Navigating Choices: Determinants and Outcomes of Surgery Refusal in Thyroid Cancer Patients Using SEER Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population and Variables
2.3. Primary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Determinants of Surgical Refusal in Cancer Patients
3.3. Disease Outcomes
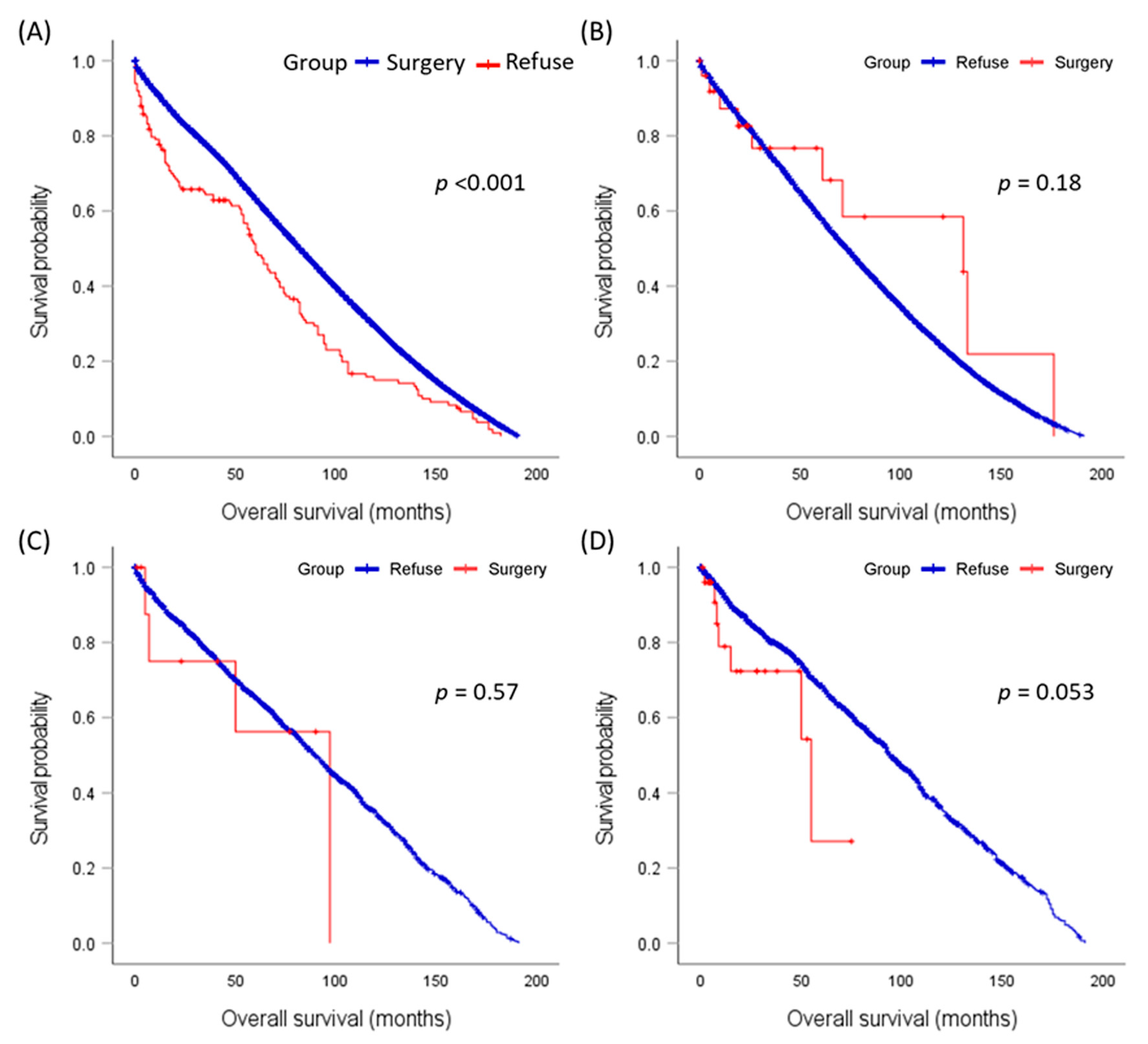
3.4. Overall Survival Analysis
3.5. Disease-Specific Survival Analysis
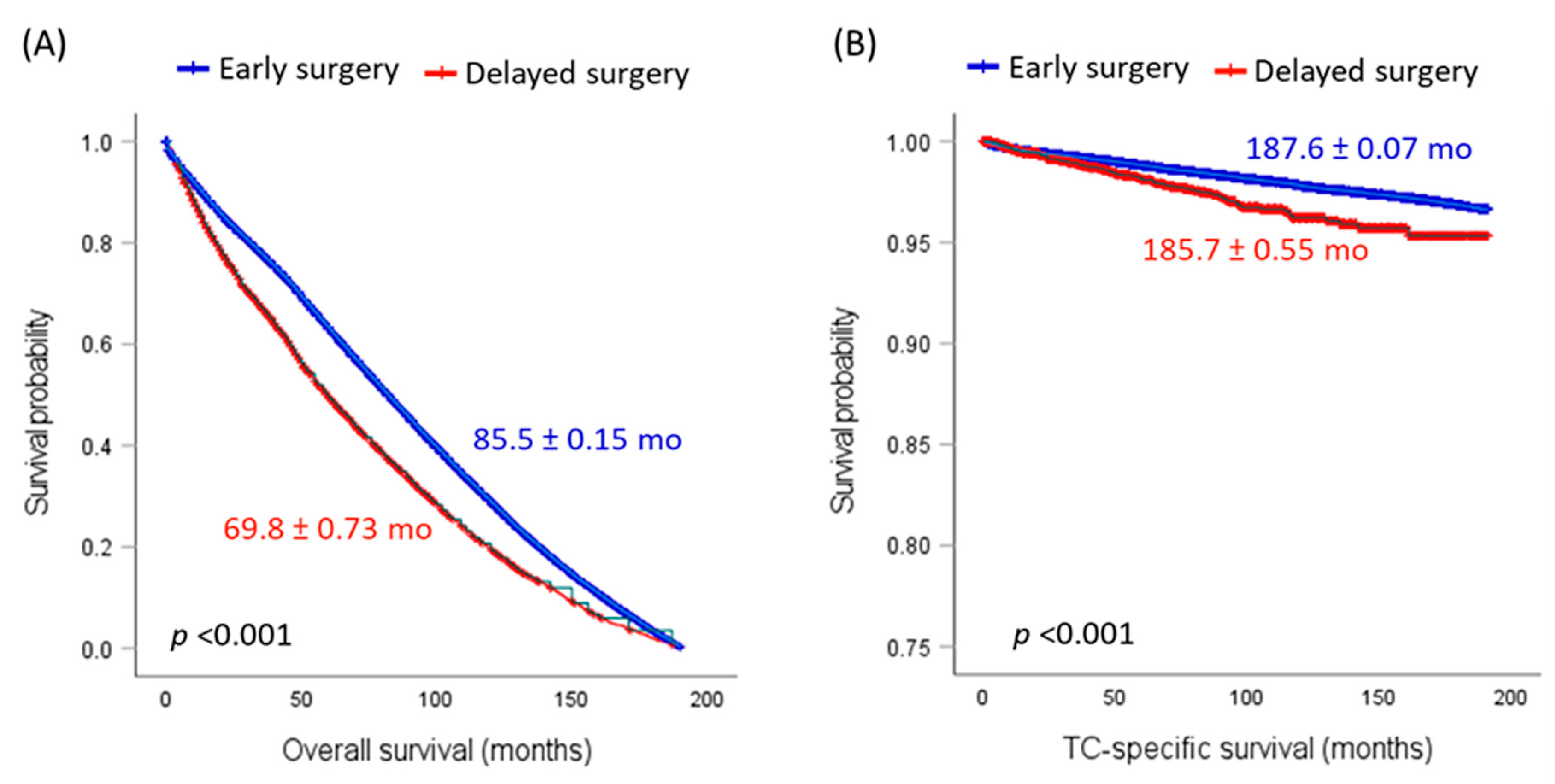
3.6. Impact of Delayed Surgery on Recurrence and Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gerwen, M.; Sinclair, C.; Rahman, M.; Genden, E.; Taioli, E. The impact of surgery refusal on thyroid cancer survival: A SEER-based analysis. Endocrine 2020, 70, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.F.; Kutlu, O.; Picado, O.; Lew, J.I. Margin Positivity and Survival Outcomes: A Review of 14,471 Patients with 1-cm to 4-cm Papillary Thyroid Carcinoma. J. Am. Coll. Surg. 2021, 232, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Coffman, A.R.; Tao, R.; Cohan, J.N.; Huang, L.C.; Pickron, T.B.; Torgeson, A.M.; Lloyd, S. Factors associated with the refusal of surgery and the associated impact on survival in patients with rectal cancer using the National Cancer Database. J. Gastrointest. Oncol. 2021, 12, 1482–1497. [Google Scholar] [CrossRef] [PubMed]
- Birkenbeuel, J.L.; Lehrich, B.M.; Goshtasbi, K.; Abiri, A.; Hsu, F.P.K.; Kuan, E.C. Refusal of Surgery in Pituitary Adenoma Patients: A Population-Based Analysis. Cancers 2022, 14, 5348. [Google Scholar] [CrossRef]
- Xu, S.; Huang, H.; Zhang, X.; Huang, Y.; Guan, B.; Qian, J.; Wang, X.; Liu, S.; Xu, Z.; Liu, J. Predictive Value of Serum Thyroglobulin for Structural Recurrence Following Lobectomy for Papillary Thyroid Carcinoma. Thyroid 2021, 31, 1391–1399. [Google Scholar] [CrossRef]
- Megwalu, U.C.; Saini, A.T. Racial disparities in papillary thyroid microcarcinoma survival. J. Laryngol. Otol. 2017, 131, 83–87. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.W. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int. J. Surg. Oncol. 2010, 2010, 381795. [Google Scholar] [CrossRef]
- Delisle, M.; Singh, S.; Howard, J.; Panda, N.; Weppler, A.M.; Wang, Y. Refusal of colorectal cancer surgery in the United States: Predictors and associated cancer-specific mortality in a Surveillance, Epidemiology, and End Results (SEER) cohort. Surg. Open Sci. 2020, 2, 12–18. [Google Scholar] [CrossRef]
- Sun, J.; Nan, Q. Survival benefit of surgical resection for stage IV gastric cancer: A SEER-based propensity score-matched analysis. Front. Surg. 2022, 9, 927030. [Google Scholar] [CrossRef]
