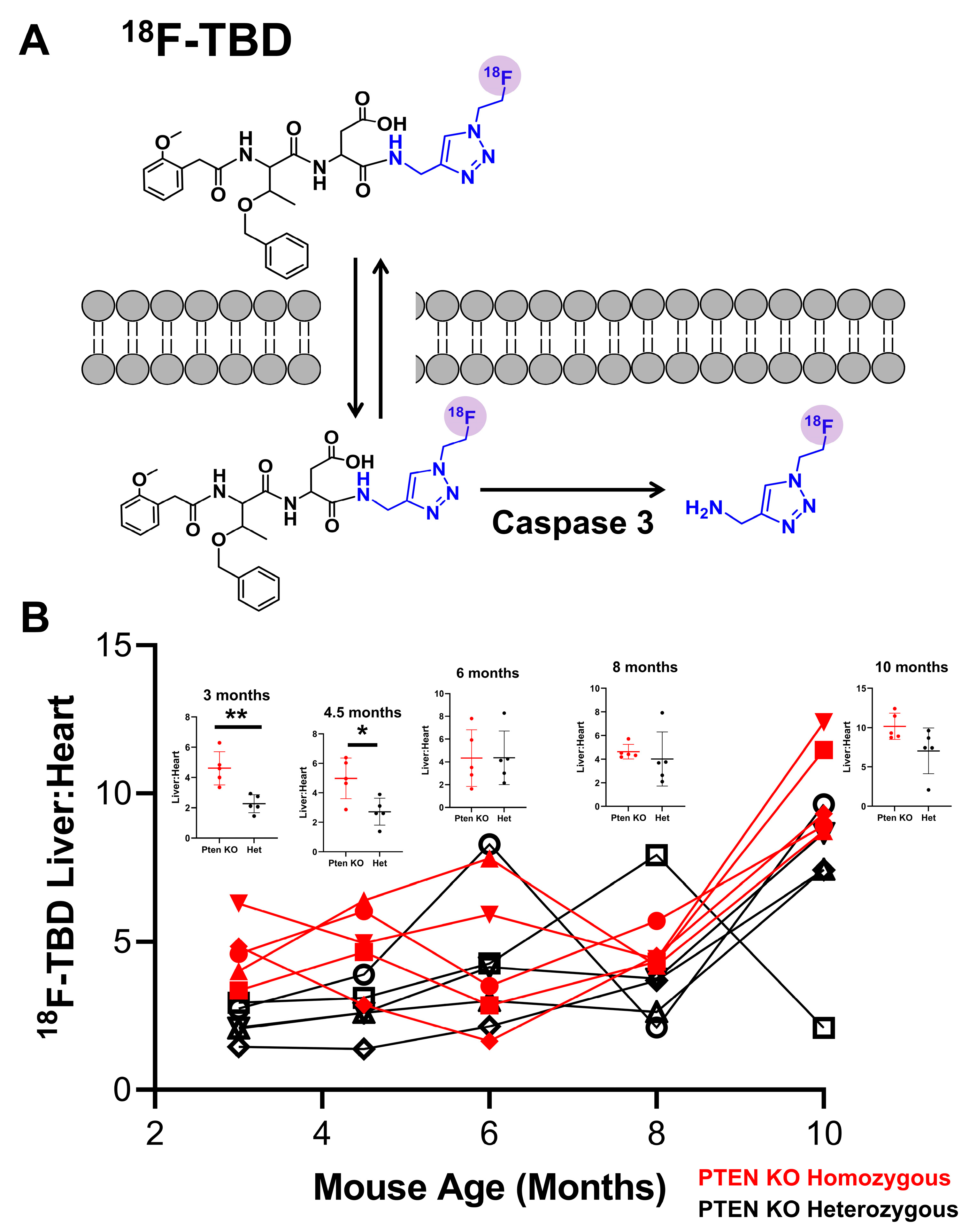
2.1. Radiochemistry of 18F-TBD
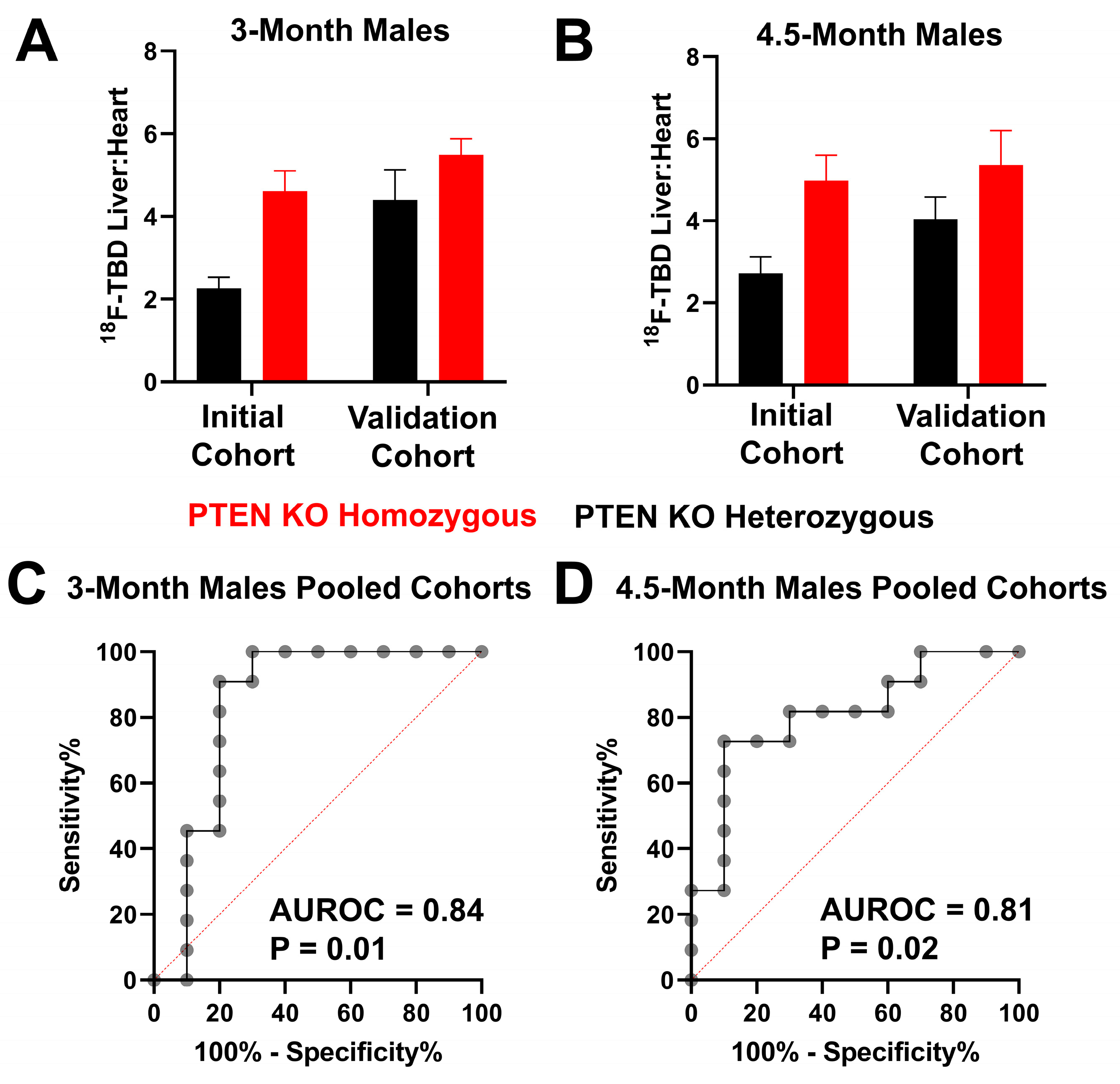
2.2. Dynamic PET Imaging with 18F-TBD
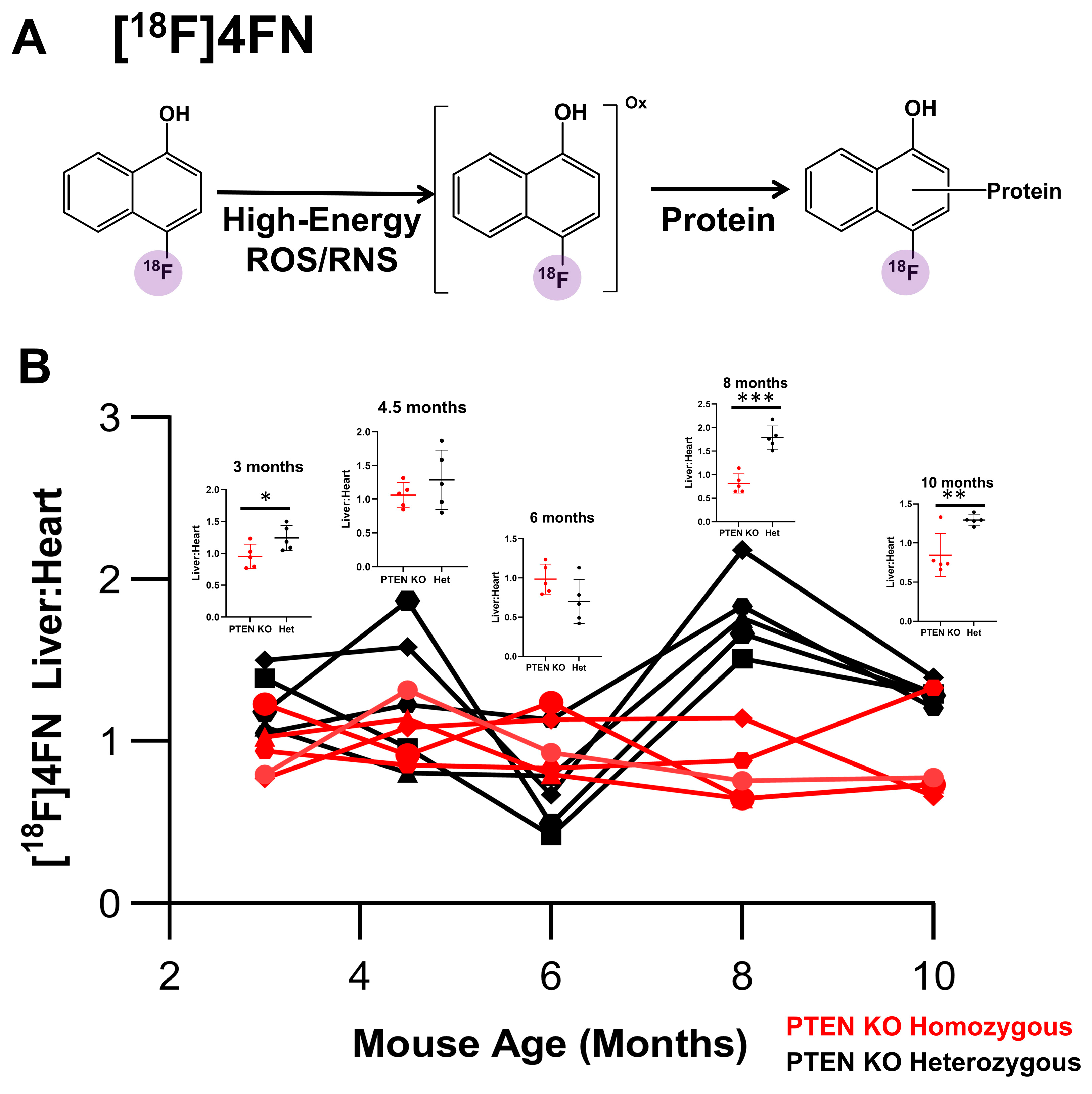
2.3. PET Imaging with [18F]4FN and [18F]FDG
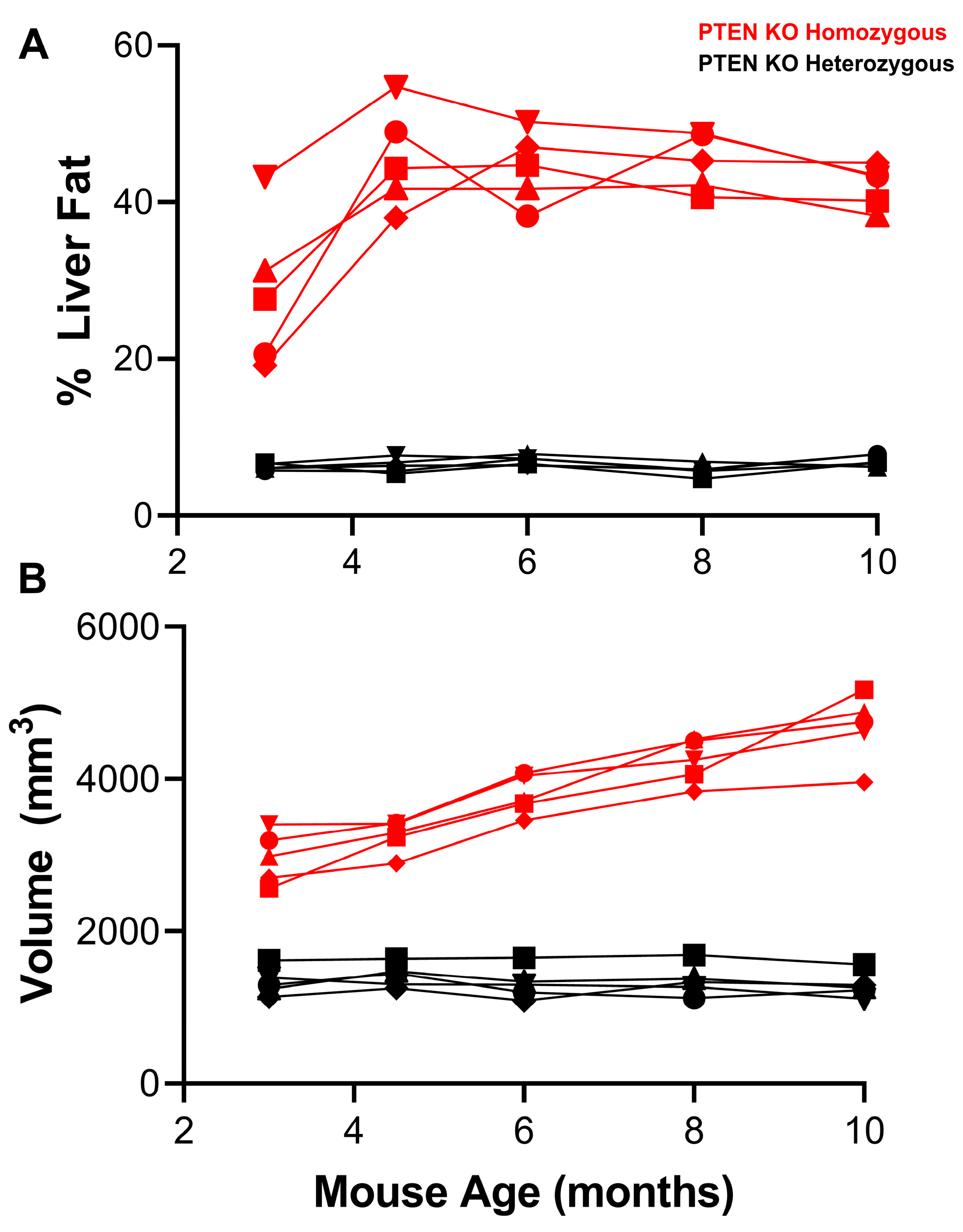
2.4. Quantitation of Liver Fat and Liver Volume Using MR
2.5. Measurement of Liver Density Using CT
2.6. PTEN Deletion Mouse Model
3. Results
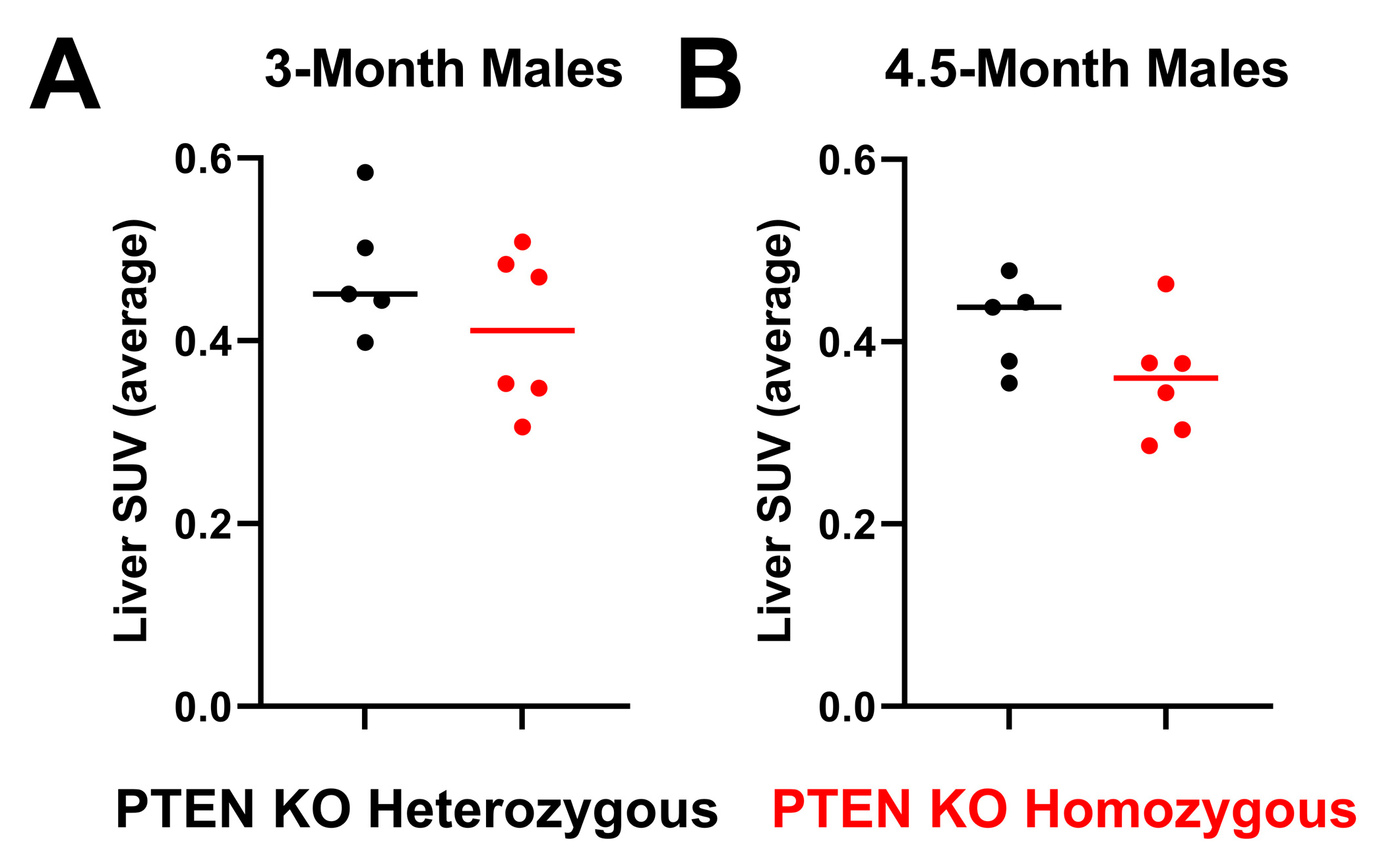
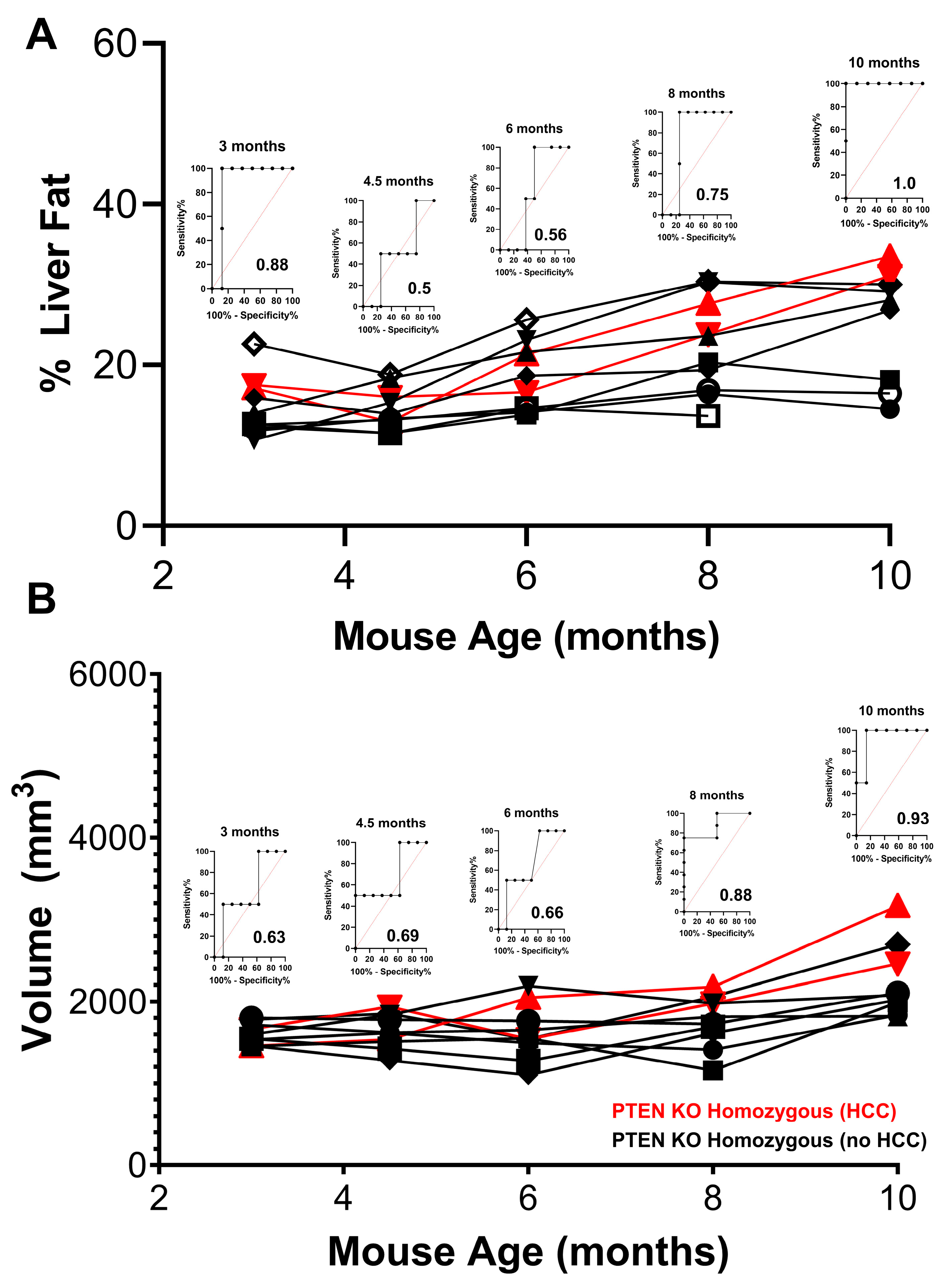
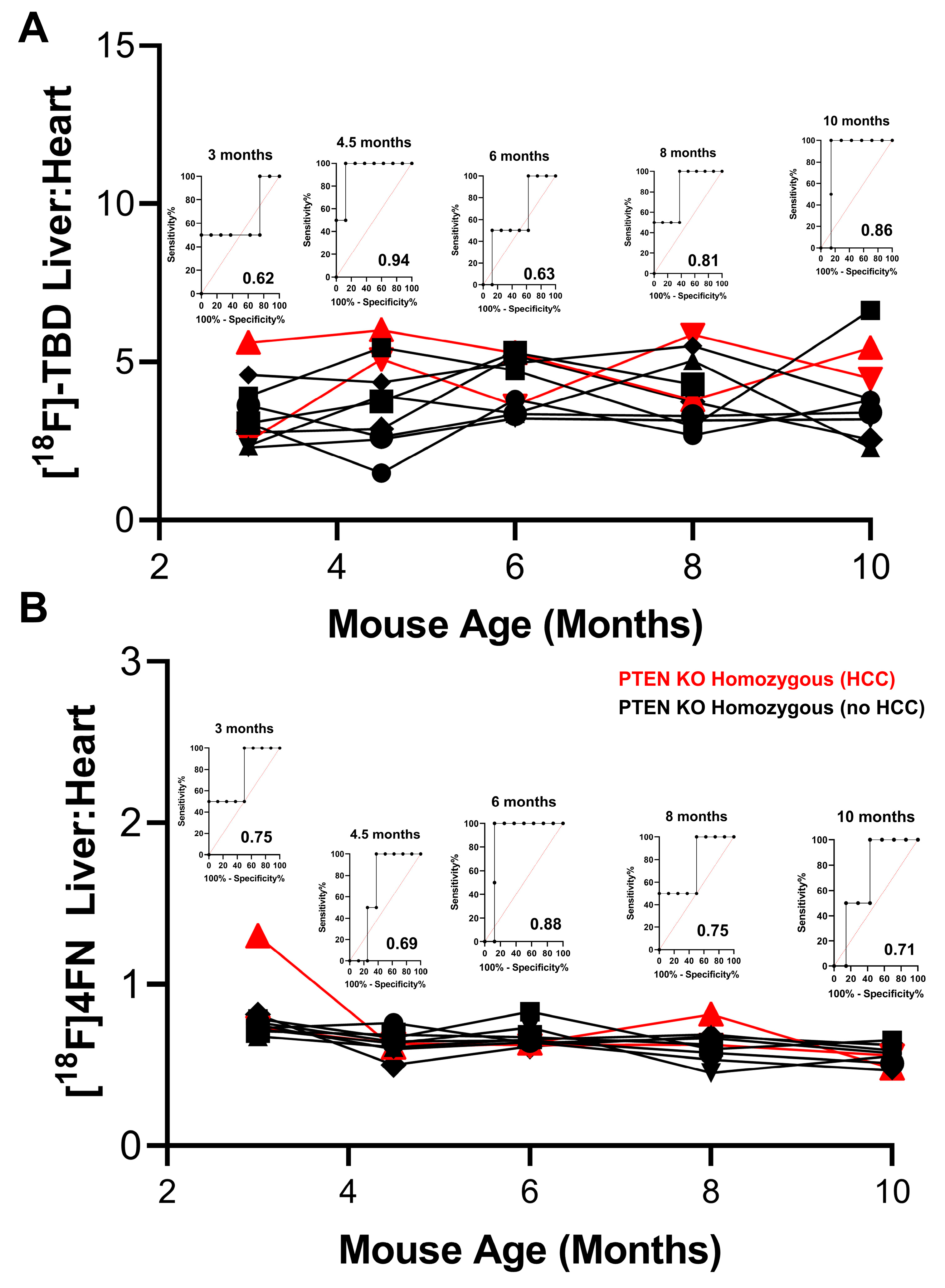
3.1. Liver PTEN Model—Males
3.2. Liver PTEN Model—Female Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ganne-Carrie, N.; Nahon, P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019, 70, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Otsuka, M.; Kishikawa, T.; Yoshikawa, T.; Yamagami, M.; Ohno, M.; Takata, A.; Shibata, C.; Ishibashi, R.; Koike, K. MicroRNAs and liver disease. J. Hum. Genet. 2017, 62, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yasser, M.B.; Abdellatif, M.; Emad, E.; Jafer, A.; Ahmed, S.; Nageb, L.; Abdelshafy, H.; Al-Anany, A.M.; Al-Arab, M.A.E.; Gibriel, A.A. Circulatory miR-221 & miR-542 expression profiles as potential molecular biomarkers in Hepatitis C Virus mediated liver cirrhosis and hepatocellular carcinoma. Virus Res. 2021, 296, 198341. [Google Scholar]
