Brain Plasticity Profiling as a Key Support to Therapeutic Decision-Making in Low-Grade Glioma Oncological Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Treatment Goals in LGGs Clinical Management
2.1. Functional Goals
2.2. Oncological Goals
2.3. Toward an Individual Onco-Functional Balance
3. Profiling Individual Brain Plasticity in the Context of LGG
3.1. The Location of the Tumor within Brain Architecture and the Level of Infiltration within Critical WM Tracts
3.2. The Velocity of Tumor Expansion
3.3. Cognitive Compensation
4. The Plasticity Potential May Interfere with the Benefits and Risk of Treatment Options
4.1. Surgery
4.2. Chemotherapy
4.3. Radiotherapy
- (1)
- with the advent of functional-guided surgical techniques, residual tumor volumes targeted by radiation therapies inevitably consist in highly functional areas with restricted potential for plastic compensation [66], especially within the WM pathways forming the “minimal common brain” [16], which is essential to maintaining long-term cognitive compensation.
- (2)
- there are now sufficient evidence that radiotherapy leads to alterations of the WM microstructure through demyelination and axonal degeneration mechanisms [117], which can easily be measured with modern neuroimaging tools (e.g., diffusion tensor imaging) [118,119]. The resulting structural and functional connectivity alterations may be correlated with early [120] and long-term [121] cognitive performance, although brain regional susceptibilities are still poorly documented [122].
4.4. Other Therapies
5. Proposal of a Multistage and Individualized Workflow Accounting for the Plasticity Potential
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Pallud, J.; Mandonnet, E.; Duffau, H.; Kujas, M.; Guillevin, R.; Galanaud, D.; Taillandier, L.; Capelle, L. Prognostic Value of Initial Magnetic Resonance Imaging Growth Rates for World Health Organization Grade II Gliomas. Ann. Neurol. 2006, 60, 380–383. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.-L.; Duan, J.-J.; Yi, L.; Guo, Y.-F.; Shi, Y.; Li, L.; Yang, Z.-Y.; Liao, X.-M.; Cai, J.; et al. Invasion of White Matter Tracts by Glioma Stem Cells Is Regulated by a NOTCH1–SOX2 Positive-Feedback Loop. Nat. Neurosci. 2019, 22, 91–105. [Google Scholar] [CrossRef]
- Zhao, B.; Li, T.; Yang, Y.; Wang, X.; Luo, T.; Shan, Y.; Zhu, Z.; Xiong, D.; Hauberg, M.E.; Bendl, J.; et al. Common Genetic Variation Influencing Human White Matter Microstructure. Science 2021, 372, eabf3736. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Mazor, T.; Chesnelong, C.; Pankov, A.; Jalbert, L.E.; Hong, C.; Hayes, J.; Smirnov, I.V.; Marshall, R.; Souza, C.F.; Shen, Y.; et al. Clonal Expansion and Epigenetic Reprogramming Following Deletion or Amplification of Mutant IDH1. Proc. Natl. Acad. Sci. USA 2017, 114, 10743–10748. [Google Scholar] [CrossRef]
- Ferreyra Vega, S.; Olsson Bontell, T.; Kling, T.; Jakola, A.S.; Carén, H. Longitudinal DNA Methylation Analysis of Adult-Type IDH-Mutant Gliomas. Acta Neuropathol. Commun. 2023, 11, 23. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain Tumour Cells Interconnect to a Functional and Resistant Network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic Synaptic Input to Glioma Cells Drives Brain Tumour Progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and Synaptic Integration of Glioma into Neural Circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef]
- Numan, T.; Breedt, L.C.; Maciel, B.d.A.P.C.; Kulik, S.D.; Derks, J.; Schoonheim, M.M.; Klein, M.; de Witt Hamer, P.C.; Miller, J.J.; Gerstner, E.R.; et al. Regional Healthy Brain Activity, Glioma Occurrence and Symptomatology. Brain 2022, 145, 3654–3665. [Google Scholar] [CrossRef]
- Romero-Garcia, R.; Mandal, A.S.; Bethlehem, R.A.I.; Crespo-Facorro, B.; Hart, M.G.; Suckling, J. Transcriptomic and Connectomic Correlates of Differential Spatial Patterning among Gliomas. Brain 2023, 146, 1200–1211. [Google Scholar] [CrossRef]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional Outcome after Language Mapping for Glioma Resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef]
- Duffau, H. Lessons from Brain Mapping in Surgery for Low-Grade Glioma: Insights into Associations between Tumour and Brain Plasticity. Lancet Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Ius, T.; Angelini, E.; Thiebaut de Schotten, M.; Mandonnet, E.; Duffau, H. Evidence for Potentials and Limitations of Brain Plasticity Using an Atlas of Functional Resectability of WHO Grade II Gliomas: Towards a “Minimal Common Brain”. NeuroImage 2011, 56, 992–1000. [Google Scholar] [CrossRef]