- Chen, G.; Jin, Y.; Guan, W.-L.; Zhang, R.-X.; Xiao, W.-W.; Cai, P.-Q.; Liu, M.; Lin, J.-Z.; Wang, F.-L.; Li, C.; et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: An open-label, single-centre phase 2 study. Lancet Gastroenterol. Hepatol. 2023, 8, 422–431. [Google Scholar] [CrossRef]
- Wu, M.; Wajeeh, H.; McPhail, M.N.; Seyam, O.; Flora, J.; Nguyen, H. Usage of Tranexamic Acid for Treatment of Subdural Hematomas. Cureus 2023, 15, e37628. [Google Scholar] [CrossRef] [PubMed]
- Angeles, M.A.; Cabarrou, B.; Gil-Moreno, A.; Pérez-Benavente, A.; Spagnolo, E.; Rychlik, A.; Martínez-Gómez, C.; Guyon, F.; Zapardiel, I.; Querleu, D.; et al. Effect of tumor burden and radical surgery on survival difference between upfront, early interval or delayed cytoreductive surgery in ovarian cancer. J. Gynecol. Oncol. 2021, 32, e78. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, K.; Locher, C.; Sabbagh, C.; Ortega-Deballon, P.; Heyd, B.; Bachellier, P.; Métairie, S.; Paye, F.; Bourlier, P.; Adam, R.; et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann. Surg. 2021, 273, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.R.; Peak, T.; Gast, J.; Arnell, M. Associations Between Masculine Norms and Healthcare Utilization in Highly Religious, Heterosexual Men. Am. J. Men’s Health 2019, 13, 1557988319856739. [Google Scholar] [CrossRef]
- Hu, X.; Ye, H.; Yan, W.; Sun, Y. Factors Associated with Patient’s Refusal of Recommended Cancer Surgery: Based on Surveillance, Epidemiology, and End Results. Front. Public Health 2022, 9, 785602. [Google Scholar] [CrossRef]
- Wu, S.S.; Lamarre, E.D.; Yalamanchali, A.; Brauer, P.R.; Hong, H.; Reddy, C.A.; Yilmaz, E.; Woody, N.; Ku, J.A.; Prendes, B.; et al. Association of Treatment Strategies and Tumor Characteristics with Overall Survival among Patients with Anaplastic Thyroid Cancer: A Single-Institution 21-Year Experience. JAMA Otolaryngol. Neck Surg. 2023, 149, 300–309. [Google Scholar] [CrossRef]
- Chaves, N.; Broekhuis, J.M.; Fligor, S.C.; A Collins, R.; Modest, A.M.; Kaul, S.; James, B.C. Delay in Surgery and Papillary Thyroid Cancer Survival in the United States: A SEER-Medicare Analysis. J. Clin. Endocrinol. Metab. 2023, dgad163. [Google Scholar] [CrossRef]
- Sahli, Z.T.; Canner, J.K.; Zeiger, M.A.; Mathur, A. Association between age and disease specific mortality in medullary thyroid cancer. Am. J. Surg. 2021, 221, 478–484. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, J.; Ming, J.; Guo, H.; Li, X.; Zhang, N.; Huang, T. Management of Very Elderly Patients with Papillary Thyroid Cancer: Analysis of Outcomes for Surgery Versus Nonsurgery. J. Surg. Res. 2020, 256, 512–519. [Google Scholar] [CrossRef]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of Overall Survival in Patients with Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef]