- Pinero, F.; Dirchwolf, M.; Pessoa, M.G. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020, 9, 1370. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Kawamura, N.; Nagai, H.; Bando, K.; Koyama, M.; Matsumoto, S.; Tajiri, T.; Onda, M.; Fujimoto, J.; Ueki, T.; Konishi, N.; et al. PTEN/MMAC1 mutations in hepatocellular carcinomas: Somatic inactivation of both alleles in tumors. Jpn. J. Cancer Res. 1999, 90, 413–418. [Google Scholar] [CrossRef]
- Hu, T.-H.; Wang, C.-C.; Huang, C.-C.; Chen, C.-L.; Hung, C.-H.; Chen, C.-H.; Wang, J.-H.; Lu, S.-N.; Lee, C.-M.; Changchien, C.-S.; et al. Down-regulation of tumor suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen index predict poor patient outcome of hepatocellular carcinoma after resection. Oncol. Rep. 2007, 18, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.H.; Huang, C.C.; Lin, P.R.; Chang, H.W.; Ger, L.P.; Lin, Y.W.; Changchien, C.S.; Lee, C.M.; Tai, M.H. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer 2003, 97, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Carracedo, A.; Pandolfi, P.P. Tenets of PTEN tumor suppression. Cell 2008, 133, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Daikoku, T.; Hirota, Y.; Tranguch, S.; Joshi, A.R.; DeMayo, F.J.; Lydon, J.P.; Ellenson, L.H.; Dey, S.K. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008, 68, 5619–5627. [Google Scholar] [CrossRef]
