Promising Highly Targeted Therapies for Cholangiocarcinoma: A Review and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
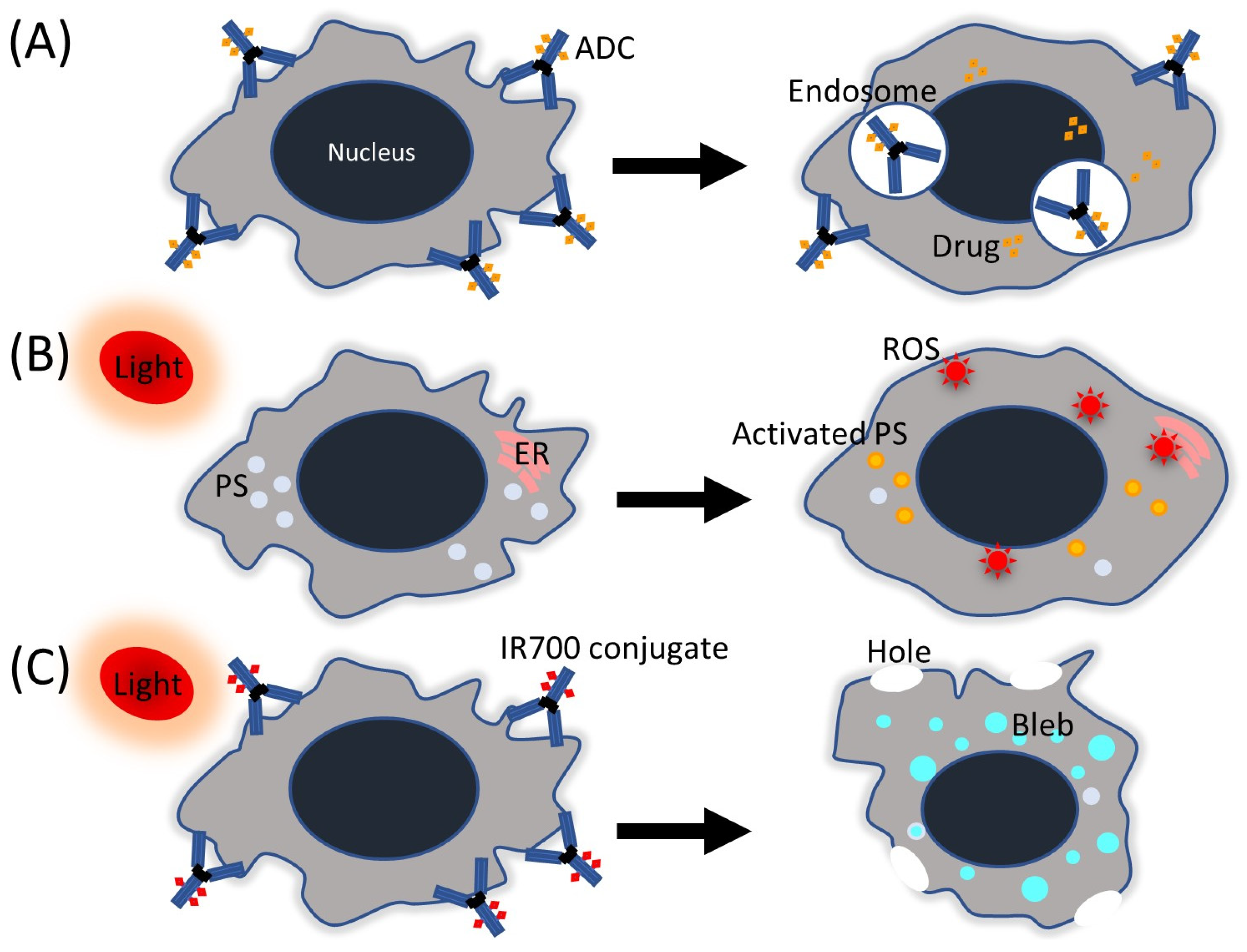
2. Antibody-Drug Conjugate
2.1. Preclinical Studies of ADCs for CCA
2.2. Human Studies of ADCs for CCA
3. Photodynamic Therapy
3.1. Photosensitizers
3.2. Light Sources for PDT
3.3. Human Clinical Studies on PDT
| Investigations for CCA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Photosensitizer | Potential Indications | Activation Wavelength | Human | Animal | In Vitro | LED Effect | LED Wavelength | Reports Regarding CCA * |
| Photodynamic therapy (PDT) | ||||||||
| HPD (partially purified) porfimer sodium | Cervical, endobronchial, oesophageal, bladder, gastric cancers, brain tumor, bile duct cancer | 630 nm | ✓ | ✓ | ✓ | ✓ | 620–650 nm | [60,61,75,76,77,78,79,80,81,82,83] |
| Phosphorus tetraphenylporphyrin | Bile duct cancer | 610 nm | ✓ | ✓ | 610 nm | [72,73,74] | ||
| m-THPC (Temoporfin) | Head and neck, prostate, pancreas, lung, skin, bile duct cancer | 652 nm | ✓ | ✓ | ✓ | [62,75] | ||
| Phthalocyanine-4 | Cutaneous/subcutaneous lesions from diverse solid tumor origins, bile duct cancer | 670 nm | ✓ | ✓ | [63] | |||
| Taporfin sodium (Talaporfin, NPe6) | Lung, liver metastasis, pancreas, colon, brain cancer, bile duct cancer, solid tumors from diverse origins | 664 nm | ✓ | ✓ | ✓ | ✓ | 660 nm | [64,65,66,73] |
| Chlorine e6 derivatives | Nasopharyngeal, sarcoma, brain, pancreaticobiliary malignancies | 660 nm | ✓ | ✓ | ✓ | [67,68,75] | ||
| Chlorin I/chlorin II | Bile duct cancer | 650 nm | ✓ | ✓ | [69] | |||
| Nanoparticle albumin-bound mTHPC | Bile duct cancer | 652 nm | ✓ | [70] | ||||
| ITLs encapsulating ZnPC and AlPC | Breast, bile duct cancer | 671 nm | ✓ | [71] | ||||
| 5-ALA | Basal-cell carcinoma, head and neck, gynaecological tumors brain, bladder tumors | 635 nm 375–400 nm | ||||||
| 5-ALA-methylesther | Basal-cell carcinoma | 635 nm | ||||||