- Corrigan, K.L.; Williamson, H.; Range, D.E.; Niedzwiecki, D.; Brizel, D.; Mowery, Y.M. Treatment Outcomes in Anaplastic Thyroid Cancer. J. Thyroid. Res. 2019, 2019, 8218949. [Google Scholar] [CrossRef] [PubMed]
- Pierie, J.-P.E.N.; Muzikansky, A.; Gaz, R.D.; Faquin, W.C.; Ott, M.J. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann. Surg. Oncol. 2002, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lu, Y.; Horie, S.; Vogel, M.; Zhang, R.; Zheng, P.; Wei, Y. Cancer-directed surgery brings survival benefits for patients with advanced prostate cancer: A population-based propensity-score matching study. J. Cancer 2023, 14, 231–238. [Google Scholar] [CrossRef]
- Silva, P.; Lemos, J.; Borges, M.; Rêgo, T.D.; Dantas, T.; Leite, C.; Lima, M.; Cunha, M.; Sousa, F. Prognostic factors on surgically and non-surgically treated oral squamous cell carcinoma: Advances in survival in fifteen years of follow up. J. Clin. Exp. Dent. 2021, 13, e240–e249. [Google Scholar] [CrossRef]
- Coffman, A.; Torgeson, A.; Lloyd, S. Correlates of Refusal of Surgery in the Treatment of Non-metastatic Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 98–108. [Google Scholar] [CrossRef] [PubMed]
- May, T.; Comeau, R.; Sun, P.; Kotsopoulos, J.; Narod, S.A.; Rosen, B.; Ghatage, P. A Comparison of Survival Outcomes in Advanced Serous Ovarian Cancer Patients Treated with Primary Debulking Surgery Versus Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer 2017, 27, 668–674. [Google Scholar] [CrossRef]
- Verkooijen, H.M.; Fioretta, G.M.; Rapiti, E.; Bonnefoi, H.; Vlastos, G.; Kurtz, J.; Schaefer, P.; Sappino, A.-P.; Schubert, H.; Bouchardy, C. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann. Surg. 2005, 242, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Garas, S.N.; Witherspoon, L.; Lukich, N.; Abdi, H.; Breau, R.H. Refusal of surgery: A case-based review of ethical and legal principles behind informed consent in Canada. Can. Urol. Assoc. J. 2021, 15, 67–69. [Google Scholar] [CrossRef]

| Characteristics | Levels | Total Patients (n = 176,472) | Surgery Performed (n = 176,002) | Patient Refused (n = 470) | p-Value |
|---|---|---|---|---|---|
| Total number | 176,472 | 176,002 (99.7) | 470 (0.3) | ||
| Age (years) | Median (IQR) | 49.0 (38–60) | 49.0 (38–60) | 59.0 (44–74) | <0.001 |
| <55 years | 110,252 (62.5) | 110,055 (62.5) | 197 (41.9) | <0.001 | |
| ≥55 years | 66,220 (37.5) | 65,947 (37.5) | 273 (58.1) | ||
| Gender | Female | 134,960 (76.5) | 134,637 (76.5) | 323 (68.7) | <0.001 |
| Male | 41,512 (23.5) | 41,365 (23.5) | 147 (31.3) | ||
| Race | White | 143,085 (82.1) | 142,752 (82.1) | 333 (72.1) | <0.001 |