- Ng, S.; Valdes, P.A.; Moritz-Gasser, S.; Lemaitre, A.-L.; Duffau, H.; Herbet, G. Intraoperative Functional Remapping Unveils Evolving Patterns of Cortical Plasticity. Brain 2023, 146, 3088–3100. [Google Scholar] [CrossRef]
- Krishna, S.; Choudhury, A.; Keough, M.B.; Seo, K.; Ni, L.; Kakaizada, S.; Lee, A.; Aabedi, A.; Popova, G.; Lipkin, B.; et al. Glioblastoma Remodelling of Human Neural Circuits Decreases Survival. Nature 2023, 617, 599–607. [Google Scholar] [CrossRef]
- Sprugnoli, G.; Rigolo, L.; Faria, M.; Juvekar, P.; Tie, Y.; Rossi, S.; Sverzellati, N.; Golby, A.J.; Santarnecchi, E. Tumor BOLD Connectivity Profile Correlates with Glioma Patients’ Survival. Neuro-Oncol. Adv. 2022, 4, vdac153. [Google Scholar] [CrossRef]
- Duffau, H. Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy. Cancers 2021, 13, 4759. [Google Scholar] [CrossRef]
- Duffau, H.; Taillandier, L. New Concepts in the Management of Diffuse Low-Grade Glioma: Proposal of a Multistage and Individualized Therapeutic Approach. Neuro-Oncology 2014, 17, 332–342. [Google Scholar] [CrossRef]
- Duffau, H. Surgery of Low-Grade Gliomas: Towards a ‘Functional Neurooncology’. Curr. Opin. Oncol. 2009, 21, 543–549. [Google Scholar] [CrossRef]
- Obara, T.; Blonski, M.; Brzenczek, C.; Mézières, S.; Gaudeau, Y.; Pouget, C.; Gauchotte, G.; Verger, A.; Vogin, G.; Moureaux, J.-M.; et al. Adult Diffuse Low-Grade Gliomas: 35-Year Experience at the Nancy France Neurooncology Unit. Front. Oncol. 2020, 10, 574679. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Darlix, A.; Zouaoui, S.; Rigau, V.; Bessaoud, F.; Figarella-Branger, D.; Mathieu-Daudé, H.; Trétarre, B.; Bauchet, F.; Duffau, H.; Taillandier, L.; et al. Epidemiology for Primary Brain Tumors: A Nationwide Population-Based Study. J. Neuro-Oncol. 2017, 131, 525–546. [Google Scholar] [CrossRef]
- Ng, S.; Zouaoui, S.; Bessaoud, F.; Rigau, V.; Roux, A.; Darlix, A.; Bauchet, F.; Mathieu-Daudé, H.; Trétarre, B.; Figarella-Branger, D.; et al. An Epidemiology Report for Primary Central Nervous System Tumors in Adolescents and Young Adults: A Nationwide Population-Based Study in France, 2008–2013. Neuro-Oncology 2019, 22, 851–863. [Google Scholar] [CrossRef]
- Jakola, A.S.; Skjulsvik, A.J.; Myrmel, K.S.; Sjåvik, K.; Unsgård, G.; Torp, S.H.; Aaberg, K.; Berg, T.; Dai, H.Y.; Johnsen, K.; et al. Surgical Resection versus Watchful Waiting in Low-Grade Gliomas. Ann. Oncol. 2017, 28, 1942–1948. [Google Scholar] [CrossRef]
- Rydén, I.; Carstam, L.; Gulati, S.; Smits, A.; Sunnerhagen, K.S.; Hellström, P.; Henriksson, R.; Bartek, J.; Salvesen, Ø.; Jakola, A.S. Return to Work Following Diagnosis of Low-Grade Glioma: A Nationwide Matched Cohort Study. Neurology 2020, 95, e856–e866. [Google Scholar] [CrossRef]
- Boele, F.W.; den Otter, P.W.M.; Reijneveld, J.C.; de Witt Hamer, P.C.; van Thuijl, H.F.; Lorenz, L.M.C.; Wesseling, P.; Lagerwaard, F.J.; Taphoorn, M.J.B.; Kouwenhoven, M.C.M.; et al. Long-Term Wellbeing and Neurocognitive Functioning of Diffuse Low-Grade Glioma Patients and Their Caregivers: A Longitudinal Study Spanning Two Decades. Neuro-Oncology 2023, 25, 351–364. [Google Scholar] [CrossRef]
- Cochereau, J.; Herbet, G.; Duffau, H. Patients with Incidental WHO Grade II Glioma Frequently Suffer from Neuropsychological Disturbances. Acta Neurochir. 2016, 158, 305–312. [Google Scholar] [CrossRef]
- De Roeck, L.; Gillebert, C.R.; van Aert, R.C.M.; Vanmeenen, A.; Klein, M.; Taphoorn, M.J.B.; Gehring, K.; Lambrecht, M.; Sleurs, C. Cognitive Outcomes after Multimodal Treatment in Adult Glioma Patients: A Meta-Analysis. Neuro-Oncology 2023, noad045. [Google Scholar] [CrossRef]
- Pallud, J.; Audureau, E.; Blonski, M.; Sanai, N.; Bauchet, L.; Fontaine, D.; Mandonnet, E.; Dezamis, E.; Psimaras, D.; Guyotat, J.; et al. Epileptic Seizures in Diffuse Low-Grade Gliomas in Adults. Brain 2014, 137, 449–462. [Google Scholar] [CrossRef]
- Nakajima, R.; Kinoshita, M.; Okita, H.; Nakada, M. Quality of Life Following Awake Surgery Depends on Ability of Executive Function, Verbal Fluency, and Movement. J. Neurooncol. 2022, 156, 173–183. [Google Scholar] [CrossRef]
- Klein, M. Neurocognitive Functioning in Adult WHO Grade II Gliomas: Impact of Old and New Treatment Modalities. Neuro-Oncology 2012, 14, iv17–iv24. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Taphoorn, M.J.B.; Heimans, J.J.; Postma, T.J.; Gundy, C.M.; Beute, G.N.; Slotman, B.J.; Klein, M. Compromised Health-Related Quality of Life in Patients with Low-Grade Glioma. J. Clin. Oncol. 2011, 29, 4430–4435. [Google Scholar] [CrossRef]