| 5-ALA benzylesther | Gastrointestinal cancer | 635 nm | ||||||
| 5-ALA hexylesther | Bladder tumors | 375–400 nm | ||||||
| Boronated protoporphyrin | Brain tumors | 630 nm | ||||||
| BPD-MA (benzoporphyrin) | Basal-cell carcinoma | 689 nm | ||||||
| HPPH | Basal-cell carcinoma, head and neck, esophagus, lung cancer | 665 nm | ||||||
| Lutetium texaphyrin | Cervical, prostate and brain tumours | 732 nm | ||||||
| Motexafin lutetium (Mlu) | Prostate cancer | 732 nm | ||||||
| Padeliporfin | Prostate cancer | 762 nm | ||||||
| Redaporfin | Head and neck cancer | 749 nm | ||||||
| Silicon phthalocyanine | Cutaneous T-cell lymphoma | 675 nm | ||||||
| SnET2 | Cutaneous metastatic breast cancer, basal-cell carcinoma, Kaposi’s sarcoma, prostate cancer | 664 nm | ||||||
| Verteporfin | Skin, pancreas cancer | 690 nm | ||||||
| Photoimmunotherapy (PIT) | ||||||||
| IR700 (IRDye 700DX N-hydroxysuccinimide ester) | Head and neck can, stomach, esophagus, pancreas, bile duct cancer | 690 nm | ✓ | ✓ | ✓ | 680–700 nm | [88,89] | |
4. Photoimmunotherapy
4.1. Antibody and Low Molecular Weight (LMW) Compound for Targeting Cancer Cells with IR700
4.2. Human Clinical Studies on PIT
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Nakanuma, Y.; Hoso, M.; Sanzen, T.; Sasaki, M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc. Res. Tech. 1997, 38, 552–570. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [PubMed]
- Kamsa-Ard, S.; Luvira, V.; Suwanrungruang, K.; Kamsa-Ard, S.; Luvira, V.; Santong, C.; Srisuk, T.; Pugkhem, A.; Bhudhisawasdi, V.; Pairojkul, C. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand from 1989 through 2013: A populationbased cancer registry study. J. Epidemiol. 2019, 29, 197–204. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Center Japan. Cancer Statistics in Japan 2023; National Cancer Center Japan: Tokyo, Japan, 2023; Available online: https://ganjoho.jp/public/qa_links/report/statistics/2023_en.html (accessed on 30 April 2023).