| Black | 11,063 (6.3) | 11,031 (6.3) | 32 (6.9) | ||
| API | 18,998 (10.9) | 18,908 (10.9) | 90 (19.5) | ||
| AI/AN | 1168 (0.7) | 1161 (0.7) | 7 (1.5) | ||
| Ethnicity | Not Hispanic/Latino | 147,128 (83.4) | 146,740 (83.4) | 388 (82.6) | 0.68 |
| Hispanic/Latino | 29,344 (16.6) | 29,262 (16.6) | 82 (17.4) | ||
| Marital status | Married/domestic partner | 108,619 (64.8) | 108,410 (64.9) | 209 (48.9) | <0.001 |
| Single | 36,539 (21.8) | 36,431 (21.8) | 108 (25.3) | ||
| Separated/divorced | 13,900 (8.3) | 13,858 (8.3) | 42 (9.8) | ||
| Widowed | 8485 (5.1) | 8417 (5.0) | 68 (15.9) | ||
| Metropolitan | Metropolitan > 1 M pop | 106,097 (60.2) | 105,788 (60.2) | 309 (66.2) | 0.11 |
| Metropolitan > 250 K–1 M | 39,729 (22.5) | 39,643 (22.5) | 86 (18.4) | ||
| Metropolitan of <250 K | 13,149 (7.5) | 13,118 (7.5) | 31 (6.6) | ||
| Nonmetropolitan adj to a Metropolitan | 9925 (5.6) | 9903 (5.6) | 22 (4.7) | ||
| Nonmetropolitan not adj to Metropolitan | 7370 (4.2) | 7351 (4.2) | 19 (4.1) | ||
| Household annual income | USD 75,000+ | 60,727 (34.4) | 60,547 (34.4) | 180 (38.3) | 0.33 |
| USD 70,000–USD 74,999 | 16,114 (9.1) | 16,069 (9.1) | 45 (9.6) | ||
| USD 65,000–USD 69,999 | 27,760 (15.7) | 27,681 (15.7) | 79 (16.8) | ||
| USD 60,000–USD 64,999 | 28,092 (15.9) | 28,016 (15.9) | 76 (16.2) | ||
| USD 55,000–USD 59,999 | 11,380 (6.4) | 11,360 (6.5) | 20 (4.3) | ||
| USD 50,000–USD 54,999 | 13,498 (7.6) | 13,466 (7.7) | 32 (6.8) | ||
| USD 45,000–USD 49,999 | 7801 (4.4) | 7783 (4.4) | 18 (3.8) | ||
| USD 40,000–USD 44,999 | 5760 (3.3) | 5748 (3.3) | 12 (2.6) | ||
| USD 35,000–USD 39,999 | 3163 (1.8) | 3158 (1.8) | 5 (1.1) | ||
| <USD 35,000 | 2163 (1.2) | 2160 (1.2) | 3 (0.6) |
| Characteristics | Levels | Total (n = 176,472) | Surgery Performed (n = 176,002) | Patient Refused (n = 470) | p-Value |
|---|---|---|---|---|---|
| Previous malignancies | No | 155,637 (88.2) | 155,264 (88.2) | 373 (79.4) | <0.001 |
| Yes | 20,830 (11.8) | 20,733 (11.8) | 97 (20.6) | ||
| Histological Type | Papillary | 166,311 (94.2) | 165,867 (94.2) | 444 (94.5) | 0.91 |
| Follicular | 10,161 (5.8) | 10,135 (5.8) | 26 (5.5) | ||
| T staging | T1 | 90,418 (59.8) | 90,309 (59.8) | 109 (48.9) | <0.001 |
| T2 | 26,415 (17.5) | 26,358 (17.5) | 57 (25.6) | ||
| T3 | 29,482 (19.5) | 29,455 (19.5) | 27 (12.1) | ||
| T4 | 4859 (3.2) | 4829 (3.2) | 30 (13.5) | ||
| N staging | N0 | 128,380 (76.3) | 128,105 (76.3) | 275 (72.4) | 0.08 |
| N1 | 39,855 (23.7) | 39,750 (23.7) | 105 (27.6) | ||
| M staging | M0 | 174,068 (98.8) | 173,641 (98.8) | 427 (91.2) | <0.001 |
| M1 | 2161 (1.2) | 2120 (1.2) | 41 (8.8) | ||
| Extension | Localized | 112,842 (64.5) | 112,594 (64.5) | 248 (62.6) | <0.001 |
