The Role of Colonoscopy in the Management of Individuals with Lynch Syndrome: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
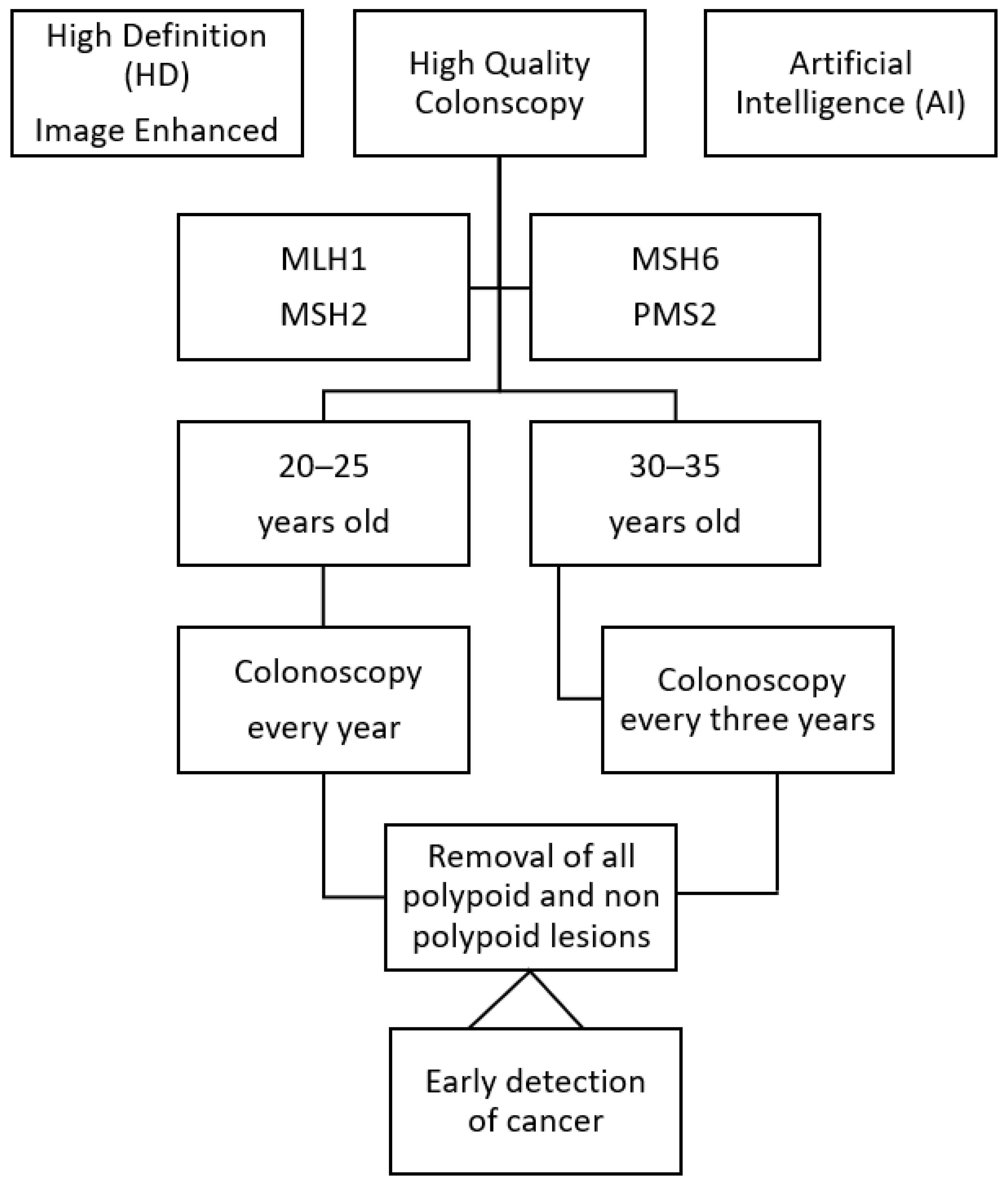
2. Four Crucial Points
3. Some Points of Clarification
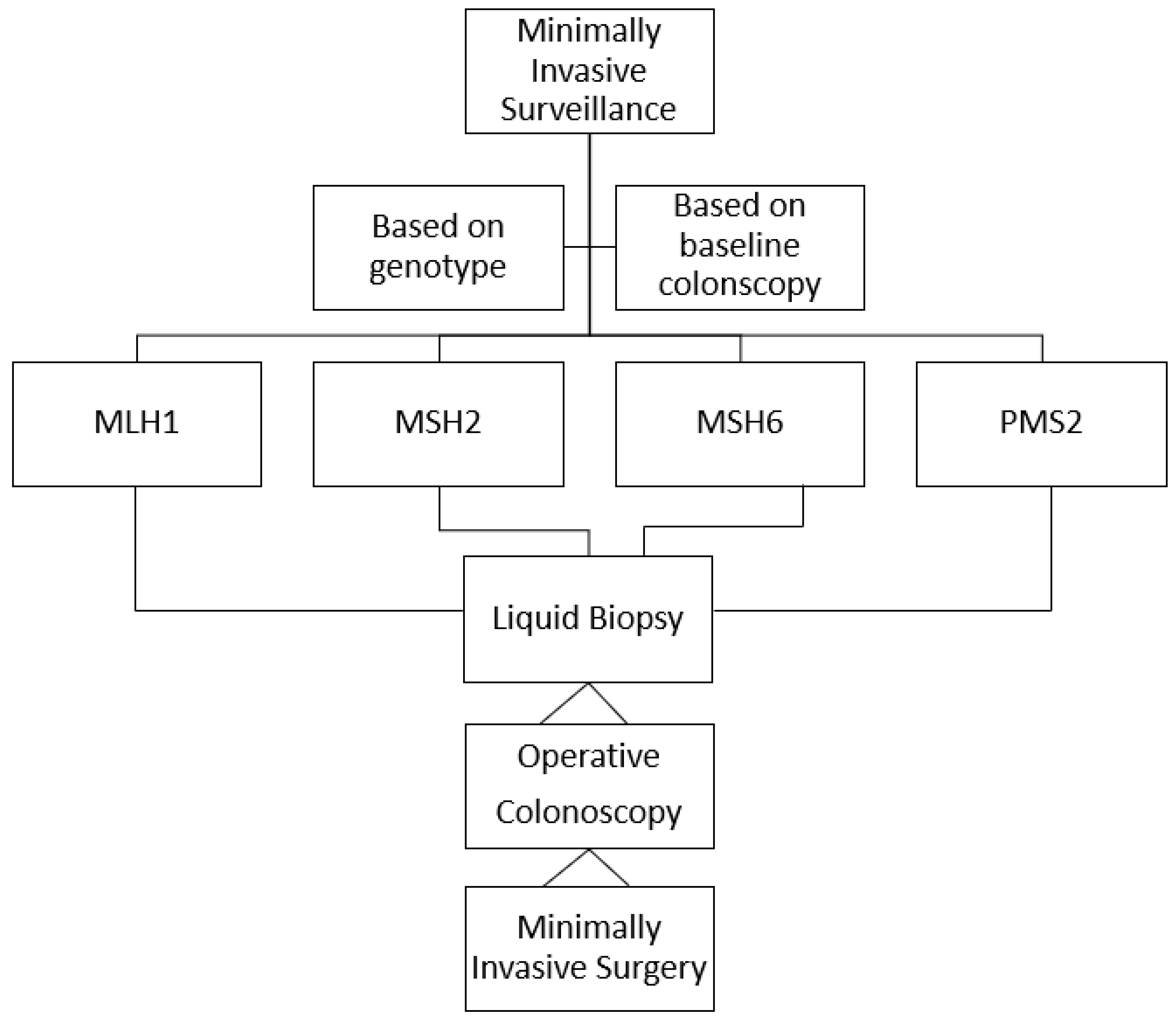
4. Open Questions
5. Conclusions
6. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bhattacharya, P.; McHugh, T.W. Lynch Syndrome; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Weisenberger, D.J.; Liang, G.; Lenz, H.-J. DNA methylation aberrancies delineate clinically distinct subsets of colorectal cancer and provide novel targets for epigenetic therapies. Oncogene 2018, 37, 566–577. [Google Scholar] [CrossRef]
- Banerjea, A.; Bustin, S.A.; Dorudi, S. The immunogenicity of colorectal cancers with high-degree microsatellite instability. World J. Surg. Oncol. 2005, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, H.J.; Aarnio, M.; Mustonen, H.; Aktan–Collan, K.; Aaltonen, L.A.; Peltomäki, P.; De La Chapelle, A.; Mecklin, J.P. Controlled 15-Year Trial on Screening for Colorectal Cancer in Families with Hereditary Non polyposis Colorectal Cancer. Gastroenterology 2000, 118, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Lynch, P. If Aggressive Surveillance in Hereditary Nonpolyposis Colorectal Cancer Is Now State of the Art, Are There Any Challenges Left? Gastroenterology 2000, 118, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.H.; Enns, R.; Heidelbaugh, J.; Barkun, A. The Clinical Guidelines Committee American Gastroenterological Association Institute. Guideline on the Diagnosis and Management of Lynch Syndrome. Gastroenterology 2015, 149, 777–782. [Google Scholar] [CrossRef] [Green Version]
