The Immune Cells in the Development of Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Immune Cells of the Tumor Microenvironment in Oral Cancer Development and Progression
2.1. T Lymphocytes
2.2. Macrophages
2.3. Dendritic Cells
2.4. Mast Cells
2.5. Myeloid-Derived Suppressive Cells (MDSCs)
2.6. Neutrophils and Eosinophils
2.7. The Immune Function of Cancer-Associated Fibroblasts
3. Immunopathogenic Mechanisms in OSCC and Precursor Lesions
3.1. Acquisition of Tolerance during the Progression to Malignancy
3.2. Expression of Immune Checkpoint Markers
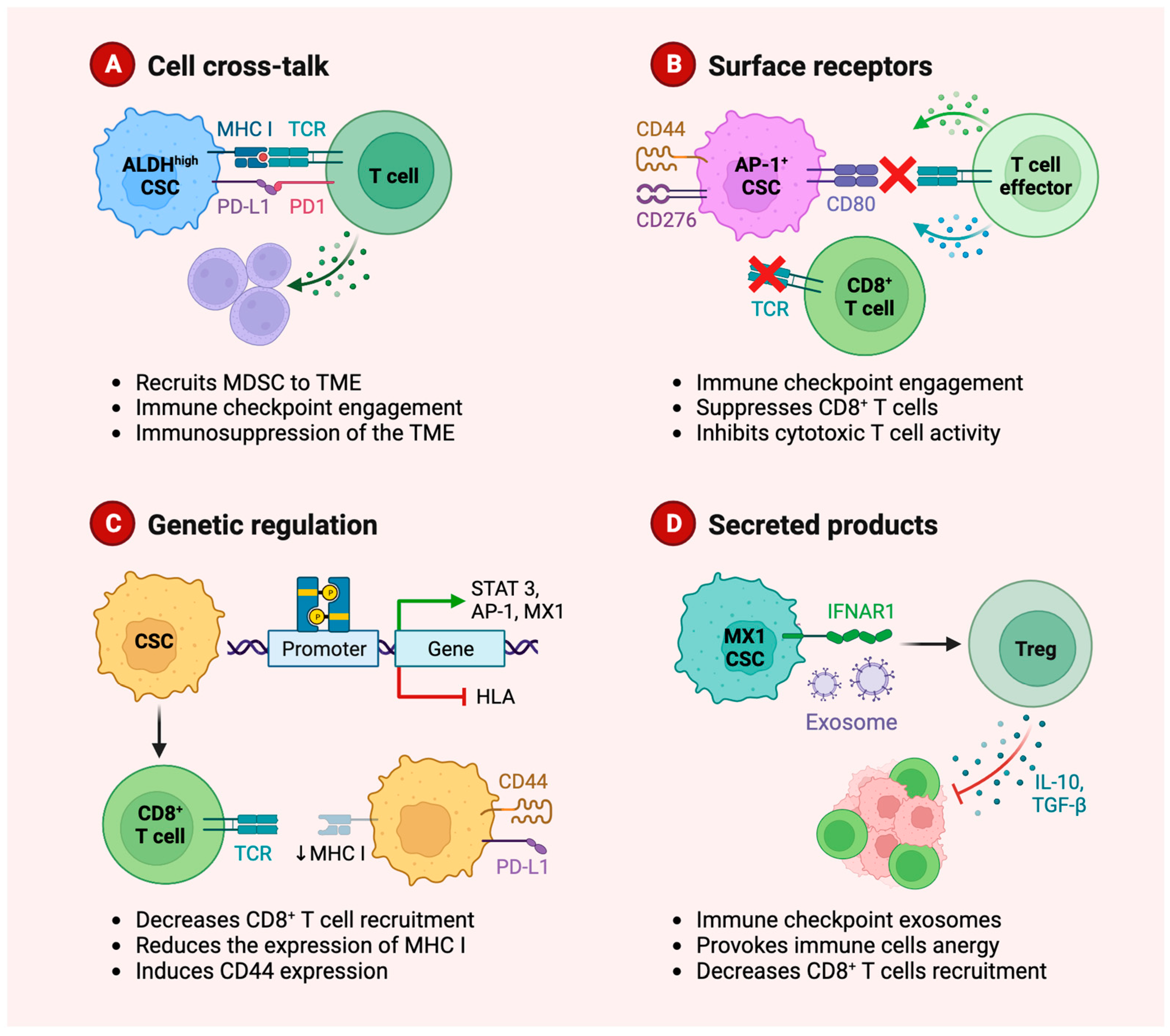
3.3. Role of Cancer Stem Cells in Immune Evasion
3.4. Role of Immune Modulatory Cytokines
4. Clinical Significance of Immune Biomarkers in OSCC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pahwa, V.; Nair, S.; Shetty, R.S.; Kamath, A. Prevalence of Oral Premalignant Lesions and Its Risk Factors among the Adult Population in Udupi Taluk of Coastal Karnataka, India. Asian Pac. J. Cancer Prev. 2018, 19, 2165–2170. [Google Scholar]
- Perez-Sayans, M.; Somoza-Martin, J.M.; Barros-Angueira, F.; Reboiras-Lopez, M.D.; Gandara Rey, J.M.; Garcia-Garcia, A. Genetic and molecular alterations associated with oral squamous cell cancer (Review). Oncol. Rep. 2009, 22, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Freeman, P.; Mielgo, A. Cancer-Associated Fibroblast Mediated Inhibition of CD8+ Cytotoxic T Cell Accumulation in Tumours: Mechanisms and Therapeutic Opportunities. Cancers 2020, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Beacham, D.A.; Cukierman, E. Stromagenesis: The changing face of fibroblastic microenvironments during tumor progression. Semin. Cancer Biol. 2005, 15, 329–341. [Google Scholar] [CrossRef]
- Lim, K.P.; Cirillo, N.; Hassona, Y.; Wei, W.; Thurlow, J.K.; Cheong, S.C.; Pitiyage, G.; Parkinson, E.K.; Prime, S.S. Fibroblast gene expression profile reflects the stage of tumour progression in oral squamous cell carcinoma. J. Pathol. 2011, 223, 459–469. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Lim, K.P.; Herman, A.; Mellone, M.; Thomas, G.J.; Pitiyage, G.N.; Parkinson, E.K.; Prime, S.S. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-β. Carcinogenesis 2013, 34, 1286–1295. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010, 29, 309–316. [Google Scholar] [CrossRef]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFalpha Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar] [CrossRef]
- Yagyuu, T.; Hatakeyama, K.; Imada, M.; Kurihara, M.; Matsusue, Y.; Yamamoto, K.; Obayashi, C.; Kirita, T. Programmed death ligand 1 (PD-L1) expression and tumor microenvironment: Implications for patients with oral precancerous lesions. Oral. Oncol. 2017, 68, 36–43. [Google Scholar] [CrossRef]
- Cirillo, N.; Morgan, D.J.; Pedicillo, M.C.; Celentano, A.; Lo Muzio, L.; McCullough, M.J.; Prime, S.S. Characterisation of the cancer-associated glucocorticoid system: Key role of 11β-hydroxysteroid dehydrogenase type 2. Br. J. Cancer 2017, 117, 984–993. [Google Scholar] [CrossRef]
- Burnet, M. Cancer: A biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1957, 1, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Kouketsu, A.; Sato, I.; Oikawa, M.; Shimizu, Y.; Saito, H.; Tashiro, K.; Yamashita, Y.; Takahashi, T.; Kumamoto, H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral. Maxillofac. Surg. 2019, 48, 1279–1288. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Migliorati, E.K.; Silverman, S.; Jr Greenspan, D.; Greenspan, J.S. Phenotypic identification of mononuclear cells in oral premalignant lesions and cancer by monoclonal antibodies. J. Oral. Pathol. 1986, 15, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Agag, M.; Smaga, M.; Vaishnaw, Y.; Idrees, M.; Shearston, K.; Farah, C.S. PD-1/PD-L1, Treg-related proteins, and tumour-infiltrating lymphocytes are associated with the development of oral squamous cell carcinoma. Pathology 2021, 54, 409–416. [Google Scholar] [CrossRef]