- Pascual, J.S.G.; Duffau, H. The Need to Consider Return to Work as a Main Outcome in Patients Undergoing Surgery for Diffuse Low-Grade Glioma: A Systematic Review. Acta Neurochir. 2022, 164, 2789–2809. [Google Scholar] [CrossRef]
- Moritz-Gasser, S.; Herbet, G.; Maldonado, I.L.; Duffau, H. Lexical Access Speed Is Significantly Correlated with the Return to Professional Activities after Awake Surgery for Low-Grade Gliomas. J. Neurooncol. 2012, 107, 633–641. [Google Scholar] [CrossRef]
- Haider, S.A.; Asmaro, K.; Kalkanis, S.N.; Lee, I.Y.; Bazydlo, M.; Nerenz, D.R.; Salloum, R.G.; Snyder, J.; Walbert, T. The Economic Impact of Glioma Survivorship: The Cost of Care from a Patient Perspective. Neurology 2020, 95, e1575–e1581. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Kim, G.H.J.; Brown, M.; Lee, J.; Salamon, N.; Steelman, L.; Hassan, I.; Pandya, S.S.; Chun, S.; Linetsky, M.; et al. Volumetric Measurements Are Preferred in the Evaluation of Mutant IDH Inhibition in Non-Enhancing Diffuse Gliomas: Evidence from a Phase I Trial of Ivosidenib. Neuro-Oncology 2022, 24, 770–778. [Google Scholar] [CrossRef]
- Berger, M.S.; Deliganis, A.V.; Dobbins, J.; Keles, G.E. The Effect of Extent of Resection on Recurrence in Patients with Low Grade Cerebral Hemisphere Gliomas. Cancer 1994, 74, 1784–1791. [Google Scholar] [CrossRef]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and Therapeutic Prognostic Factors in Adult Hemispheric World Health Organization Grade II Gliomas: A Series of 1097 Cases. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef]
- Roelz, R.; Strohmaier, D.; Jabbarli, R.; Kraeutle, R.; Egger, K.; Coenen, V.A.; Weyerbrock, A.; Reinacher, P.C. Residual Tumor Volume as Best Outcome Predictor in Low Grade Glioma—A Nine-Years Near-Randomized Survey of Surgery vs. Biopsy. Sci. Rep. 2016, 6, 32286. [Google Scholar] [CrossRef]
- Ius, T.; Ng, S.; Young, J.S.; Tomasino, B.; Polano, M.; Ben-Israel, D.; Kelly, J.J.P.; Skrap, M.; Duffau, H.; Berger, M.S. The Benefit of Early Surgery on Overall Survival in Incidental Low-Grade Glioma Patients: A Multicenter Study. Neuro-Oncology 2022, 24, 624–638. [Google Scholar] [CrossRef]
- Wijnenga, M.M.J.; French, P.J.; Dubbink, H.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Smits, M.; Gahrmann, R.; Rutten, G.-J.; Verheul, J.B.; et al. The Impact of Surgery in Molecularly Defined Low-Grade Glioma: An Integrated Clinical, Radiological, and Molecular Analysis. Neuro-Oncology 2018, 20, 103–112. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Zhang, Y.; Phillips, J.J.; Morshed, R.A.; Young, J.S.; McCoy, L.; Lafontaine, M.; Luks, T.; Ammanuel, S.; Kakaizada, S.; et al. Interactive Effects of Molecular, Therapeutic, and Patient Factors on Outcome of Diffuse Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 2029–2042. [Google Scholar] [CrossRef]
- Kavouridis, V.K.; Boaro, A.; Dorr, J.; Cho, E.Y.; Iorgulescu, J.B.; Reardon, D.A.; Arnaout, O.; Smith, T.R. Contemporary Assessment of Extent of Resection in Molecularly Defined Categories of Diffuse Low-Grade Glioma: A Volumetric Analysis. J. Neurosurg. 2020, 133, 1291–1301. [Google Scholar] [CrossRef]
- Baumert, B.G.; Hegi, M.E.; Van Den Bent, M.J.; Von Deimling, A.; Gorlia, T.; Hoang-Xuan, K.; Brandes, A.A.; Kantor, G.; Taphoorn, M.J.B.; Hassel, M.B.; et al. Temozolomide Chemotherapy versus Radiotherapy in High-Risk Low-Grade Glioma (EORTC 22033-26033): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol. 2016, 17, 1521–1532. [Google Scholar] [CrossRef]
- Oberheim Bush, N.A.; Chang, S. Treatment Strategies for Low-Grade Glioma in Adults. JOP 2016, 12, 1235–1241. [Google Scholar] [CrossRef]
- Buckner, J.C.; Shaw, E.G.; Pugh, S.L.; Chakravarti, A.; Gilbert, M.R.; Barger, G.R.; Coons, S.; Ricci, P.; Bullard, D.; Brown, P.D.; et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N. Engl. J. Med. 2016, 374, 1344–1355. [Google Scholar] [CrossRef]
- Geurts, M.; Van Den Bent, M.J. On High-Risk, Low-Grade Glioma: What Distinguishes High from Low?: Cancer Case Conundrums. Cancer 2019, 125, 174–176. [Google Scholar] [CrossRef]
- Svenjeby, C.; Carstam, L.; Werlenius, K.; Bontell, T.O.; Rydén, I.; Jacobsson, J.; Dénes, A.; Jakola, A.S.; Corell, A. Changes in Clinical Management of Diffuse IDH-Mutated Lower-Grade Gliomas: Patterns of Care in a 15-Year Period. J. Neurooncol. 2022, 160, 535–543. [Google Scholar] [CrossRef]