- Kwabi-Addo, B.; Giri, D.; Schmidt, K.; Podsypanina, K.; Parsons, R.; Greenberg, N.; Ittmann, M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc. Natl. Acad. Sci. USA 2001, 98, 11563–11568. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Gao, C.; Fabien, L.; Jia, Y.; Lu, J.; Silberstein, L.E.; Pinkus, G.S.; Ye, K.; Chai, L.; et al. Relevant mouse model for human monocytic leukemia through Cre/lox-controlled myeloid-specific deletion of PTEN. Leukemia 2010, 24, 1077–1080. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, A.; Kataoka, E.; Sasaki, T.; Hamada, K.; Sasaki, J.; Mizuno, K.; Hasegawa, G.; Kishimoto, H.; Iizuka, M.; et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Investig. 2004, 113, 1774–1783. [Google Scholar] [CrossRef]
- Anezaki, Y.; Ohshima, S.; Ishii, H.; Kinoshita, N.; Dohmen, T.; Kataoka, E.; Sato, W.; Iizuka, M.; Goto, T.; Sasaki, J.; et al. Sex difference in the liver of hepatocyte-specific Pten-deficient mice: A model of nonalcoholic steatohepatitis. Hepatol. Res. 2009, 39, 609–618. [Google Scholar] [CrossRef]
- Ren, Q.N.; Zhang, H.; Sun, C.Y.; Zhou, Y.F.; Yang, X.F.; Long, J.W.; Li, X.X.; Mai, S.J.; Zhang, M.Y.; Zhang, H.Z.; et al. Phosphorylation of androgen receptor by mTORC1 promotes liver steatosis and tumorigenesis. Hepatology 2022, 75, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Engel, B.J.; Gammon, S.T.; Chaudhari, R.; Lu, Z.; Pisaneschi, F.; Yang, H.; Ornelas, A.; Yan, V.; Kelderhouse, L.; Najjar, A.M.; et al. Caspase-3 Substrates for Noninvasive Pharmacodynamic Imaging of Apoptosis by PET/CT. Bioconjug. Chem. 2018, 29, 3180–3195. [Google Scholar] [CrossRef] [PubMed]