- Bondad-Palmario, G.G. Histological and immunochemical studies of oral leukoplakia: Phenotype and distribution of immunocompetent cells. J. Philipp. Dent. Assoc. 1995, 47, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hidalgo, A.; Murrah, V.; Fedoriw, Y.; Padilla, R.J. Relationship of infiltrating intraepithelial T lymphocytes in the diagnosis of oral lichen planus versus oral epithelial dysplasia: A pilot study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2019, 127, e123–e135. [Google Scholar] [CrossRef] [PubMed]
- Gannot, G.; Gannot, I.; Vered, H.; Buchner, A.; Keisari, Y. Increase in immune cell infiltration with progression of oral epithelium from hyperkeratosis to dysplasia and carcinoma. Br. J. Cancer 2002, 86, 1444–1448. [Google Scholar] [CrossRef]
- Loning, T.; Burkhardt, A. Plasma cells and immunoglobulin-synthesis in oral precancer and cancer. Correlation with dysplasia, cancer differentiation, radio- and chemotherapy. Virchows Arch. A Pathol. Anat. Histol. 1979, 384, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Bergmann, C.; Szczepanski, M.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007, 13 Pt 1, 4345–4354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; Han, J.; Qiu, X.; Pan, J.; Chen, J. Increased frequency of CD4+ CD25+ FOXP3+ cells correlates with the progression of 4-nitroquinoline1-oxide-induced rat tongue carcinogenesis. Clin. Oral. Investig. 2014, 18, 1725–1730. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression Induced by Chronic Inflammation and the Progression to Oral Squamous Cell Carcinoma. Mediators Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Lee, B.K.B.; Lau, S.H.; Kallarakkal, T.G.; Zaini, Z.M.; Zain, R.B.; Sathasivam, H.P.; Yeong, J.P.S.; Savelyeva, N.; Thomas, G.; et al. 911 Immune profiling reveals enrichment of distinct immune signatures in high-risk oral potentially malignant disorders. J. ImmunoTherapy Cancer 2021, 9, A957. [Google Scholar] [CrossRef]
- Chang, D.T.; Jones, J.A.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Lymphocyte/macrophage interactions: Biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J. Biomed. Mater. Res. A 2008, 87, 676–687. [Google Scholar] [CrossRef]
- Kawsar, H.I.; Weinberg, A.; Hirsch, S.A.; Venizelos, A.; Howell, S.; Jiang, B.; Jin, G. Overexpression of human beta-defensin-3 in oral dysplasia: Potential role in macrophage trafficking. Oral. Oncol. 2009, 45, 696–702. [Google Scholar] [CrossRef]
- Rangel, R.; Pickering, C.R.; Sikora, A.G.; Spiotto, M.T. Genetic Changes Driving Immunosuppressive Microenvironments in Oral Premalignancy. Front. Immunol. 2022, 13, 840923. [Google Scholar] [CrossRef]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Öztürk, H.; Neubauer, K.; Wickenhauser, C.; Kesting, M.; Ries, J. Malignant transformation of oral leukoplakia is associated with macrophage polarization. J. Transl. Med. 2020, 18, 11. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.I.; Nishio, M.; Komori, T.; Yokozaki, H. CD163(+) macrophages infiltration correlates with the immunosuppressive cytokine interleukin 10 expression in tongue leukoplakia. Clin. Exp. Dent. Res. 2019, 5, 627–637. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.I.; Kanzawa, M.; Akashi, M.; Yokozaki, H. Intraepithelial Macrophage Expressing CD163 Is a Histopathological Clue to Evaluate the Malignant Potency of Oral Lichenoid Condition: A Case Report and Immunohistochemical Investigation. Diagnostics 2020, 10, 624. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- Yagyuu, T.; Funayama, N.; Imada, M.; Kirita, T. Effect of smoking status and programmed death-ligand 1 expression on the microenvironment and malignant transformation of oral leukoplakia: A retrospective cohort study. PLoS ONE 2021, 16, e0250359. [Google Scholar] [CrossRef] [PubMed]