| Regional | 57,949 (33.1) | 57,863 (33.1) | 86 (21.7) | ||
| Distant | 4273 (2.4) | 4211 (2.4) | 62 (15.7) |
| Determinant of Surgical Refusal | Odds Ratio | Lower Limit | Upper Limit | p-Value |
|---|---|---|---|---|
| Age: ≥55 vs. <55 years old | 1.570 | 1.122 | 2.197 | 0.009 |
| Sex: Male vs. Female | 1.679 | 1.215 | 2.320 | 0.002 |
| Race: White vs. Black | 1.770 | 1.069 | 2.929 | 0.026 |
| Race: White vs. API | 2.754 | 1.931 | 3.928 | <0.001 |
| Race: White vs. AI/AN | 1.119 | 0.155 | 8.096 | 0.91 |
| Single vs. Married | 1.899 | 1.321 | 2.729 | 0.001 |
| Separated/divorced vs. Married | 1.309 | 0.741 | 2.314 | 0.35 |
| Widowed vs. Married | 4.268 | 2.782 | 6.547 | <0.001 |
| Residency: Urban vs. Rural | 1.271 | 0.714 | 2.264 | 0.42 |
| Income ≥USD 75,000 vs. <USD 75,000 | 1.038 | 0.761 | 1.417 | 0.81 |
| Prior primary malignancy vs. None | 1.169 | 0.787 | 1.735 | 0.43 |
| Histopathology: Follicular vs. Papillary | 0.316 | 0.128 | 0.785 | 0.013 |
| T staging: T3/4 vs. T1/2 | 1.728 | 1.204 | 2.478 | 0.003 |
| N staging: N1 vs. N0 | 0.782 | 0.538 | 1.137 | 0.19 |
| M staging: M1 vs. M0 | 6.402 | 3.717 | 11.026 | <0.001 |
| Characteristics | Levels | Total (n = 176,472) | Surgery Performed (n = 176,002) | Patient Refused (n = 470) | p-Value |
|---|---|---|---|---|---|
| Management | |||||
| Radiotherapy | RAI | 76,331 (43.3) | 76,327 (43.4) | 4 (0.9) | <0.001 |
| Beam radiation | 1863 (1.1) | 1845 (1.0) | 18 (3.8) | ||
| Radioactive implants | 1278 (0.7) | 1278 (0.7) | 0 (0.0) | ||
| Unspecified radiotherapy | 981 (0.6) | 981 (0.6) | 1 (0.0) | ||
| Time to treatment | <1 month | 111,162 (63.5) | 111,109 (63.5) | 53 (60.2) | <0.001 |
| 1–3 months | 57,943 (33.1) | 57,924 (33.1) | 19 (21.6) | ||
| 4–6 months | 4562 (2.6) | 4552 (2.6) | 10 (11.4) | ||
| ≥6 months | 1429 (0.8) | 1423 (0.8) | 6 (6.8) | ||
| Clinical outcomes | |||||
| Second primary malignancy | Positive | 13,597 (8.7) | 13,568 (8.7) | 29 (7.8) | 0.72 |
| Survival status | Died | 15,568 (8.8) | 15,418 (8.8) | 150 (31.9) | <0.001 |
| Risk Factors for Mortality | Frequency | HR (Univariable) | HR (Multivariable) |
|---|---|---|---|
| Age: ≥55 vs. <55 years old | 69,710 (38.2) | 7.74 (7.46–8.02, p < 0.001) | 6.20 (5.96–6.45, p < 0.001) |
| Sex: Male vs. Female | 43,599 (23.9) | 2.22 (2.16–2.29, p < 0.001) | 1.55 (1.50–1.60, p < 0.001) |
| Ethnicity: Hispanic/Latino vs. None | 30,509 (16.7) | 0.78 (0.74–0.82, p < 0.001) | 0.95 (0.91–1.00, p = 0.07) |
| Household income: >USD 75,000 vs. <USD 75,000 | 62,906 (34.4) | 0.79 (0.77–0.82, p < 0.001) | 0.84 (0.81–0.87, p < 0.001) |
| Residency: Urban vs. Rural | 164,605 (90.2) | 0.71 (0.68–0.75, p < 0.001) | 0.79 (0.75–0.82, p < 0.001) |