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 26–54. [Google Scholar]
- Sutherland, M.; Ahmed, O.; Zaidi, A.; Ahmed, S. Current progress in systemic therapy for biliary tract cancers. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 1094–1107. [Google Scholar] [CrossRef]
- Hosseini Shabanan, S.; Nezami, N.; Abdelsalam, M.E.; Sheth, R.A.; Odisio, B.C.; Mahvash, A.; Habibollahi, P. Selective Internal Radiation Therapy with Yttrium-90 for Intrahepatic Cholangiocarcinoma: A Systematic Review on Post-Treatment Dosimetry and Concomitant Chemotherapy. Curr. Oncol. 2022, 29, 3825–3848. [Google Scholar]
- Taggar, A.S.; Mann, P.; Folkert, M.R.; Aliakbari, S.; Myrehaug, S.D.; Dawson, L.A. A systematic review of intraluminal high dose rate brachytherapy in the management of malignant biliary tract obstruction and cholangiocarcinoma. Radiother. Oncol. 2021, 165, 60–74. [Google Scholar] [CrossRef]
- Wang, N.; Huang, A.; Kuang, B.; Xiao, Y.; Xiao, Y.; Ma, H. Progress in Radiotherapy for Cholangiocarcinoma. Front. Oncol. 2022, 12, 868034. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; TOPAZ-1 Investigators; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin. Cancer Res. 2018, 246, 1277–1286. [Google Scholar]
- Wiggers, J.K.; Ruys, A.T.; Groot Koerkamp, B.; Beuers, U.; ten Kate, F.J.; van Gulik, T.M. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2014, 29, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Javle, M. Molecular profiling of biliary tract cancer: A target rich disease. J. Gastrointest. Oncol. 2016, 7, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Wang, Y.; Carpino, G.; Mendel, G.; Alpini, G.; Gaudio, E.; Reid, L.M.; Alvaro, D. The biliary tree—A reservoir of multipotent stem cells. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 231–240. [Google Scholar] [PubMed]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar]
- Mathe, G.; Lou, T.B.; Bernard, J. Effet sur la leucemie 1210 de la souris dune combinaison par diazotation da-methopterine et de gamma- globulines de hamsters porteurs de cette leucemie par heterogreffe. Presse Med. 1958, 66, 571. [Google Scholar]
- Yu, J.F.; Song, Y.P.; Tian, W. How to select IgG subclasses in developing anti- tumor therapeutic antibodies. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar]
- Kang, T.H.; Jung, S.T. Reprogramming the Constant Region of Immunoglobulin G Subclasses for Enhanced Therapeutic Potency against Cancer. Biomolecules 2020, 10, 382. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar]
- Senter, P.D. Potent antibody drug conjugates for cancer therapy. Curr. Opin. Chem. Biol. 2009, 13, 235–244. [Google Scholar] [CrossRef]
- Agarwal, P.; Bertozzi, C.R. Site- specific antibodydrug conjugates: The nexus of biorthogonal chemistry, protein engineering, and drug development. Bioconjugate Chem. 2015, 26, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Yamashita-Kashima, Y.; Yoshimura, Y.; Fujimura, T.; Shu, S.; Yanagisawa, M.; Yorozu, K.; Furugaki, K.; Higuchi, R.; Shoda, J.; Harada, N. Molecular targeting of HER2-overexpressing biliary tract cancer cells with trastuzumab emtansine, an antibody-cytotoxic drug conjugate. Cancer Chemother. Pharmacol. 2019, 83, 659–671. [Google Scholar] [PubMed]
- Shinoda, M.; Kudo, T.; Suzuki, M.; Katayose, Y.; Sakurai, N.; Saeki, H.; Kodama, H.; Fukuhara, K.; Imai, K.; Hinoda, Y.; et al. Effective adoptive immunotherapy by T-LAK cells retargeted with bacterial superantigen-conjugated antibody to MUC1 in xenografted severe combined immunodeficient mice. Cancer Res. 1998, 58, 2838–2843. [Google Scholar] [PubMed]