- Pisaneschi, F.; Gammon, S.T.; Paolillo, V.; Qureshy, S.A.; Piwnica-Worms, D. Imaging of innate immunity activation in vivo with a redox-tuned PET reporter. Nat. Biotechnol. 2022, 40, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Pisaneschi, F.; Kelderhouse, L.E.; Hardy, A.; Engel, B.J.; Mukhopadhyay, U.; Gonzalez-Lepera, C.; Gray, J.P.; Ornelas, A.; Takahashi, T.T.; Roberts, R.W.; et al. Automated, Resin-Based Method to Enhance the Specific Activity of Fluorine-18 Clicked PET Radiotracers. Bioconjug. Chem. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Garbow, J.R.; Lin, X.; Sakata, N.; Chen, Z.; Koh, D.; Schonfeld, G. In vivo MRS measurement of liver lipid levels in mice. J. Lipid Res. 2004, 45, 1364–1371. [Google Scholar] [CrossRef]
- Melloul, E.; Raptis, D.A.; Boss, A.; Pfammater, T.; Tschuor, C.; Tian, Y.; Graf, R.; Clavien, P.A.; Lesurtel, M. Small animal magnetic resonance imaging: An efficient tool to assess liver volume and intrahepatic vascular anatomy. J. Surg. Res. 2014, 187, 458–465. [Google Scholar] [CrossRef]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Stiles, B.; Wang, Y.; Stahl, A.; Bassilian, S.; Lee, W.P.; Kim, Y.J.; Sherwin, R.; Devaskar, S.; Lesche, R.; Magnuson, M.A.; et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc. Natl. Acad. Sci. USA 2004, 101, 2082–2087. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Dedes, N.; Kouroumalis, E.; Theocharis, S. The Role of the NLRP3 Inflammasome in HCC Carcinogenesis and Treatment: Harnessing Innate Immunity. Cancers 2022, 14, 3150. [Google Scholar] [CrossRef]