- Darlix, A.; Rigau, V.; Fraisse, J.; Gozé, C.; Fabbro, M.; Duffau, H. Postoperative Follow-up for Selected Diffuse Low-Grade Gliomas with WHO Grade III/IV Foci. Neurology 2020, 94, e830–e841. [Google Scholar] [CrossRef]
- Duffau, H.; Mandonnet, E. The “Onco-Functional Balance” in Surgery for Diffuse Low-Grade Glioma: Integrating the Extent of Resection with Quality of Life. Acta Neurochir. 2013, 155, 951–957. [Google Scholar] [CrossRef]
- Di Perri, D.; Jmil, S.; Lawson, T.M.; Van Calster, L.; Whenham, N.; Renard, L. Health-Related Quality of Life and Cognitive Failures in Patients with Lower-Grade Gliomas Treated with Radiotherapy. Cancer/Radiothérapie 2023, 27, 219–224. [Google Scholar] [CrossRef]
- Blonski, M.; Taillandier, L.; Herbet, G.; Maldonado, I.L.; Beauchesne, P.; Fabbro, M.; Campello, C.; Gozé, C.; Rigau, V.; Moritz-Gasser, S.; et al. Combination of Neoadjuvant Chemotherapy Followed by Surgical Resection as a New Strategy for WHO Grade II Gliomas: A Study of Cognitive Status and Quality of Life. J. Neurooncol. 2012, 106, 353–366. [Google Scholar] [CrossRef]
- Vanacôr, C.; Duffau, H. Analysis of Legal, Cultural, and Socioeconomic Parameters in Low-Grade Glioma Management: Variability Across Countries and Implications for Awake Surgery. World Neurosurg. 2018, 120, 47–53. [Google Scholar] [CrossRef]
- Ng, S.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Return to Work Following Surgery for Incidental Diffuse Low-Grade Glioma: A Prospective Series With 74 Patients. Neurosurgery 2019, 87, 720–729. [Google Scholar] [CrossRef]
- Pallud, J.; Mandonnet, E.; Deroulers, C.; Fontaine, D.; Badoual, M.; Capelle, L.; Guillet-May, F.; Page, P.; Peruzzi, P.; Jouanneau, E.; et al. Pregnancy Increases the Growth Rates of World Health Organization Grade II Gliomas. Ann. Neurol. 2010, 67, 398–404. [Google Scholar] [CrossRef]
- Ng, S.; Duffau, H. Factors Associated with Long-Term Survival in Women Who Get Pregnant After Surgery for WHO Grade II Glioma. Neurology 2022, 99, e89–e97. [Google Scholar] [CrossRef]
- Mahncke, H.W.; DeGutis, J.; Levin, H.; Newsome, M.R.; Bell, M.D.; Grills, C.; French, L.M.; Sullivan, K.W.; Kim, S.-J.; Rose, A.; et al. A Randomized Clinical Trial of Plasticity-Based Cognitive Training in Mild Traumatic Brain Injury. Brain 2021, 144, 1994–2008. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during Stroke Recovery: From Synapse to Behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of Brain Plasticity in Stroke: A Novel Model for Neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Almairac, F.; Deverdun, J.; Cochereau, J.; Coget, A.; Lemaitre, A.-L.; Moritz-Gasser, S.; Duffau, H.; Herbet, G. Homotopic Redistribution of Functional Connectivity in Insula-Centered Diffuse Low-Grade Glioma. NeuroImage Clin. 2021, 29, 102571. [Google Scholar] [CrossRef]
- Ng, S.; Deverdun, J.; Lemaitre, A.L.; Giampiccolo, D.; Le Bars, E.; Moritz-Gasser, S.; de Champfleur, N.M.; Duffau, H.; Herbet, G. Precuneal Gliomas Promote Behaviorally Relevant Remodeling of the Functional Connectome. J. Neurosurg. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Almairac, F.; Duffau, H.; Herbet, G. Contralesional Macrostructural Plasticity of the Insular Cortex in Patients with Glioma: A VBM Study. Neurology 2018, 91, e1902–e1908. [Google Scholar] [CrossRef]
- Yuan, T.; Ying, J.; Zuo, Z.; Gui, S.; Gao, Z.; Li, G.; Zhang, Y.; Li, C. Structural Plasticity of the Bilateral Hippocampus in Glioma Patients. Aging 2020, 12, 10259–10274. [Google Scholar] [CrossRef]
- Pasquini, L.; Jenabi, M.; Peck, K.K.; Holodny, A.I. Language Reorganization in Patients with Left-Hemispheric Gliomas Is Associated with Increased Cortical Volume in Language-Related Areas and in the Default Mode Network. Cortex 2022, 157, 245–255. [Google Scholar] [CrossRef]
- Herbet, G.; Maheu, M.; Costi, E.; Lafargue, G.; Duffau, H. Mapping Neuroplastic Potential in Brain-Damaged Patients. Brain 2016, 139, 829–844. [Google Scholar] [CrossRef]
- Tate, M.C.; Herbet, G.; Moritz-Gasser, S.; Tate, J.E.; Duffau, H. Probabilistic Map of Critical Functional Regions of the Human Cerebral Cortex: Broca’s Area Revisited. Brain 2014, 137, 2773–2782. [Google Scholar] [CrossRef]
- Sarubbo, S.; Tate, M.; De Benedictis, A.; Merler, S.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Mapping Critical Cortical Hubs and White Matter Pathways by Direct Electrical Stimulation: An Original Functional Atlas of the Human Brain. NeuroImage 2020, 205, 116237. [Google Scholar] [CrossRef]