| Histopathology: Papillary vs. Follicular | 10,662 (5.8) | 1.63 (1.55–1.71, p < 0.001) | 1.31 (1.24–1.38, p < 0.001) |
| Prior primary malignancy vs. None | 22,555 (12.4) | 3.98 (3.85–4.10, p < 0.001) | 2.27 (2.19–2.35, p < 0.001) |
| Surgery: Patient refused vs. Operated | 470 (0.3) | 5.98 (5.09–7.02, p < 0.001) | 3.48 (2.52–4.82, p < 0.001) |
| Extension: Regional vs. Localized | 58,810 (32.8) | 1.28 (1.24–1.32, p < 0.001) | 1.39 (1.34–1.44, p < 0.001) |
| Extension: Distant vs. Localized | 5102 (2.8) | 6.80 (6.48–7.13, p < 0.001) | 4.75 (4.48–5.03, p < 0.001) |
| Delayed treatment: ≥4 vs. <4 months | 6096 (3.5) | 1.64 (1.52–1.78, p < 0.001) | 1.26 (1.16–1.36, p < 0.001) |
| Risk Factors for Disease-Specific Mortality | Frequency | HR (Univariable) | HR (Multivariable) |
|---|---|---|---|
| Age: ≥55 vs. <55 years old | 56,586 (34.5) | 9.11 (8.36–9.92, p < 0.001) | 8.43 (7.71–9.22, p < 0.001) |
| Sex: Male vs. Female | 36,915 (22.5) | 2.72 (2.53–2.91, p < 0.001) | 1.61 (1.50–1.73, p < 0.001) |
| Ethnicity: Hispanic/Latino vs. None | 28,060 (17.1) | 1.11 (1.01–1.22, p = 0.028) | 1.21 (1.10–1.33, p < 0.001) |
| Household income: >USD 75,000 vs. <USD 75,000 | 56,955 (34.8) | 0.85 (0.79–0.91, p < 0.001) | 0.91 (0.85–0.99, p = 0.024) |
| Residency: Urban vs. Rural | 148,123 (90.5) | 0.76 (0.69–0.85, p < 0.001) | 0.80 (0.71–0.89, p < 0.001) |
| Histopathology: Papillary vs. Follicular | 9156 (5.6) | 2.31 (2.08–2.56, p < 0.001) | 1.58 (1.42–1.76, p < 0.001) |
| Prior primary malignancy vs. None | 16,955 (10.3) | 2.63 (2.42–2.87, p < 0.001) | 1.50 (1.37–1.63, p < 0.001) |
| Surgery: Patient refused vs. Operated | 470 (0.3) | 17.3 (9.55–31.4, p < 0.001) | 3.52 (1.93–6.42, p < 0.001) |
| Extension: Regional vs. Localized | 53,950 (33.2) | 4.07 (3.73–4.44, p < 0.001) | 4.44 (4.07–4.85, p < 0.001) |
| Extension: Distant vs. Localized | 3715 (2.3) | 39.9 (36.3–43.9, p < 0.001) | 33.8 (30.7–37.4, p < 0.001) |
| Delayed treatment: ≥4 vs. <4 months | 5483 (3.4) | 1.52 (1.28–1.81, p < 0.001) | 0.96 (0.80–1.14, p = 0.64) |
| Cause of Death | Early Surgery (n = 140,149) | Delayed Surgery (n = 5257) | HR (95% CI) | p-Value |
|---|---|---|---|---|
| Alive | 130,253 | 4760 | Reference | |
| Death | 9896 | 497 | 1.37 (1.25–1.51) | <0.001 |
| Thyroid cancer | 2062 | 97 | 1.28 (1.05–1.58) | 0.016 |
| Nonthyroid cancer | 7834 | 400 | 1.39 (1.25–1.55) | <0.001 |
| Other malignancies | 2670 | 185 | 1.89 (1.62–2.21) | <0.001 |
| Diseases of Heart | 1511 | 60 | 1.08 (0.83–1.4) | 0.53 |
| Cerebrovascular Diseases | 373 | 15 | 1.1 (0.65–1.84) | 0.71 |
| Pneumonia and Influenza | 140 | 13 | 2.54 (1.43–4.48) | 0.001 |
| Accidents and Adverse Effects | 329 | 12 | 0.99 (0.56–1.77) | 0.007 |