- van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Balaguer, F.; Dekker, E.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Ricciardiello, L.; Rupińska, M.; et al. Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 1082–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Provenzale, D.; Llor, X.; Halverson, A.L.; Grady, W.; Chung, D.C.; Haraldsdottir, S.; Markowitz, A.J.; Slavin, T.P., Jr.; Hampel, H.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2. J. Natl. Compr. Canc. Netw. 2019, 17, 1032–1041. [Google Scholar] [CrossRef] [Green Version]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; DeSouza, B.; Dunlop, M.; E East, J.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Hereditary CRC guidelines eDelphi consensus group. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef]
- Olivier, R.; Randrian, V.; Tougeron, D.; Jea Saurin, J.C. Endoscopy to Diagnose and Prevent Digestive Cancers in Lynch Syndrome. Cancers 2021, 13, 3505. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.; et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: First report from the prospective Lynch syndrome database. Gut 2017, 66, 464–472. [Google Scholar] [CrossRef]
- Perrod, G.; Rahmi, G.; Cellier, C. Colorectal cancer screening in Lynch syndrome: Indication, techniques and future perspectives. Dig. End. 2021, 33, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, T.T.; Dominguez-Valentin, M.; Sampson, J.R.; Møller, P. Prospective observational data informs understanding and future management of Lynch syndrome: Insights from the Prospective Lynch Syndrome Database (PLSD). Familial Cancer 2021, 20, 35–39. [Google Scholar] [CrossRef]
- Drogan, X.C.; Kupfer, S.S. Colorectal Cancer Screening Recommendations and Outcomes in Lynch Syndrome. Gastrointest Endosc. Clin. N. Am. 2022, 32, 59–74. [Google Scholar] [CrossRef]
- Tanabe, H.; Moriichi, K.; Mizukami, Y.; Fujiya, M.; Okumura, T. Artificial intelligence–assisted detection of colorectal polyps in Lynch syndrome. Gastrointest Endosc. 2022, 95, 1276–1277. [Google Scholar] [CrossRef]