- Yokota, K.; Serada, S.; Tsujii, S.; Toya, K.; Takahashi, T.; Matsunaga, T.; Fujimoto, M.; Uemura, S.; Namikawa, T.; Murakami, I.; et al. Anti-Glypican-1 Antibody-drug Conjugate as Potential Therapy Against Tumor Cells and Tumor Vasculature for Glypican-1-Positive Cholangiocarcinoma. Mol. Cancer Ther. 2021, 20, 1713–1722. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127. [Google Scholar]
- Wang, Z. ErbB receptors and cancer. In ErbB Receptor Signaling: Methods and Protocols; Wang, Z., Ed.; Springer: New York, NY, USA, 2017; pp. 3–35. [Google Scholar]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef]
- West, E.J.; Scott, K.J.; Jennings, V.A.; Melcher, A.A. Immune activation by combination human lymphokine-activated killer and dendritic cell therapy. Br. J. Cancer 2011, 105, 787–795. [Google Scholar] [CrossRef]
- White, J.; Herman, A.; Pullen, A.M.; Kubo, R.; Kappler, J.W.; Marrack, P. The Vβ-specific superantigen staphylococcal enlerotoxin B: Stimulation of mature T cells and clonal deletion in neonatal mice. Cell 1989, 56, 27–35. [Google Scholar] [CrossRef]
- Marrack, P.; Kappler, J. The staphylococcal enterotoxins and their relatives. Science 1990, 248, 705–711. [Google Scholar]
- Ioannides, C.G.; Fisk, B.; Jerome, K.R.; Irimura, T.; Wharton, J.T.; Finn, O.J. Cytoloxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J. Immunol. 1993, 151, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Imai, K.; Yachi, A. Immunohistological and immunochemical characterization of a novel pancreatic cancer-associated antigen MUSE11. Cancer Res. 1989, 49, 7141–7146. [Google Scholar] [PubMed]
- Yamashita, K.; Yonezawa, S.; Tanaka, S.; Shirahama, H.; Sakoda, K.; Imai, K.; Xing, P.X.; McKenzie, I.F.; Hilkens, J.; Kim, Y.S.; et al. Immunohistochemical study of mucin carbohydrates and core proteins in hepatolithiasis and cholangiocarcinoma. Int. J. Cancer 1993, 55, 82–91. [Google Scholar] [PubMed]
- Lund, M.E.; Campbell, D.H.; Walsh, B.J. The role of Glypican-1 in the tumour microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 163–176. [Google Scholar]
- Smith, L.M.; Nesterova, A.; Ryan, M.C.; Duniho, S.; Jonas, M.; Anderson, M.; Zabinski, R.F.; Sutherland, M.K.; Gerber, H.P.; Van Orden, K.L.; et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br. J. Cancer. 2008, 99, 100–109. [Google Scholar] [CrossRef]
- Mondaca, S.; Razavi, P.; Xu, C.; Offin, M.; Myers, M.; Scaltriti, M.; Hechtman, J.F.; Bradley, M.; O’Reilly, E.M.; Berger, M.F.; et al. Genomic Characterization of ERBB2-Driven Biliary Cancer and a Case of Response to Ado-Trastuzumab Emtansine. JCO Precis. Oncol. 2019, 3, PO.19.00223. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar]
- Kim, S.B.; Meric-Bernstam, F.; Kalyan, A.; Babich, A.; Liu, R.; Tanigawa, T.; Sommer, A.; Osada, M.; Reetz, F.; Laurent, D.; et al. First-in-Human Phase I Study of Aprutumab Ixadotin, a Fibroblast Growth Factor Receptor 2 Antibody-Drug Conjugate (BAY 1187982) in Patients with Advanced Cancer. Target Oncol. 2019, 14, 591–601. [Google Scholar] [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Ojima, H.; Nakamura, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar]
- Graham, R.P.; Barr Fritcher, E.G.; Pestova, E.; Schulz, J.; Sitailo, L.A.; Vasmatzis, G.; Murphy, S.J.; McWilliams, R.R.; Hart, S.N.; Halling, K.C.; et al. Fibroblast growth factor receptor 2 teanslocations in intrahepatic cholangiocarcinoma. Hum. Pathol. 2014, 45, 1630–1638. [Google Scholar] [CrossRef]