- Canet, M.J.; Hardwick, R.N.; Lake, A.D.; Dzierlenga, A.L.; Clarke, J.D.; Cherrington, N.J. Modeling human nonalcoholic steatohepatitis-associated changes in drug transporter expression using experimental rodent models. Drug Metab. Dispos. 2014, 42, 586–595. [Google Scholar] [CrossRef]
- De Lombaerde, S.; Devisscher, L.; Verhoeven, J.; Neyt, S.; Van Vlierberghe, H.; Vanhove, C.; De Vos, F. Validation of hepatobiliary transport PET imaging in liver function assessment: Evaluation of 3beta-[(18)F]FCA in mouse models of liver disease. Nucl. Med. Biol. 2019, 68–69, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Garcia, V.; Chavez-Lopez Mde, G.; Quintanar-Jurado, V.; Gabino-Lopez, N.B.; Hernandez-Gallegos, E.; Soriano-Rosas, J.; Perez-Carreon, J.I.; Camacho, J. Differential Expression of Ion Channels and Transporters During Hepatocellular Carcinoma Development. Dig. Dis. Sci. 2015, 60, 2373–2383. [Google Scholar] [CrossRef]
- Ali, I.; Slizgi, J.R.; Kaullen, J.D.; Ivanovic, M.; Niemi, M.; Stewart, P.W.; Barritt, A.S.T.; Brouwer, K.L.R. Transporter-Mediated Alterations in Patients With NASH Increase Systemic and Hepatic Exposure to an OATP and MRP2 Substrate. Clin. Pharmacol. Ther. 2018, 104, 749–756. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mukherjee, S.; Sankara Sivaprasad, L.V.J.; Naik, T.; Gautam, S.S.; Murali, B.V.; Hadambar, A.A.; Gunti, G.R.; Kuchibhotla, V.; Deyati, A.; et al. Transporter Activity Changes in Nonalcoholic Steatohepatitis: Assessment with Plasma Coproporphyrin I and III. J. Pharmacol. Exp. Ther. 2021, 376, 29–39. [Google Scholar] [CrossRef]
- Atilano-Roque, A.; Roda, G.; Fogueri, U.; Kiser, J.J.; Joy, M.S. Effect of Disease Pathologies on Transporter Expression and Function. J. Clin. Pharmacol. 2016, 56 (Suppl. S7), S205–S221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, P.; Hu, K.; Dai, G.; Li, J.; Zheng, D.; Yuan, H.; He, L.; Xie, P.; Tu, M.; et al. Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation. J. Cell. Mol. Med. 2021, 25, 1568–1582. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Li, T.; Sanchez-Duffhues, G.; Zhou, F.; Zhang, L. Involvement of inflammation and its related microRNAs in hepatocellular carcinoma. Oncotarget 2017, 8, 22145–22165. [Google Scholar] [CrossRef]
- Ucar, F.; Sezer, S.; Erdogan, S.; Akyol, S.; Armutcu, F.; Akyol, O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013, 18, 127–133. [Google Scholar] [CrossRef]
- Li, N.; Qin, J.; Lan, L.; Zhang, H.; Liu, F.; Wu, Z.; Ni, H.; Wang, Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol. Ther. 2015, 16, 297–306. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, C.W. Protein phosphatases regulate the liver microenvironment in the development of hepatocellular carcinoma. Exp. Mol. Med. 2022, 54, 1799–1813. [Google Scholar] [CrossRef]