- Sealock, R.J.; Othman, M.O. Commentary. Gastrointest Endosc. 2022, 95, 1277. [Google Scholar]
- Hasegawa, I.; Yamamura, T.; Suzuki, H.; Maeda, K.; Sawada, T.; Mizutani, Y.; Ishikawa, E.; Ishikawa, T.; Kakushima, N.; Furukawa, K.; et al. Detection of Colorectal Neoplasms Using Linked Color Imaging: A Prospective, Randomized, Tandem Colonoscopy Trial. Clin. Gastroenterol. Hepatol. 2021, 19, 1708–1716. [Google Scholar] [CrossRef]
- Hüneburg, R.; Bucksch, K.; Schmeißer, F.; Heling, D.; Marwitz, T.; Aretz, S.; Kaczmarek, D.J.; Kristiansen, G.; Hommerding, O.; Strassburg, C.P.; et al. Real-time use of artificial intelligence (CADEYE) in colorectal cancer surveillance of patients with Lynch syndrome—A randomized controlled pilot trial. United Eur. Gastroenterol. J. 2023, 11, 60–68. [Google Scholar] [CrossRef]
- Rivero-Sánchez, L.; Gavri, A.; Herrero, J.; Remedios, D.; Alvarez, V.; Albéniz, E.; Gordillo, J.; Puig, I.; López-Vicente, J.; Huerta, A.; et al. The “diagnose and leave in” strategy for diminutive rectosigmoid polyps in Lynch syndrome: A post hoc analysis from a randomized controlled trial. Endoscopy 2022, 54, 27–34. [Google Scholar] [CrossRef]
- Rex, D.K.; Kahi, C.; O’Brien, M.; Levin, T.R.; Pohl, H.; Rastogi, A.; Burgart, L.; Imperiale, T.; Ladabaum, U.; Cohen, J.; et al. The ASGE-PIVI on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gatrointest Endosc. 2011, 73, 419–422. [Google Scholar] [CrossRef]
- Boonstra, J.J.; de Vos tot Nederveen Cappel, W.H.; Langers, A.M.J.; van der Sluis, H.; Hardwick, J.H.; Vasen, H.F.A. Colonoscopy in Lynch syndrome: The need for a new quality score. Fam. Cancer 2016, 16, 239–241. [Google Scholar] [CrossRef]