- Mandonnet, E.; Delattre, J.-Y.; Tanguy, M.-L.; Swanson, K.R.; Carpentier, A.F.; Duffau, H.; Cornu, P.; Van Effenterre, R.; Alvord, E.C.; Capelle, L. Continuous Growth of Mean Tumor Diameter in a Subset of Grade II Gliomas. Ann. Neurol. 2003, 53, 524–528. [Google Scholar] [CrossRef]
- Pallud, J.; Blonski, M.; Mandonnet, E.; Audureau, E.; Fontaine, D.; Sanai, N.; Bauchet, L.; Peruzzi, P.; Frénay, M.; Colin, P.; et al. Velocity of Tumor Spontaneous Expansion Predicts Long-Term Outcomes for Diffuse Low-Grade Gliomas. Neuro-Oncology 2013, 15, 595–606. [Google Scholar] [CrossRef]
- Desmurget, M.; Bonnetblanc, F.; Duffau, H. Contrasting Acute and Slow-Growing Lesions: A New Door to Brain Plasticity. Brain 2006, 130, 898–914. [Google Scholar] [CrossRef]
- Aabedi, A.A.; Lipkin, B.; Kaur, J.; Kakaizada, S.; Valdivia, C.; Reihl, S.; Young, J.S.; Lee, A.T.; Krishna, S.; Berger, M.S.; et al. Functional Alterations in Cortical Processing of Speech in Glioma-Infiltrated Cortex. Proc. Natl. Acad. Sci. USA 2021, 118, e2108959118. [Google Scholar] [CrossRef]
- Duffau, H. Introducing the Concept of Brain Metaplasticity in Glioma: How to Reorient the Pattern of Neural Reconfiguration to Optimize the Therapeutic Strategy. J. Neurosurg. 2022, 136, 613–617. [Google Scholar] [CrossRef]
- Ng, S.; Duffau, H.; Herbet, G. Perspectives in Human Brain Plasticity Sparked by Glioma Invasion: From Intraoperative (Re)Mappings to Neural Reconfigurations. Neural Regen. Res. 2023, in press. [Google Scholar]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a Strategy Favoring Early Surgical Resection vs a Strategy Favoring Watchful Waiting in Low-Grade Gliomas. JAMA 2012, 308, 1881. [Google Scholar] [CrossRef]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of Intraoperative Stimulation Brain Mapping on Glioma Surgery Outcome: A Meta-Analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef]
- Duffau, H. Contribution of Intraoperative Electrical Stimulations in Surgery of Low Grade Gliomas: A Comparative Study between Two Series without (1985–1996) and with (1996–2003) Functional Mapping in the Same Institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef]
- Duffau, H.; Ng, S.; Lemaitre, A.-L.; Moritz-Gasser, S.; Herbet, G. Constant Multi-Tasking with Time Constraint to Preserve Across-Network Dynamics Throughout Awake Surgery for Low-Grade Glioma: A Necessary Step to Enable Patients Resuming an Active Life. Front. Oncol. 2022, 12, 924762. [Google Scholar] [CrossRef]
- Lemaitre, A.-L.; Herbet, G.; Ng, S.; Moritz-Gasser, S.; Duffau, H. Cognitive Preservation Following Awake Mapping-Based Neurosurgery for Low-Grade Gliomas: A Longitudinal, within-Patient Design Study. Neuro-Oncology 2022, 24, 781–793. [Google Scholar] [CrossRef]
- Duffau, H. Awake Mapping with Transopercular Approach in Right Insular–Centered Low-Grade Gliomas Improves Neurological Outcomes and Return to Work. Neurosurgery 2022, 91, 182–190. [Google Scholar] [CrossRef]
- Yordanova, Y.N.; Moritz-Gasser, S.; Duffau, H. Awake Surgery for WHO Grade II Gliomas within “Noneloquent” Areas in the Left Dominant Hemisphere: Toward a “Supratotal” Resection: Clinical Article. J. Neurosurg. 2011, 115, 232–239. [Google Scholar] [CrossRef]
- Rossi, M.; Gay, L.; Ambrogi, F.; Conti Nibali, M.; Sciortino, T.; Puglisi, G.; Leonetti, A.; Mocellini, C.; Caroli, M.; Cordera, S.; et al. Association of Supratotal Resection with Progression-Free Survival, Malignant Transformation, and Overall Survival in Lower-Grade Gliomas. Neuro-Oncology 2021, 23, 812–826. [Google Scholar] [CrossRef]
- Sarubbo, S.; De Benedictis, A.; Merler, S.; Mandonnet, E.; Balbi, S.; Granieri, E.; Duffau, H. Towards a Functional Atlas of Human White Matter: Functional Atlas of White Matter. Hum. Brain Mapp. 2015, 36, 3117–3136. [Google Scholar] [CrossRef]
- Ng, S.; Herbet, G.; Lemaitre, A.-L.; Cochereau, J.; Moritz-Gasser, S.; Duffau, H. Neuropsychological Assessments before and after Awake Surgery for Incidental Low-Grade Gliomas. J. Neurosurg. 2021, 135, 871–880. [Google Scholar] [CrossRef]
- Southwell, D.G.; Hervey-Jumper, S.L.; Perry, D.W.; Berger, M.S. Intraoperative Mapping during Repeat Awake Craniotomy Reveals the Functional Plasticity of Adult Cortex. J. Neurosurg. 2016, 124, 1460–1469. [Google Scholar] [CrossRef]
- Picart, T.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Iterative Surgical Resections of Diffuse Glioma with Awake Mapping: How to Deal with Cortical Plasticity and Connectomal Constraints? Neurosurgery 2018, 85, 105–116. [Google Scholar] [CrossRef]