- Bouaoud, J.; Foy, J.-P.; Tortereau, A.; Michon, L.; Lavergne, V.; Gadot, N.; Boyault, S.; Valantin, J.; De Souza, G.; Zrounba, P.; et al. Early changes in the immune microenvironment of oral potentially malignant disorders reveal an unexpected association of M2 macrophages with oral cancer free survival. Oncoimmunology 2021, 10, 1944554. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Haraguchi, S.; Hiori, M.; Shimada, J.; Ohmori, Y. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer 2015, 15, 573. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Sun, J.; Wang, T.; Liu, Q.; Wu, G.; Qian, Y.; Yang, W.; Wang, Y.; Wang, W. Cigarette smoke promotes oral leukoplakia via regulating glutamine metabolism and M2 polarization of macrophage. Int. J. Oral. Sci. 2021, 13, 25. [Google Scholar] [CrossRef]
- Stasikowska-Kanicka, O.; Wagrowska-Danilewicz, M.; Danilewicz, M. T cells are involved in the induction of macrophage phenotypes in oral leukoplakia and squamous cell carcinoma-a preliminary report. J. Oral. Pathol. Med. 2018, 47, 136–143. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, J.; Lu, R.; Zhou, G. Signal regulatory protein alpha associated with the progression of oral leukoplakia and oral squamous cell carcinoma regulates phenotype switch of macrophages. Oncotarget 2016, 7, 81305–81321. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.I.; Nishio, M.; Akashi, M.; Yokozaki, H. Alteration of Macrophage Infiltrating Compartment: A Novel View on Oral Carcinogenesis. Pathobiology 2021, 88, 327–337. [Google Scholar] [CrossRef]
- Mori, K.; Hiroi, M.; Shimada, J.; Ohmori, Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Shigeoka, M.; Koma, Y.I.; Kodama, T.; Nishio, M.; Akashi, M.; Yokozaki, H. Intraepithelial CD163(+) macrophages in tongue leukoplakia biopsy: A promising tool for cancer screening. Oral. Dis. 2020, 26, 527–536. [Google Scholar] [CrossRef]
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Franklin, R.A.; Luo, C.T.; Oh, S.A.; Toure, A.; Pritykin, Y.; Huse, M.; Leslie, C.S.; et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 2016, 164, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 2002, 195, F9–F14. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Chen, I.C.; Wu, Y.H.; Wu, Y.C.; Chen, H.M.; Yu-Fong Chang, J. Langerhans cell counts in oral epithelial dysplasia and their correlation to clinicopathological parameters. J. Formos. Med. Assoc. 2017, 116, 457–463. [Google Scholar] [CrossRef]
- Araújo, C.P.; Gurgel, C.A.S.; Ramos, E.A.G.; Freitas, V.S.; Júnior, A.d.A.B.; Ramalho, L.M.P.; dos Santos, J.N. Accumulation of CD1a-positive Langerhans cells and mast cells in actinic cheilitis. J. Mol. Histol. 2010, 41, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Pottier, C.; Larsimont, D.; Journé, F.; Delvenne, P.; Saussez, S. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral. Oncol. 2016, 62, 1–10. [Google Scholar] [CrossRef]
- Rani, S.V.; Aravindha, B.; Leena, S.; Balachander, N.; Malathi, L.K.; Masthan, M.K. Role of abnormal Langerhans cells in oral epithelial dysplasia and oral squamous cell carcinoma: A pilot study. J. Nat. Sci. Biol. Med. 2015, 6 (Suppl. 1), S128–S133. [Google Scholar] [CrossRef]