- Mittendorf, K.F.; Hunter, J.E.; Schneider, J.L.; Shuster, E.; Rope, A.F.; Zepp, J.; Gilmore, M.J.; Muessig, K.R.; Davis, J.V.; Kauffman, T.L.; et al. Recommended care and care adherence following a diagnosis of Lynch syndrome: A mixed-methods study. Hered. Cancer Clin. Pract. 2019, 17, 31. [Google Scholar] [CrossRef]
- Dove-Edwin, I.; Sasieni, P.; Adams, J.; Thomas, H.J.W. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ 2005, 331, 1047. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, C.; Vasen, H.F.; Seppälä, T.; Aretz, S.; Bigirwamungu-Bargeman, M.; de Boer, S.Y.; Bucksch, K.; Büttner, R.; Holinski-Feder, E.; Holzapfel, S.; et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy Among 3 Countries With Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018, 155, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.M. Implementation of evidence-based patient navigation programs. Acta Oncol. 2017, 56, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paskett, E.D.; Bernardo, B.M.; Young, G.S.; Katz, M.L.; Reiter, P.L.; Tatum, C.M.; Oliveri, J.M.; DeGraffinreid, C.R.; Gray, D.M.; Pearlman, R.; et al. Comparative Effectiveness of Two Interventions to Increase Colorectal Cancer Screening for Those at Increased Risk Based on Family History: Results of a Randomized Trial. Cancer Epidemiol. Biomark. Prev. 2020, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cragun, D.; Beckstead, J.; Farmer, M.; Hooker, G.; Dean, M.; Matloff, E.; Reid, S.; Tezak, A.; Weidner, A.; Whisenant, J.G.; et al. IMProving care After inherited Cancer Testing (IMPACT) study: Protocol of a randomized trial evaluating the efficacy of two interventions designed to improve cancer risk management and family communication of genetic test results. BMC Cancer 2021, 21, 1099. [Google Scholar] [CrossRef]
- Prospective Lynch Syndrome Database (PLSD). Available online: https://www.ehtg.org/plsd.php (accessed on 1 November 2022).