| Diabetes Mellitus | 218 | 10 | 1.25 (0.66–2.36) | 0.7 |
| Septicemia | 109 | 9 | 2.25 (1.14–4.46) | 0.018 |
| Chronic Obstructive Pulmonary Disease | 299 | 8 | 0.73 (0.36–1.47) | 0.38 |
| Alzheimer’s (ICD-9 and 10 only) | 178 | 7 | 1.07 (0.51–2.29) | 0.84 |
| Hypertension without Heart Disease | 93 | 7 | 2.05 (0.95–4.44) | 0.06 |
| Nephritis, Nephrotic Syndrome, and Nephrosis | 188 | 5 | 0.73 (0.29–1.76) | 0.48 |
| Suicide and Self-Inflicted Injury | 90 | 3 | 0.91 (0.28–2.88) | 0.87 |
| Chronic Liver Disease and Cirrhosis | 72 | 2 | 0.76 (0.18–3.09) | 0.7 |
| Other Infectious and Parasitic Diseases, including HIV | 75 | 2 | 0.79 (0.18–2.97) | 0.66 |
| Study Characteristics | Data Source | Study Population | Sample Size | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Author, Year (Ref.) | Country | Total | Surgery | No Surgery | Cancer-Directed Surgery Cohort | No Surgery Cohort | ||
| Wu, 2023 [16] | USA | Institutional (2000–2021) | ATC | 97 | 44 | 53 | Higher OS | Lower OS |
| Chaves, 2022 [17] | USA | SEER (1999–2018) | Medicare beneficiaries, PTC | 8170 | 8170 | - | Surgery (<90 days): high OS, DSS Surgery (>180 days): lower OS, DSS | |
| Sahli, 2021 [18] | USA | SEER | MTC (Exclude lobectomy) | 1367 | 1301 | 66 | Lower disease-specific mortality | Higher disease-specific mortality |
| Zhou, 2020 [19] | CHN | SEER (1973–2015) | >85 years old, PTC | 1196 | 871 | 325 | Higher OS | Lower OS |
| Van Gerwen, 2020 [1] | USA | SEER (1988–2015) | Localized and regional, PTC | 45,136 | 44,990 | 146 | Higher DSS time | Lower DSS times * |
| Maniakas, 2020 [20] | USA | Tertiary Care Center (2000–2019) | ATC | 479 | 55 | 424 | 1-year survival; 94% | 1-year survival; 52% |
| Corrigan, 2019 [21] | USA | Institutional (1990–2015) | ATC | 28 | 19 | 9 | Higher OS | Lower OS |
| Megwalu, 2017 [6] | USA | SEER (1988–2009) | >65 years old, PTC | 2323 | - | - | 5-year survival rate; 91% | 5-year survival rate; 23% |
| Pierie, 2002 [22] | USA | Tertiary Care Center (1969–1999) | ATC | 67 | 44 | 23 | Higher 6-month, 1- and 3-year survival rates | Lower 6-month, 1 and 3-year survival rates |
| Study Characteristics | Data Source | Study Population | Sample Size | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Author, Year (Ref.) | Country | Total | Surgery | No Surgery | Cancer-Directed Surgery Cohort | No Surgery Cohort | ||
| Chen, 2023 [23] | China | Institutional (2010–2019) | Localized and advanced Prostate cancer | 19,729 | 6339 | 13,390 | Reduced rates of cancer-specific and overall mortality | Increased rates of cancer-specific and overall mortality |
| Birkenbeuel, 2022 [4] | USA | NCDB (2004–2015) | Pituitary macroadenoma | 34,226 | 33,946 | 280 | Increased OS | Reduced OS * |