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol. 2011, 46 (Suppl. S1), 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Fallon, M.B. Gender and racial differences in nonalcoholic fatty liver disease. World J. Hepatol. 2014, 6, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.; Livanos, A.; Guo, A.; Pomenti, S.; Yeh, J.; Dakhoul, L.; Burney, H.; Kettler, C.; Liu, H.; Miller, E.; et al. Gender Matters: Characteristics of Hepatocellular Carcinoma in Women From a Large, Multicenter Study in the United States. Am. J. Gastroenterol. 2020, 115, 1486–1495. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Jung, Y. Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Cells 2019, 8, 1259. [Google Scholar] [CrossRef]
- Naqvi, S.; Godfrey, A.K.; Hughes, J.F.; Goodheart, M.L.; Mitchell, R.N.; Page, D.C. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science 2019, 365, eaaw7317. [Google Scholar] [CrossRef]
- Clayton, J.A. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav. 2018, 187, 2–5. [Google Scholar] [CrossRef]
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.J.; Woodruff, T.; McCarthy, M.M.; Sohrabji, F.; Schiebinger, L.; Wetherington, C.L.; et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Panasyuk, G.; Espeillac, C.; Chauvin, C.; Pradelli, L.A.; Horie, Y.; Suzuki, A.; Annicotte, J.S.; Fajas, L.; Foretz, M.; Verdeguer, F.; et al. PPARgamma contributes to PKM2 and HK2 expression in fatty liver. Nat. Commun. 