- Engel, C.; Rahner, N.; Schulmann, K.; Holinski–Feder, E.; Goecke, T.O.; Schackert, H.K.; Kloor, M.; Steinke, V.; Vogelsang, H.; Möslein, G.; et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin. Gastroenterol. Hepatol. 2010, 8, 174–182. [Google Scholar] [CrossRef]
- Lindberg, L.J.; Ladelund, S.; Frederiksen, B.L.; Smith-Hansen, L.; Bernstein, I. Outcome of 24 years national surveillance in different hereditary colorectal cancer subgroups leading to more individualised surveillance. J. Med. Genet. 2017, 54, 297–304. [Google Scholar] [CrossRef]
- Ahadova, A.; Pfuderer, P.L.; Ahtiainen, M.; Ballhausen, A.; Bohaumilitzky, L.; Kösegi, S.; Müller, N.; Tang, Y.L.; Kosmalla, K.; Witt, J.; et al. Distinct Mutational Profile of Lynch Syndrome Colorectal Cancers Diagnosed under Regular Colonoscopy Surveillance. J. Clin. Med. 2021, 10, 2458. [Google Scholar] [CrossRef]
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef] [Green Version]
- Aissaoui, S.; Cartellier, C.; Seytier, T.; Giraud, S.; Calender, A. Genetic mutation risk calculation in Lynch syndrome inheri-tance: Evaluating the utility of the PREMM model in Lyon: The first French study. Bull. Cancer 2017, 104, 288–294. [Google Scholar] [CrossRef]
- Duraturo, F.; Liccardo, R.; De Rosa, M.; Izzo, P. Genetics, diagnosis and treatment of Lynch syndrome: Old lessons and current challenges. Oncol. Lett. 2019, 17, 3048–3054. [Google Scholar] [CrossRef] [Green Version]
- Kastrinos, F.; Ingram, M.A.; Silver, E.R.; Oh, A.; Laszkowska, M.; Rustgi, A.K.; Hur, C. Gene-Specific Variation in Colorectal Cancer Surveillance Strategies for Lynch Syndrome. Gastroenterology 2021, 161, 453–462. [Google Scholar] [CrossRef]
- Andesdottir, A.K.; Einarsson, H.; Jonsdottir, H.; Jonasson, J.G.; Bjornsson, E.S.; Haraldsdottir, S. Metachronous colorectal cancer in Icelandic MSH6 and PMS2 Lynch syndrome carriers in 1955–2017: A population-based study. Gastroenterology 2023, in press. [Google Scholar] [CrossRef]
- Maratt, J.K.; Rubenstein, J.H. Tailoring Colorectal Cancer Surveillance in Lynch Syndrome:More Is Not Always Better. Gastroenterology 2021, 161, 411–422. [Google Scholar] [CrossRef]
- Ahadova, A.; Seppälä, T.T.; Engel, C.; Gallon, R.; Burn, J.; Holinski-Feder, E.; Steinke-Lange, V.; Möslein, G.; Nielsen, M.; Ten Broeke, S.W.; et al. The “unnatural” history of colorectalcancer in Lynch syndrome: Lessons from colonoscopy surveillance. Int. J. Cancer 2021, 148, 800–811. [Google Scholar] [CrossRef]
- Rondagh, E.J.A.; Gulikers, S.; Gómez-García, E.B.; Vanlingen, Y.; Detisch, Y.; Winkens, B.; Vasen, H.F.A.; Masclee, A.A.M.; Sanduleanu, S. Nonpolypoid colorectal neoplasms: A challenge in endoscopic surveillance of patients with Lynch syndrome. Endoscopy 2013, 45, 257–264. [Google Scholar] [CrossRef]
- Shia, J.; Schultz, N.; Kuk, D.; Vakiani, E.; Middha, S.; Segal, N.H.; Hechtman, J.F.; Berger, M.F.; Stadler, Z.K.; Weiser, M.R.; et al. Morphological characterization of colorectal cancers in The Cancer Genome Atlas reveals distinct morphology-molecular associations: Clinical and biological implications. Mod. Pathol. 