- Ng, S.; Lemaitre, A.-L.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Recurrent Low-Grade Gliomas: Does Reoperation Affect Neurocognitive Functioning? Neurosurgery 2022, 90, 221–232. [Google Scholar] [CrossRef]
- Capo, G.; Skrap, M.; Guarracino, I.; Isola, M.; Battistella, C.; Ius, T.; Tomasino, B. Cognitive Functions in Repeated Glioma Surgery. Cancers 2020, 12, 1077. [Google Scholar] [CrossRef]
- Shofty, B.; Haim, O.; Costa, M.; Kashanian, A.; Shtrozberg, S.; Ram, Z.; Grossman, R. Impact of Repeated Operations for Progressive Low-Grade Gliomas. Eur. J. Surg. Oncol. 2020, 46, 2331–2337. [Google Scholar] [CrossRef]
- Morshed, R.A.; Young, J.S.; Han, S.J.; Hervey-Jumper, S.L.; Berger, M.S. Perioperative Outcomes Following Reoperation for Recurrent Insular Gliomas. J. Neurosurg. 2019, 131, 467–473. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Hebb, A.; Barber, J.; Rostomily, R.; Silbergeld, D. Outcomes in Reoperated Low-Grade Gliomas. Neurosurgery 2015, 77, 175–184. [Google Scholar] [CrossRef]
- Martino, J.; Taillandier, L.; Moritz-Gasser, S.; Gatignol, P.; Duffau, H. Re-Operation Is a Safe and Effective Therapeutic Strategy in Recurrent WHO Grade II Gliomas within Eloquent Areas. Acta Neurochir. 2009, 151, 427–436. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Berger, M.S.; Lamborn, K.R.; Aldape, K.; McDermott, M.W.; Prados, M.D.; Chang, S.M. Repeated Operations for Infiltrative Low-Grade Gliomas without Intervening Therapy. J. Neurosurg. 2003, 98, 1165–1169. [Google Scholar] [CrossRef]
- Ribeiro, L.; Ng, S.; Duffau, H. Recurrent Insular Low-Grade Gliomas: Factors Guiding the Decision to Reoperate. J. Neurosurg. 2022, 138, 1216–1226. [Google Scholar] [CrossRef]
- Hamdan, N.; Duffau, H. Extending the Multistage Surgical Strategy for Recurrent Initially Low-Grade Gliomas: Functional and Oncological Outcomes in 31 Consecutive Patients Who Underwent a Third Resection under Awake Mapping. J. Neurosurg. 2022, 136, 1035–1044. [Google Scholar] [CrossRef]
- Cairncross, J.G.; Macdonald, D.R. Successful Chemotherapy for Recurrent Malignant Oligodendroglioma. Ann. Neurol. 1988, 23, 360–364. [Google Scholar] [CrossRef]
- Mason, W.P.; Krol, G.S.; DeAngelis, L.M. Low-Grade Oligodendroglioma Responds to Chemotherapy. Neurology 1996, 46, 203–207. [Google Scholar] [CrossRef]
- Buckner, J.C.; Gesme, D.; O’Fallon, J.R.; Hammack, J.E.; Stafford, S.; Brown, P.D.; Hawkins, R.; Scheithauer, B.W.; Erickson, B.J.; Levitt, R.; et al. Phase II Trial of Procarbazine, Lomustine, and Vincristine as Initial Therapy for Patients with Low-Grade Oligodendroglioma or Oligoastrocytoma: Efficacy and Associations with Chromosomal Abnormalities. J. Clin. Oncol. 2003, 21, 251–255. [Google Scholar] [CrossRef]
- Nokia, M.S.; Anderson, M.L.; Shors, T.J. Chemotherapy Disrupts Learning, Neurogenesis and Theta Activity in the Adult Brain. Eur. J. Neurosci. 2012, 36, 3521–3530. [Google Scholar] [CrossRef]
- Smits, A.; Duffau, H. Seizures and the Natural History of World Health Organization Grade II Gliomas: A Review. Neurosurgery 2011, 68, 1326–1333. [Google Scholar] [CrossRef]
- Castellano, A.; Donativi, M.; Rudà, R.; De Nunzio, G.; Riva, M.; Iadanza, A.; Bertero, L.; Rucco, M.; Bello, L.; Soffietti, R.; et al. Evaluation of Low-Grade Glioma Structural Changes after Chemotherapy Using DTI-Based Histogram Analysis and Functional Diffusion Maps. Eur. Radiol. 2016, 26, 1263–1273. [Google Scholar] [CrossRef]
- Blonski, M.; Pallud, J.; Gozé, C.; Mandonnet, E.; Rigau, V.; Bauchet, L.; Fabbro, M.; Beauchesne, P.; Baron, M.-H.; Fontaine, D.; et al. Neoadjuvant Chemotherapy May Optimize the Extent of Resection of World Health Organization Grade II Gliomas: A Case Series of 17 Patients. J. Neurooncol. 2013, 113, 267–275. [Google Scholar] [CrossRef]
- Jo, J.; Williams, B.; Smolkin, M.; Wintermark, M.; Shaffrey, M.E.; Lopes, M.B.; Schiff, D. Effect of Neoadjuvant Temozolomide upon Volume Reduction and Resection of Diffuse Low-Grade Glioma. J. Neurooncol. 2014, 120, 155–161. [Google Scholar] [CrossRef]
- Darlix, A.; Mandonnet, E.; Freyschlag, C.F.; Pinggera, D.; Forster, M.-T.; Voss, M.; Steinbach, J.; Loughrey, C.; Goodden, J.; Banna, G.; et al. Chemotherapy and Diffuse Low-Grade Gliomas: A Survey within the European Low-Grade Glioma Network. Neuro-Oncol. Pract. 2019, 6, 264–273. [Google Scholar] [CrossRef]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K.; et al. Mechanisms and Therapeutic Implications of Hypermutation in Gliomas. Nature 2020, 580, 517–523. [Google Scholar] [CrossRef]