- Upadhyay, J.; Rao, N.N.; Upadhyay, R.B. A comparative analysis of langerhans cell in oral epithelial dysplasia and oral squamous cell carcinoma using antibody CD-1a. J. Cancer Res. Ther. 2012, 8, 591–597. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Fonseca, F.P.; de Almeida, O.P.; de Almeida Mariz, B.A.L.; Lopes, M.A.; Radhakrishnan, R.; Sharma, M.; Kowalski, L.P.; Vargas, P.A. CD1a+ and CD207+ cells are reduced in oral submucous fibrosis and oral squamous cell carcinoma. Med. Oral. Patol. Oral. Cir. Bucal 2020, 25, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Telagi, N.; Ahmed Mujib, B.R.; Kulkarni, P.G.; Naik, R. The master switch: Comparative study of mast cell in oral epithelial dysplasia, oral submucous fibrosis and oral squamous cells carcinoma and their association with inflammation and angiogenesis. J. Oral. Maxillofac. Pathol. 2015, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Madhuri RAnkle, A.D.K.; Nayak, R. Mast cells are increased in leukoplakia, oral submucous fibrosis, oral lichen planus and oral squamous cell carcinoma. J. Oral. Maxillofac. Pathol. 2007, 11, 18–22. [Google Scholar]
- Bhatt, A.; Dholakia, H. Mast cell density in oral submucous fibrosis. J. Indian. Dent. Assoc. 1977, 49, 187–191. [Google Scholar]
- Zhao, Z.Z.; Savage, N.W.; Pujic, Z.; Walsh, L.J. Immunohistochemical localization of mast cells and mast cell-nerve interactions in oral lichen planus. Oral. Dis. 1997, 3, 71–76. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Sugerman, P.B.; Zhou, X.J.; Walsh, L.J.; Savage, N.W. Mast cell degranulation and the role of T cell RANTES in oral lichen planus. Oral. Dis. 2001, 7, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Jontell, M.; Hansson, H.A.; Nygren, H. Mast cells in oral lichen planus. J. Oral. Pathol. 1986, 15, 273–275. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Savage, N.W.; Sugerman, P.B.; Walsh, L.J. Mast cell/T cell interactions in oral lichen planus. J. Oral. Pathol. Med. 2002, 31, 189–195. [Google Scholar] [CrossRef]
- Ashish Shrestha, S.K.; Raut, T. Evaluation of Mast Cells in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Int. J. Dent. 2021, 2021, 5. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef]
- Dvorak, A.M. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem. Immunol. Allergy 2005, 85, 135–184. [Google Scholar] [PubMed]
- Coussens, L.M.; Raymond, W.W.; Bergers, G.; Laig-Webster, M.; Behrendtsen, O.; Werb, Z.; Caughey, G.H.; Hanahan, D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes. Dev. 1999, 13, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, E.Z.; Markopoulos, A.K.; Antoniades, D.Z. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent. J. 2008, 2, 126–132. [Google Scholar] [CrossRef]
- Iamaroon, A.; Pongsiriwet, S.; Jittidecharaks, S.; Pattanaporn, K.; Prapayasatok, S.; Wanachantararak, S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2003, 32, 195–199. [Google Scholar] [CrossRef]
- Saxena, S.; Singh, A.; Singh, P.; Sundaragiri, K.S.; Sankhla, B.; Bhargava, A. Evaluating the Role of Immunological Cells (Tissue Eosinophils and Mast Cells) in Progression of Oral Squamous Cell Carcinoma. Mymensingh Med. J. 2018, 27, 382–388. [Google Scholar]
- Laishram, D.; Rao, K.; Devi, H.S.U.; Priya, N.S.; Smitha, T.; Sheethal, H.S. Mast cells and angiogenesis in malignant and premalignant oral lesions: An immunohistochemical study. J. Oral. Maxillofac. Pathol. 2017, 21, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jyothsna, M.; Rammanohar, M.; Kumar, K. Histomorphometric Analysis of Angiogenesis using CD31 Immunomarker and Mast Cell Density in Oral Premalignant and Malignant Lesions: A Pilot Study. J. Clin. Diagn. Res. 2017, 11, ZC37–ZC40. [Google Scholar] [CrossRef]