2017, 30, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Argillander, T.E.; Koornstra, J.J.; van Kouwen, M.; Langers, A.M.; Nagengast, F.M.; Vecht, J.; de Vos Tot Nederveen Cappel, W.H.; Dekker, E.; van Duijvendijk, P.; Vasen, H.F. Features of incident colorectal cancer in Lynch syndrome. United Eur. Gastroenterol. J. 2018, 6, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Seppälä, T.T.; Ahadova, A.; Dominguez-Valentin, M.; Macrae, F.; Evans, D.G.; Therkildsen, C.; Sampson, J.; Scott, R.; Burn, J.; Möslein, G.; et al. Lack of association between screening interval and cancer stage in Lynch syndrome may be accounted for by over-diagnosis; a prospective Lynch syndrome database report. Hered Cancer Clin. Pract. 2019, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Lepore Signorile, M.; Disciglio, V.; Di Carlo, G.; Pisani, A.; Simone, C.; Ingravallo, G. From Genetics to Histomolecular Characterization: An Insight into Colorectal Carcinogenesis in Lynch Syndrome. Int. J. Mol. Sci. 2021, 22, 6767. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Nevala, S.; Valo, S.; Ristimäki, A.; Sarhadi, V.; Knuutila, S.; Nyström, M.; Renkonen-Sinisalo, L.; Lepistö, A.; Mecklin, J.-P.; Peltomäki, P. DNA methylation changes and somatic mutations as tumorigenic events in Lynch syndrome-associated adenomas retaining mismatch repair protein expression. eBioMedicine 2019, 39, 280–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatamori, H.; Chino, A.; Arai, M.; Ide, D.; Saito, S.; Igarashi, M.; Kita, M.; Nakajima, T.; Kawachi, H.; Fujisaki, J. Malignant potential of colorectal neoplasms in Lynch syndrome: An analysis of 325 lesions endoscopically treated at a single institute. Jpn. J. Clin. Oncol. 2021, 51, 737–743. [Google Scholar] [CrossRef]
- Lynch, H.T.; Smyrk, T. Hereditary NonPolyposis Colorectal Cancer (Lynch Syndrome). An Updated Rewiev. Cancer 1996, 78, 1149–1167. [Google Scholar] [CrossRef]
- Schwitalle, Y.; Kloor, M.; Eiermann, S.; Linnebacher, M.; Kienle, P.; Knaebel, H.P.; Tariverdian, M.; Benner, A.; von Knebel Doeberitz, M. Immune Response Against Frameshift-Induced Neopeptides in HNPCC Patients and Healthy HNPCC Mutation Carriers. Gastroenterology 2008, 134, 988–997. [Google Scholar] [CrossRef]
- Annapragada, A.; Sikora, A.; Bollard, C.; Conejo-Garcia, J.; Cruz, C.R.; Demehri, S.; Demetriou, M.; Demirdjian, L.; Fong, L.; Horowitz, M.; et al. Cancer Moonshot Immuno-Oncology Translational Network (IOTN): Accelerating the clinical translation of basic discoveries for improving immunotherapy and immunoprevention of cancer. J. Immunol. Ther. Cancer 2020, 8, e000796. [Google Scholar] [CrossRef]
- Ahadova, A.; Gallon, R.; Gebert, J.; Ballhausen, A.; Endris, V.; Kirchner, M.; Stenzinger, A.; Burn, J.; von Knebel Doeberitz, M.; Bläker, H.; et al. Three molecular pathways model colorectal carcinogenesis in Lynch syndrome. Int. J. Cancer 2018, 143, 139–150. [Google Scholar] [CrossRef] [Green Version]
- van Liere, E.; de Boer, N.K.H.; Dekker, E.; van Leerdam, M.E.; de Meij, T.G.J.; Ramsoekh, D. Systematic review: Non-endoscopic surveillance for colorectal neoplasia in individuals with Lynch syndrome. Aliment. Pharm. Ther. 2022, 55, 778–788. [Google Scholar] [CrossRef]
- Srivastava, S.; Wagner, P.D. The early detection research network: A National Infrastructure to support the discovery, development, and validation of cancer biomarkers. Cancer Epidemiol. Biomark Prev. 2020, 29, 2401–2410. [Google Scholar] [CrossRef]