- Yu, Y.; Villanueva-Meyer, J.; Grimmer, M.R.; Hilz, S.; Solomon, D.A.; Choi, S.; Wahl, M.; Mazor, T.; Hong, C.; Shai, A.; et al. Temozolomide-Induced Hypermutation Is Associated with Distant Recurrence and Reduced Survival after High-Grade Transformation of Low-Grade IDH -Mutant Gliomas. Neuro-Oncology 2021, 23, 1872–1884. [Google Scholar] [CrossRef]
- Van den Bent, M.; Afra, D.; de Witte, O.; Hassel, M.B.; Schraub, S.; Hoang-Xuan, K.; Malmström, P.-O.; Collette, L.; Piérart, M.; Mirimanoff, R.; et al. Long-Term Efficacy of Early versus Delayed Radiotherapy for Low-Grade Astrocytoma and Oligodendroglioma in Adults: The EORTC 22845 Randomised Trial. Lancet 2005, 366, 985–990. [Google Scholar] [CrossRef]
- Douw, L.; Klein, M.; Fagel, S.S.; van den Heuvel, J.; Taphoorn, M.J.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and Radiological Effects of Radiotherapy in Patients with Low-Grade Glioma: Long-Term Follow-Up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Drijver, A.J.; Van Den Bent, M.J.; Bromberg, J.C.; Hoang-Xuan, K.; Taphoorn, M.J.B.; Reijneveld, J.C.; Ben Hassel, M.; Vauleon, E.; Eekers, D.B.P.; et al. Memory in Low-Grade Glioma Patients Treated with Radiotherapy or Temozolomide: A Correlative Analysis of EORTC Study 22033-26033. Neuro-Oncology 2021, 23, 803–811. [Google Scholar] [CrossRef]
- Reijneveld, J.C.; Taphoorn, M.J.B.; Coens, C.; Bromberg, J.E.C.; Mason, W.P.; Hoang-Xuan, K.; Ryan, G.; Hassel, M.B.; Enting, R.H.; Brandes, A.A.; et al. Health-Related Quality of Life in Patients with High-Risk Low-Grade Glioma (EORTC 22033-26033): A Randomised, Open-Label, Phase 3 Intergroup Study. Lancet Oncol. 2016, 17, 1533–1542. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Won, M.; Shaw, E.G.; Hu, C.; Brachman, D.G.; Buckner, J.C.; Stelzer, K.J.; Barger, G.R.; Brown, P.D.; Gilbert, M.R.; et al. Effect of the Addition of Chemotherapy to Radiotherapy on Cognitive Function in Patients with Low-Grade Glioma: Secondary Analysis of RTOG 98-02. J. Clin. Oncol. 2014, 32, 535–541. [Google Scholar] [CrossRef]
- Wahl, M.; Phillips, J.J.; Molinaro, A.M.; Lin, Y.; Perry, A.; Haas-Kogan, D.A.; Costello, J.F.; Dayal, M.; Butowski, N.; Clarke, J.L.; et al. Chemotherapy for Adult Low-Grade Gliomas: Clinical Outcomes by Molecular Subtype in a Phase II Study of Adjuvant Temozolomide. Neuro-Oncology 2016, 19, 242–251. [Google Scholar] [CrossRef]
- Bady, P.; Kurscheid, S.; Delorenzi, M.; Gorlia, T.; Van Den Bent, M.J.; Hoang-Xuan, K.; Vauléon, É.; Gijtenbeek, A.; Enting, R.; Thiessen, B.; et al. The DNA Methylome of DDR Genes and Benefit from RT or TMZ in IDH Mutant Low-Grade Glioma Treated in EORTC 22033. Acta Neuropathol. 2018, 135, 601–615. [Google Scholar] [CrossRef]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric Advantages of Proton Therapy over Conventional Radiotherapy with Photons in Young Patients and Adults with Low-Grade Glioma. Strahlenther. Onkol. 2016, 192, 759–769. [Google Scholar] [CrossRef]
- Schultheiss, T.E.; Stephens, L.C. Permanent Radiation Myelopathy. Br. J. Radiol. 1992, 65, 737–753. [Google Scholar] [CrossRef]
- Hope, T.R.; Vardal, J.; Bjørnerud, A.; Larsson, C.; Arnesen, M.R.; Salo, R.A.; Groote, I.R. Serial Diffusion Tensor Imaging for Early Detection of Radiation-Induced Injuries to Normal-Appearing White Matter in High-Grade Glioma Patients: Tracking Early RBI with DTI. J. Magn. Reson. Imaging 2015, 41, 414–423. [Google Scholar] [CrossRef]
- Zhu, T.; Chapman, C.H.; Tsien, C.; Kim, M.; Spratt, D.E.; Lawrence, T.S.; Cao, Y. Effect of the Maximum Dose on White Matter Fiber Bundles Using Longitudinal Diffusion Tensor Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 696–705. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, H.; Lv, X.-F.; Xie, F.; Liu, L.; Qiu, S.; Li, L.; Shen, D. Radiation-Induced Brain Structural and Functional Abnormalities in Presymptomatic Phase and Outcome Prediction: Radiation-Induced Brain Abnormalities. Hum. Brain Mapp. 2018, 39, 407–427. [Google Scholar] [CrossRef]
- Chapman, C.H.; Nagesh, V.; Sundgren, P.C.; Buchtel, H.; Chenevert, T.L.; Junck, L.; Lawrence, T.S.; Tsien, C.I.; Cao, Y. Diffusion Tensor Imaging of Normal-Appearing White Matter as Biomarker for Radiation-Induced Late Delayed Cognitive Decline. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2033–2040. [Google Scholar] [CrossRef]