- Ramsridhar, S.; Narasimhan, M. Immunohistochemical Evaluation of Mast Cells in Leukoplakia and Oral Squamous Cell Carcinoma. J. Clin. Diagn. Res. 2016, 10, ZC100–ZC103. [Google Scholar] [CrossRef]
- Sathyakumar, M.; Sriram, G.; Saraswathi, T.; Sivapathasundharam, B. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral precancerous lesion-leukoplakia. J. Oral. Maxillofac. Pathol. 2012, 16, 343–348. [Google Scholar] [CrossRef]
- Kinra, M.; Ramalingam, K.; Sarkar, A.; Rehman, F.; Girish, K. Comparison of mast cell count and mast cell density in normal mucosa, oral leukoplakia, oral lichen planus, oral submucous fibrosis and oral squamous cell carcinoma–a study on 50cases. J. Pharm. Sci. Inn. 2012, 1, 4–11. [Google Scholar]
- Sabarinath, B.; Sriram, G.; Saraswathi, T.R.; Sivapathasundharam, B. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral submucous fibrosis. Indian. J. Dent. Res. 2011, 22, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Neto, H.H.; Leite, A.F.; Costa, N.L.; Alencar, R.C.; Lara, V.S.; Silva, T.A.; Leles, C.R.; Mendonça, F.E.; Batista, A.C. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral. Oncol. 2007, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, V.; Vij, R.; Aggarwal, R.; Sharma, B.; Nagpal, M. Evaluation of mast cells in oral premalignant and malignant lesions: A histochemical study. Natl. J. Maxillofac. Surg. 2018, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fan, H.-Y.; Tang, Y.-L.; Wang, S.-S.; Cao, M.-X.; Wang, H.-F.; Dai, L.-L.; Wang, K.; Yu, X.-H.; Wu, J.-B.; et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef]
- Wen, L.; Mu, W.; Lu, H.; Wang, X.; Fang, J.; Jia, Y.; Li, Q.; Wang, D.; Wen, S.; Guo, J.; et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020, 99, 666–675. [Google Scholar] [CrossRef]
- Bhaskaran, N.; Jayaraman, S.; Quigley, C.; Mamileti, P.; Ghannoum, M.; Weinberg, A.; Thuener, J.; Pan, Q.; Pandiyan, P. The Role of Dectin-1 Signaling in Altering Tumor Immune Microenvironment in the Context of Aging. Front. Oncol. 2021, 11, 669066. [Google Scholar] [CrossRef]
- Madhura, M.G.; Gajalakshmi, S.; Kumar, B.V.; Suma, S.; Sarita, Y.; Shweta, R.D. Role of tissue eosinophils in oral Leukoplakia: A pilot study. J. Oral. Maxillofac. Pathol. 2015, 19, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Garley, M.; Surazynski, A.; Grubczak, K.; Iwaniuk, A.; Borys, J.; Moniuszko, M.; Ratajczak-Wrona, W. Neutrophil extracellular traps (NETs) formation induced by TGF-beta in oral lichen planus—Possible implications for the development of oral cancer. Immunobiology 2020, 225, 151901. [Google Scholar] [CrossRef]
- Jain, M.; Kasetty, S.; Sudheendra, U.S.; Tijare, M.; Khan, S.; Desai, A. Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Patholog Res. Int. 2014, 2014, 507512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deepthi, G.; Kulkarni, P.G. SRKN Eosinophils: An imperative histopathological prognostic indicator for oral squamous cell carcinoma. J. Oral. Maxillofac. Pathol. 2019, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallegos, D.; Jiang, D.; Rinkevich, Y. Fibroblasts as confederates of the immune system. Immunol. Rev. 2021, 302, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernandez, L.A.; Gomez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chansard, A.; Dubrulle, N.; De Molliens, M.P.; Falanga, P.B.; Stephen, T.; Hasan, M.; Van Zandbergen, G.; Aulner, N.; Shorte, S.L.; David-Watine, B. Unveiling Interindividual Variability of Human Fibroblast Innate Immune Response Using Robust Cell-Based Protocols. Front. Immunol. 