| Sun, 2022 [9] | China | SEER (2010–2015) | Stage IV gastric cancer | 6284, matched 864 | 432 | 432 | Prolonged median survival time | Reduced median survival time |
| Silva, 2021 [24] | Brazil | Hospital Haroldo Juaçaba (2000–2014) | Oral Squamous Cell Carcinoma | 934 | - | - | Higher OS | Lower OS |
| Coffman, 2021 [3] | USA | NCDB (2004–2015) | Rectal adenocarcinoma | 55,704 | 54,266 | 1438 | Median survival time 84.4 months | Median survival time 48.5 months * |
| Delisle, 2020 [8] | USA | SEER (2004–2015) | Colorectal | 153,698 | 152,731 | 983 | Lower disease-specific mortality | Higher disease-specific mortality |
| Coffman, 2019 [25] | USA | NCDB (2004–2013) | Nonmetastatic pancreatic adenocarcinoma | 48,902 | 47,107 | 1795 | Higher median survival times | Lower median survival times * |
| May 2017 [26] | Canada | Tom Baker Cancer Center (not provided) | Stage IIIC or IV serous ovarian carcinoma | 303 | 142 | 161 | 5-year survival; 39% | 5-year survival; 27% |
| Wang, 2010 [7] | USA | SEER (not provided) | Hepatocellular carcinoma | 4373 | 4231 | 142 | Lower mortality risk | 2.5-fold increased mortality risk * |
| Verkooijen, 2005 [27] | Switzerland | Geneva Cancer Database (1975–2000) | <80 years old, nonmetastatic | 5339 | 5269 | 70 | Lower mortality risk | 2.1-fold increased mortality risk * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, M.H.; Toraih, E.A.; Ohiomah, I.E.; Siddeeque, N.; Comeaux, M.; Landau, M.B.; Anker, A.; Jishu, J.A.; Fawzy, M.S.; Kandil, E. Navigating Choices: Determinants and Outcomes of Surgery Refusal in Thyroid Cancer Patients Using SEER Data. Cancers 2023, 15, 3699. https://doi.org/10.3390/cancers15143699
Hussein MH, Toraih EA, Ohiomah IE, Siddeeque N, Comeaux M, Landau MB, Anker A, Jishu JA, Fawzy MS, Kandil E. Navigating Choices: Determinants and Outcomes of Surgery Refusal in Thyroid Cancer Patients Using SEER Data. Cancers. 2023; 15(14):3699. https://doi.org/10.3390/cancers15143699
Chicago/Turabian StyleHussein, Mohammad H., Eman A. Toraih, Ifidon E. Ohiomah, Nabeela Siddeeque, Marie Comeaux, Madeleine B. Landau, Allison Anker, Jessan A. Jishu, Manal S. Fawzy, and Emad Kandil. 2023. "Navigating Choices: Determinants and Outcomes of Surgery Refusal in Thyroid Cancer Patients Using SEER Data" Cancers 15, no. 14: 3699. https://doi.org/10.3390/cancers15143699
APA StyleHussein, M. H., Toraih, E. A., Ohiomah, I. E., Siddeeque, N., Comeaux, M., Landau, M. B., Anker, A., Jishu, J. A., Fawzy, M. S., & Kandil, E. (2023). Navigating Choices: Determinants and Outcomes of Surgery Refusal in Thyroid Cancer Patients Using SEER Data. Cancers, 15(14), 3699. https://doi.org/10.3390/cancers15143699