- Connor, M.; Karunamuni, R.; McDonald, C.; Seibert, T.; White, N.; Moiseenko, V.; Bartsch, H.; Farid, N.; Kuperman, J.; Krishnan, A.; et al. Regional Susceptibility to Dose-Dependent White Matter Damage after Brain Radiotherapy. Radiother. Oncol. 2017, 123, 209–217. [Google Scholar] [CrossRef]
- Salans, M.; Tibbs, M.D.; Karunamuni, R.; Yip, A.; Huynh-Le, M.-P.; Macari, A.C.; Reyes, A.; Tringale, K.; McDonald, C.R.; Hattangadi-Gluth, J.A. Longitudinal Change in Fine Motor Skills after Brain Radiotherapy and in Vivo Imaging Biomarkers Associated with Decline. Neuro-Oncology 2021, 23, 1393–1403. [Google Scholar] [CrossRef]
- Dinkel, J.G.; Lahmer, G.; Mennecke, A.; Hock, S.W.; Richter-Schmidinger, T.; Fietkau, R.; Distel, L.; Putz, F.; Dörfler, A.; Schmidt, M.A. Effects of Hippocampal Sparing Radiotherapy on Brain Microstructure—A Diffusion Tensor Imaging Analysis. Brain Sci. 2022, 12, 879. [Google Scholar] [CrossRef]
- Jaspers, J.; Mèndez Romero, A.; Hoogeman, M.S.; Van Den Bent, M.; Wiggenraad, R.G.J.; Taphoorn, M.J.B.; Eekers, D.B.P.; Lagerwaard, F.J.; Lucas Calduch, A.M.; Baumert, B.G.; et al. Evaluation of the Hippocampal Normal Tissue Complication Model in a Prospective Cohort of Low Grade Glioma Patients—An Analysis Within the EORTC 22033 Clinical Trial. Front. Oncol. 2019, 9, 991. [Google Scholar] [CrossRef]
- Raschke, F.; Witzmann, K.; Seidlitz, A.; Wesemann, T.; Jentsch, C.; Platzek, I.; Van Den Hoff, J.; Kotzerke, J.; Beuthien-Baumann, B.; Baumann, M.; et al. Time- and Dose-Dependent Volume Decreases in Subcortical Grey Matter Structures of Glioma Patients after Radio(Chemo)Therapy. Clin. Transl. Radiat. Oncol. 2022, 36, 99–105. [Google Scholar] [CrossRef]
- Duffau, H. Why Brain Radiation Therapy Should Take Account of the Individual Structural and Functional Connectivity: Toward an Irradiation “à La Carte”. Crit. Rev. Oncol./Hematol. 2020, 154, 103073. [Google Scholar] [CrossRef]
- Platten, M.; Bunse, L.; Wick, A.; Bunse, T.; Le Cornet, L.; Harting, I.; Sahm, F.; Sanghvi, K.; Tan, C.L.; Poschke, I.; et al. A Vaccine Targeting Mutant IDH1 in Newly Diagnosed Glioma. Nature 2021, 592, 463–468. [Google Scholar] [CrossRef]
- Rohle, D.; Popovici-Muller, J.; Palaskas, N.; Turcan, S.; Grommes, C.; Campos, C.; Tsoi, J.; Clark, O.; Oldrini, B.; Komisopoulou, E.; et al. An Inhibitor of Mutant IDH1 Delays Growth and Promotes Differentiation of Glioma Cells. Science 2013, 340, 626–630. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Lu, M.; Wen, P.Y.; Taylor, J.W.; Maher, E.A.; Arrillaga-Romany, I.; Peters, K.B.; Ellingson, B.M.; Rosenblum, M.K.; Chun, S.; et al. Vorasidenib and Ivosidenib in IDH1-Mutant Low-Grade Glioma: A Randomized, Perioperative Phase 1 Trial. Nat. Med. 2023, 29, 615–622. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Van Den Bent, M.J.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Hamer, R.P.; Yeo, T.T. Current Status of Neuromodulation-Induced Cortical Prehabilitation and Considerations for Treatment Pathways in Lower-Grade Glioma Surgery. Life 2022, 12, 466. [Google Scholar] [CrossRef]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 3, 1181–1228. [Google Scholar] [CrossRef]
- Ille, S.; Kelm, A.; Schroeder, A.; Albers, L.E.; Negwer, C.; Butenschoen, V.M.; Sollmann, N.; Picht, T.; Vajkoczy, P.; Meyer, B.; et al. Navigated Repetitive Transcranial Magnetic Stimulation Improves the Outcome of Postsurgical Paresis in Glioma Patients—A Randomized, Double-Blinded Trial. Brain Stimul. 2021, 14, 780–787. [Google Scholar] [CrossRef]

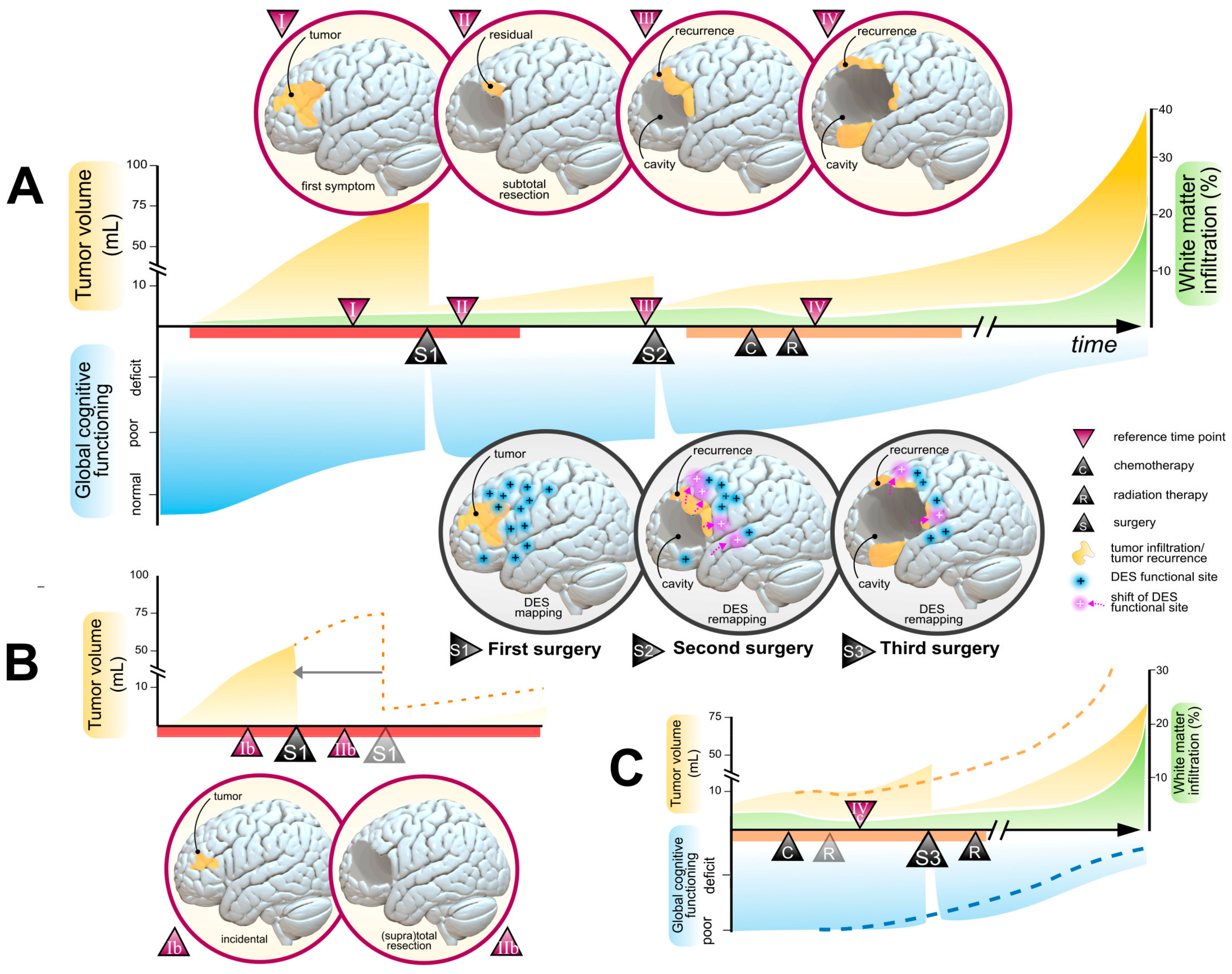
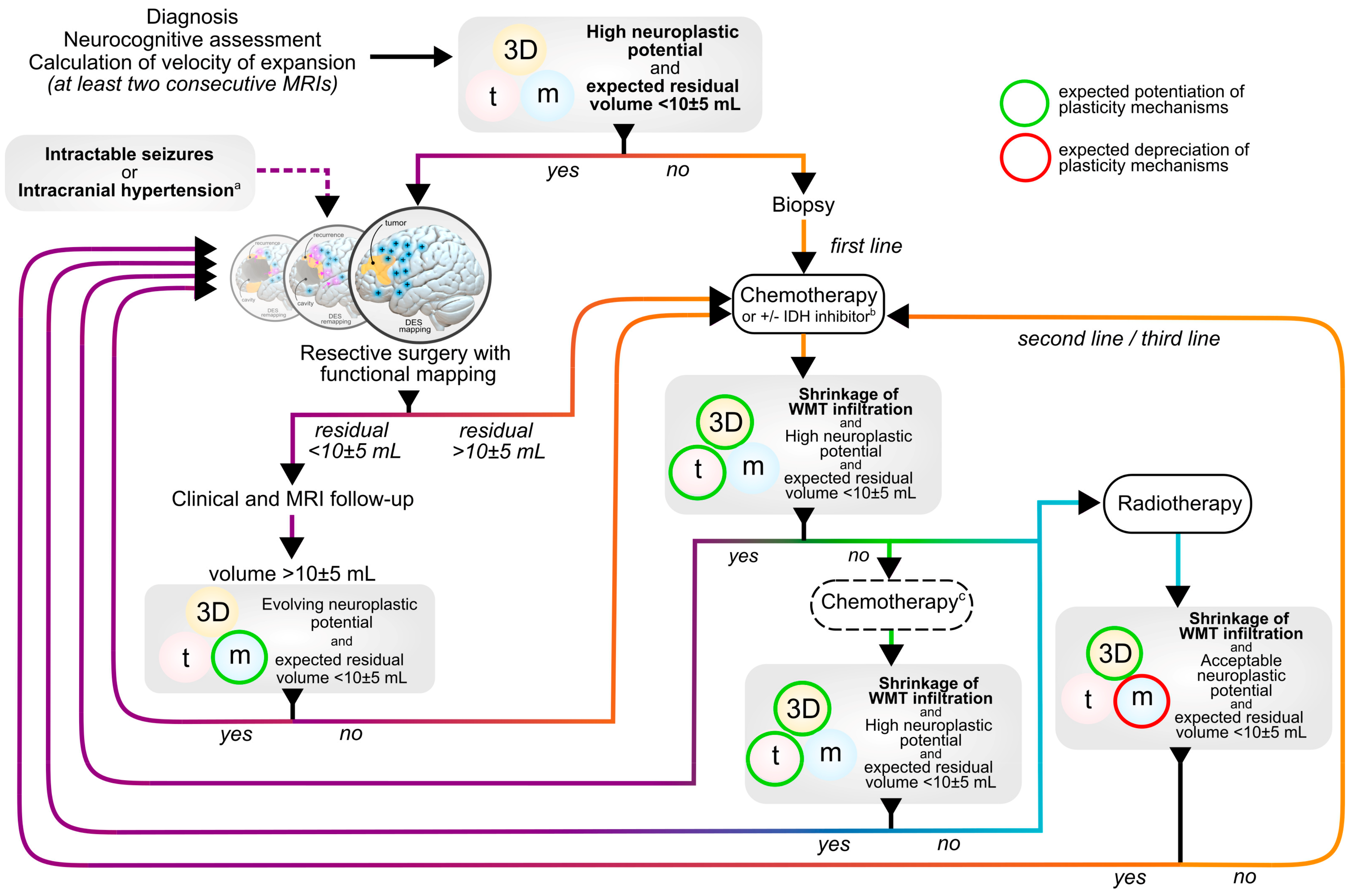
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, S.; Duffau, H. Brain Plasticity Profiling as a Key Support to Therapeutic Decision-Making in Low-Grade Glioma Oncological Strategies. Cancers 2023, 15, 3698. https://doi.org/10.3390/cancers15143698
Ng S, Duffau H. Brain Plasticity Profiling as a Key Support to Therapeutic Decision-Making in Low-Grade Glioma Oncological Strategies. Cancers. 2023; 15(14):3698. https://doi.org/10.3390/cancers15143698
Chicago/Turabian StyleNg, Sam, and Hugues Duffau. 2023. "Brain Plasticity Profiling as a Key Support to Therapeutic Decision-Making in Low-Grade Glioma Oncological Strategies" Cancers 15, no. 14: 3698. https://doi.org/10.3390/cancers15143698
APA StyleNg, S., & Duffau, H. (2023). Brain Plasticity Profiling as a Key Support to Therapeutic Decision-Making in Low-Grade Glioma Oncological Strategies. Cancers, 15(14), 3698. https://doi.org/10.3390/cancers15143698