2020, 11, 569331. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.; Sainson, R.C. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin. Cancer Biol. 2014, 25, 69–77. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Chaudhary, M.; Gadbail, A.R.; Vidhale, G.; Mankar, M.P.; Gondivkar, S.M.; Gawande, M.; Patil, S. Comparison of myofibroblasts expression in oral squamous cell carcinoma, verrucous carcinoma, high risk epithelial dysplasia, low risk epithelial dysplasia and normal oral mucosa. Head Neck Pathol. 2012, 6, 305–313. [Google Scholar] [CrossRef]
- Gupta, K.; Metgud, R.; Gupta, J. Evaluation of stromal myofibroblasts in oral leukoplakia, oral submucous fibrosis, and oral squamous cell carcinoma--an immunohistochemical study. J. Cancer Res. Ther. 2015, 11, 893–898. [Google Scholar] [CrossRef]
- Angadi, P.V.; Kale, A.D.; Hallikerimath, S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J. Oral. Pathol. Med. 2011, 40, 208–213. [Google Scholar] [CrossRef]
- Kapse, S.C.; Rathod, N.; Baad, R.; Mandlik, J.; Sharma, A.S.; Bommanavar, S. Quantitative assessment of myofibroblast in severe dysplasia, microinvasion and oral squamous cell carcinoma: An immunohistochemical study. J. Contemp. Dent. Pract. 2013, 14, 34–38. [Google Scholar]
- Vered, M.; Allon, I.; Buchner, A.; Dayan, D. Stromal myofibroblasts accompany modifications in the epithelial phenotype of tongue dysplastic and malignant lesions. Cancer Microenviron. 2009, 2, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, G.; Sherlin, H.J.; Ramani, P.; Premkumar, P.; Natesan, A. Stromal myofibroblasts in oral squamous cell carcinoma and potentially malignant disorders. Indian. J. Cancer 2015, 52, 87–92. [Google Scholar] [CrossRef] [PubMed]
- de-Assis, E.M.; Pimenta, L.G.; Costa-e-Silva, E.; Souza, P.E.; Horta, M.C. Stromal myofibroblasts in oral leukoplakia and oral squamous cell carcinoma. Med. Oral. Patol. Oral. Cir. Bucal 2012, 17, e733–e738. [Google Scholar] [CrossRef][Green Version]
- Etemad-Moghadam, S.; Khalili, M.; Tirgary, F.; Alaeddini, M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J. Oral. Pathol. Med. 2009, 38, 639–643. [Google Scholar] [CrossRef]
- Parajuli, H.; Teh, M.-T.; Abrahamsen, S.; Christoffersen, I.; Neppelberg, E.; Lybak, S.; Osman, T.; Johannessen, A.C.; Gullberg, D.; Skarstein, K.; et al. Integrin alpha11 is overexpressed by tumour stroma of head and neck squamous cell carcinoma and correlates positively with alpha smooth muscle actin expression. J. Oral. Pathol. Med. 2017, 46, 267–275. [Google Scholar] [CrossRef]
- Coletta, R.D.; Salo, T. Myofibroblasts in oral potentially malignant disorders: Is it related to malignant transformation? Oral. Dis. 2018, 24, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Dayan, D.; Salo, T.; Salo, S.; Nyberg, P.; Nurmenniemi, S.; Costea, D.E.; Vered, M. Molecular crosstalk between cancer cells and tumor microenvironment components suggests potential targets for new therapeutic approaches in mobile tongue cancer. Cancer Med. 2012, 1, 128–140. [Google Scholar] [CrossRef]
- Kellermann, M.G.; Sobral, L.M.; da Silva, S.D.; Zecchin, K.G.; Graner, E.; Lopes, M.A.; Kowalski, L.P.; Coletta, R.D. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral. Oncol. 2008, 44, 509–517. [Google Scholar] [CrossRef]