2012, 3, 672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel, B.J.; Paolillo, V.; Uddin, M.N.; Gonzales, K.A.; McGinnis, K.M.; Sutton, M.N.; Patnana, M.; Grindel, B.J.; Gores, G.J.; Piwnica-Worms, D.; et al. Gender Differences in a Mouse Model of Hepatocellular Carcinoma Revealed Using Multi-Modal Imaging. Cancers 2023, 15, 3787. https://doi.org/10.3390/cancers15153787
Engel BJ, Paolillo V, Uddin MN, Gonzales KA, McGinnis KM, Sutton MN, Patnana M, Grindel BJ, Gores GJ, Piwnica-Worms D, et al. Gender Differences in a Mouse Model of Hepatocellular Carcinoma Revealed Using Multi-Modal Imaging. Cancers. 2023; 15(15):3787. https://doi.org/10.3390/cancers15153787
Chicago/Turabian StyleEngel, Brian J., Vincenzo Paolillo, Md. Nasir Uddin, Kristyn A. Gonzales, Kathryn M. McGinnis, Margie N. Sutton, Madhavi Patnana, Brian J. Grindel, Gregory J. Gores, David Piwnica-Worms, and et al. 2023. "Gender Differences in a Mouse Model of Hepatocellular Carcinoma Revealed Using Multi-Modal Imaging" Cancers 15, no. 15: 3787. https://doi.org/10.3390/cancers15153787
APA StyleEngel, B. J., Paolillo, V., Uddin, M. N., Gonzales, K. A., McGinnis, K. M., Sutton, M. N., Patnana, M., Grindel, B. J., Gores, G. J., Piwnica-Worms, D., Beretta, L., Pisaneschi, F., Gammon, S. T., & Millward, S. W. (2023). Gender Differences in a Mouse Model of Hepatocellular Carcinoma Revealed Using Multi-Modal Imaging. Cancers, 15(15), 3787. https://doi.org/10.3390/cancers15153787