- Ballester, V.; Taylor, W.R.; Slettedahl, S.W.; Mahoney, D.W.; Yab, T.C.; A Sinicrope, F.; Boland, C.R.; Lidgard, G.P.; Cruz-Correa, M.R.; Smyrk, T.C.; et al. Novel methylated DNA markers accurately discriminate Lynch syndrome associated colorectal neoplasia. Epigenomics 2020, 12, 2173–2187. [Google Scholar] [CrossRef]
| Authors | Study Type | Numbers | Results |
|---|---|---|---|
| Dove-Edwin I et al. [23] | Prospective observational 2005 | 1678 patiens | Interval colonoscopy on the basis of family history |
| Engel C et al. [30] | Prospective coohrt 2010 | 1126 patients | Annual colonoscopy is recommended |
| Lindberg LJ et al. [31] | Prospective observational 2017 | 13,444 surveillance sessions | Elevated cancer risk despite biannual surveillance |
| Engel C et al. [25] | Population 3 countries 2018 | 2747 patients | Similar cancer risk at different intervals of surveillance colonscopy |
| Ahadova A et al. [32] | Prospective 2021 | 28 incident cancer 7 prevalent cancer | Timing of colonoscopy does not modify cancer risk |
| Bultman SJ [33] | Retrospective 2021 | 325 removed lesions | Malignant-transformation interval is similar in all segments of the colon |
| Authors | Study Type | Patients Enrolled | Results |
|---|---|---|---|
| Møller P et al. [11] | Prospective observational 2017 | 6350 | Cancer risk related to specific gene mutation and gender of individuals with LS |
| Dominguez-Valentin M, et al. [24] | Prospective observational 2020 | 6350 | Cancer risk related to specific gene mutation, gender, and age of individuals with LS |
| Kastrinos F et al. [36] | Simulation model 2021 | 6350 | CRC surveillance intervals on the basis of the type of specific gene mutation |
| Authors | Study Type | Patients Enrolled | Results |
|---|---|---|---|
| Argillander TE et al. [42] | Retrospective 2018 | 905 | Fast progression of adenoma ≥ carcinoma sequence |
| Ballester V et al. [53] | Sequential case–control 2020 | 53 | Potential use of MDMs (methylated DNA markers in screening and surveillance) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, V.; Rega, D.; Marone, P.; Di Girolamo, E.; Civiletti, C.; Tatangelo, F.; Duraturo, F.; De Rosa, M.; de Bellis, M.; Delrio, P. The Role of Colonoscopy in the Management of Individuals with Lynch Syndrome: A Narrative Review. Cancers 2023, 15, 3780. https://doi.org/10.3390/cancers15153780
D’Angelo V, Rega D, Marone P, Di Girolamo E, Civiletti C, Tatangelo F, Duraturo F, De Rosa M, de Bellis M, Delrio P. The Role of Colonoscopy in the Management of Individuals with Lynch Syndrome: A Narrative Review. Cancers. 2023; 15(15):3780. https://doi.org/10.3390/cancers15153780
Chicago/Turabian StyleD’Angelo, Valentina, Daniela Rega, Pietro Marone, Elena Di Girolamo, Corrado Civiletti, Fabiana Tatangelo, Francesca Duraturo, Marina De Rosa, Mario de Bellis, and Paolo Delrio. 2023. "The Role of Colonoscopy in the Management of Individuals with Lynch Syndrome: A Narrative Review" Cancers 15, no. 15: 3780. https://doi.org/10.3390/cancers15153780
APA StyleD’Angelo, V., Rega, D., Marone, P., Di Girolamo, E., Civiletti, C., Tatangelo, F., Duraturo, F., De Rosa, M., de Bellis, M., & Delrio, P. (2023). The Role of Colonoscopy in the Management of Individuals with Lynch Syndrome: A Narrative Review. Cancers, 15(15), 3780. https://doi.org/10.3390/cancers15153780