- Kawashiri, S.; Tanaka, A.; Noguchi, N.; Hase, T.; Nakaya, H.; Ohara, T.; Kato, K.; Yamamoto, E. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck 2009, 31, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Kelner, N.; Rodrigues, P.C.; Bufalino, A.; Fonseca, F.P.; dos Santos-Silva, A.R.; Miguel, M.C.C.; Pinto, C.A.L.; Leme, A.F.P.; Graner, E.; Salo, T.; et al. Activin A immunoexpression as predictor of occult lymph node metastasis and overall survival in oral tongue squamous cell carcinoma. Head Neck 2015, 37, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, F.; De Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. 2011: The immune hallmarks of cancer. Cancer Immunol Immunother 2011, 60, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Tosetti, F.; Benelli, R.; Noonan, D.M. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005, 65, 10637–10641. [Google Scholar] [CrossRef]
- Duray, A.; Demoulin, S.; Hubert, P.; Delvenne, P.; Saussez, S. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010, 2010, 701657. [Google Scholar] [CrossRef]
- Ries, J.; Agaimy, A.; Vairaktaris, E.; Kwon, Y.; Neukam, F.W.; Strassburg, L.H.; Nkenke, E. Evaluation of MAGE-A expression and grade of dysplasia for predicting malignant progression of oral leukoplakia. Int. J. Oncol. 2012, 41, 1085–1093. [Google Scholar] [CrossRef]
- Young, M.R.; Neville, B.W.; Chi, A.C.; Lathers, D.M.; Boyd Gillespie, M.; Day, T.A. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2007, 56, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Lee, N.V.; Liu, K.Y.; Poh, C.F. Prospects in the Application of Photodynamic Therapy in Oral Cancer and Premalignant Lesions. Cancers 2016, 8, 83. [Google Scholar] [CrossRef]
- Poh, C.M.; Zheng, J.; Channappanavar, R.; Chang, Z.W.; Nguyen, T.H.O.; Rénia, L.; Kedzierska, K.; Perlman, S.; Poon, L.L.M. Multiplex Screening Assay for Identifying Cytotoxic CD8(+) T Cell Epitopes. Front. Immunol. 2020, 11, 400. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Elmusrati, A.; Wang, J.; Wang, C.Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral. Sci. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lutz, R.; Olmos, M.; Glajzer, J.; Baran, C.; Nobis, C.P.; Möst, T.; Eckstein, M.; Kesting, M.; Ries, J. Beyond PD-L1-Identification of Further Potential Therapeutic Targets in Oral Cancer. Cancers 2022, 14, 1812. [Google Scholar] [CrossRef]
- Cirillo, N.; Wu, C.; Prime, S.S. Heterogeneity of Cancer Stem Cells in Tumorigenesis, Metastasis, and Resistance to Antineoplastic Treatment of Head and Neck Tumours. Cells 2021, 10, 3068. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.M.; Xia, W.; Hsu, Y.H.; Chan, L.C.; Yu, W.H.; Cha, J.H.; Chen, C.T.; Liao, H.W.; Kuo, C.W.; Khoo, K.H.; et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018, 9, 1908. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, J.H.; Longmire, M.; Wang, H.; Kohrt, H.E.; Chang, H.Y.; Sunwoo, J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin. Cancer Res. 2016, 22, 3571–3581. [Google Scholar] [CrossRef]
- Lechner, A.; Schlößer, H.; Rothschild, S.I.; Thelen, M.; Reuter, S.; Zentis, P.; Shimabukuro-Vornhagen, A.; Theurich, S.; Wennhold, K.; Garcia-Marquez, M.; et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 44418–44433. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Huang, W.; Zhong, L.L.D.; Wang, N.; Wang, S.; Yang, B.; Wang, X.; Pan, B.; Situ, H.; et al. Sini San Inhibits Chronic Psychological Stress-Induced Breast Cancer Stemness by Suppressing Cortisol-Mediated GRP78 Activation. Front. Pharmacol. 2021, 12, 714163. [Google Scholar] [CrossRef]
- Ponandai-Srinivasan, S.; Lalitkumar, P.G.; Garcia, L.; Varghese, S.J.; Carlson, J.W.; Gemzell-Danielsson, K.; Floter Radestad, A. Mifepristone mediates anti-proliferative effect on ovarian mesenchymal stem/stromal cells from female BRCA1−/2− carriers. Acta Obstet. Gynecol. Scand. 2019, 98, 250–261. [Google Scholar] [CrossRef]
- Chimal-Ramírez, G.K.; Espinoza-Sánchez, N.A.; Fuentes-Pananá, E.M. Protumor activities of the immune response: Insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J. Oncol. 2013, 2013, 835956. [Google Scholar] [CrossRef]
- Kondoh, N.; Mizuno-Kamiya, M. The Role of Immune Modulatory Cytokines in the Tumor Microenvironments of Head and Neck Squamous Cell Carcinomas. Cancers 2022, 14, 2884. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef]
- Hassona, Y.; Cirillo, N.; Heesom, K.; Parkinson, E.K.; Prime, S.S. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br. J. Cancer 2014, 111, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Hassona Zhang, P.; Chua, N.Q.E.; Dang, S.; Davis, A.; Chong, K.W.; Prime, S.S.; Cirillo, N. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int. J. Mol. Sci. 2022, 23, 1637. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of cancer-associated immune cells in human solid tumors. eLife 2018, 7, e36967. [Google Scholar] [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
- Almangush, A.; De Keukeleire, S.; Rottey, S.; Ferdinande, L.; Vermassen, T.; Leivo, I.; Mäkitie, A.A. Tumor-Infiltrating Lymphocytes in Head and Neck Cancer: Ready for Prime Time? Cancers 2022, 14, 1558. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Lo Russo, L.; Cirillo, N.; Lo Muzio, L. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral. Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef]
- Mariani, P.; Russo, D.; Maisto, M.; Troiano, G.; Caponio, V.C.A.; Annunziata, M.; Laino, L. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J. Oral. Pathol. Med. 2022, 51, 39–51. [Google Scholar] [CrossRef]
- Riley, R.D.; Hayden, J.A.; Steyerberg, E.W.; Moons, K.G.; Abrams, K.; Kyzas, P.A.; Malats, N.; Briggs, A.; Schroter, S.; Altman, D.G.; et al. Prognosis Research Strategy (PROGRESS) 2, prognostic factor research. PLoS Med. 2013, 10, e1001380. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Moons, K.G.; van der Windt, D.A.; Hayden, J.A.; Perel, P.; Schroter, S.; Riley, R.D.; Hemingway, H.; Altman, D.G.; PROGRESS Group. Prognosis Research Strategy (PROGRESS) 3, prognostic model research. PLoS Med. 2013, 10, e1001381. [Google Scholar] [CrossRef]
- Russo, D.; Mariani, P.; Caponio, V.C.A.; Lo Russo, L.; Fiorillo, L.; Zhurakivska, K.; Lo Muzio, L.; Laino, L.; Troiano, G. Development and Validation of Prognostic Models for Oral Squamous Cell Carcinoma: A Systematic Review and Appraisal of the Literature. Cancers 2021, 13, 5755. [Google Scholar] [CrossRef] [PubMed]
- Almangush, A.; Bello, I.O.; Heikkinen, I.; Hagström, J.; Haglund, C.; Kowalski, L.P.; Coletta, R.D.; Mäkitie, A.A.; Salo, T.; Leivo, I. Improving Risk Stratification of Early Oral Tongue Cancer with TNM-Immune (TNM-I) Staging System. Cancers 2021, 13, 3235. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponio, V.C.A.; Zhurakivska, K.; Lo Muzio, L.; Troiano, G.; Cirillo, N. The Immune Cells in the Development of Oral Squamous Cell Carcinoma. Cancers 2023, 15, 3779. https://doi.org/10.3390/cancers15153779
Caponio VCA, Zhurakivska K, Lo Muzio L, Troiano G, Cirillo N. The Immune Cells in the Development of Oral Squamous Cell Carcinoma. Cancers. 2023; 15(15):3779. https://doi.org/10.3390/cancers15153779
Chicago/Turabian StyleCaponio, Vito Carlo Alberto, Khrystyna Zhurakivska, Lorenzo Lo Muzio, Giuseppe Troiano, and Nicola Cirillo. 2023. "The Immune Cells in the Development of Oral Squamous Cell Carcinoma" Cancers 15, no. 15: 3779. https://doi.org/10.3390/cancers15153779
APA StyleCaponio, V. C. A., Zhurakivska, K., Lo Muzio, L., Troiano, G., & Cirillo, N. (2023). The Immune Cells in the Development of Oral Squamous Cell Carcinoma. Cancers, 15(15), 3779. https://doi.org/10.3390/cancers15153779