- Maruki, Y.; Morizane, C.; Arai, Y.; Ikeda, M.; Ueno, M.; Ioka, T.; Naganuma, A.; Furukawa, M.; Mizuno, N.; Uwagawaet, T.; et al. Molecular detection and clinicopathological characteristics of advanced/recurrent biliary tract carcinomas harboring the FGFR2 rearrangements: A prospective observational study (PRELUDE Study). J. Gastroenterol. 2021, 56, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Raab, O. Uber die Wirkung, fluorescirender Stoffe auf infusorien. Z. Biol. 1900, 39, 524–546. [Google Scholar]
- von Tappeiner, H.; Jodlbauer, A. Über die Wirkung der photodynamischen (fluorescierenden) Stoffe auf Protozoen und Enzyme. Dtsch. Arch. Klin. Med. 1904, 39, 427–487. [Google Scholar]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.; Granelli, S.G.; McDonagh, A.F.; Nielsen, S.; Wilson, C.B.; Jaenicke, R. Photodynamic therapy of malignant tumours. Lancet 1972, 2, 1175–1177. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta. Rev. Cancer 2019, 1872, 188308. [Google Scholar]
- Yang, H.; Ma, Y.; Chen, G.; Zhou, H.; Yamazaki, T.; Klein, C.; Pietrocola, F.; Vacchelli, E.; Souquere, S.; Sauvat, A.; et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology 2016, 5, e1149673. [Google Scholar] [CrossRef]
- Aaes, T.L.; Kaczmarek, A.; Delvaeye, T.; De Craene, B.; De Koker, S.; Heyndrickx, L.; Delrue, I.; Taminau, J.; Wiernicki, B.; De Groote, P.; et al. Vaccination with Necroptotic cancer cells induces efficient antitumor immunity. Cell Rep. 2016, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin derivative: A possible aid in the diagnosis and treatment of carcinoma of the bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380. [Google Scholar] [CrossRef] [PubMed]
- Auler, H.; Banzer, G. Untersuchungen uber die Rolle der Porphyrine bei geschwulstkranken Menschen und Tieren. Z. Für Krebsforsch. 1942, 53, 65–68. [Google Scholar] [CrossRef]
- Figge, F.H.J.; Weiland, G.S. The affinity of neoplastic embryonic and traumatized tissue for porphyrins and metalloporphyrins. Anat. Rec. 1948, 100, 659. [Google Scholar]
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation therapy II: Cure of animal tumors with haematoporphyrin and light. J. Natl. Cancer Inst. 1975, 55, 115–121. [Google Scholar] [CrossRef]
- Rumalla, A.; Baron, T.H.; Wang, K.K.; Gores, G.J.; Stadheim, L.M.; de Groen, P.C. Endoscopic application of photodynamic therapy for cholangiocarcinoma. Gastrointest. Endosc. 2001, 53, 500–504. [Google Scholar] [CrossRef]
- Oertel, M.; Schastak, S.I.; Tannapfel, A.; Hermann, R.; Sack, U.; Mössner, J.; Berr, F. Novel bacteriochlorine for high tissue-penetration: Photodynamic properties in human biliary tract cancer cells in vitro and in a mouse tumour model. J. Photochem. Photobiol. B 2003, 71, 1–10. [Google Scholar] [CrossRef]
- Wagner, A.; Denzer, U.W.; Neureiter, D.; Kiesslich, T.; Puespoeck, A.; Rauws, E.A.; Emmanuel, K.; Degenhardt, N.; Frick, U.; Beuers, U.; et al. Temoporfin improves efficacy of photodynamic therapy in advanced biliary tract carcinoma: A multicenter prospective phase II study. Hepatology 2015, 62, 1456–1465. [Google Scholar] [CrossRef]
- Schmidt, J.; Kuzyniak, W.; Berkholz, J.; Steinemann, G.; Ogbodu, R.; Hoffmann, B.; Nouailles, G.; Gürek, A.G.; Nitzsche, B.; Höpfner, M. Novel zinc- and silicon-phthalocyanines as photosensitizers for photodynamic therapy of cholangiocarcinoma. Int. J. Mol. Med. 2018, 42, 534–546. [Google Scholar] [CrossRef]
- Nanashima, A.; Hiyoshi, M.; Imamura, N.; Hamada, T.; Nishida, T.; Kawakami, H.; Ban, T.; Kubota, Y.; Nakashima, K.; Yano, K.; et al. Two cases of bile duct carcinoma patients who underwent the photodynamic therapy using talaporfin sodium (Laserphyrin®). Clin. J. Gastroenterol. 2020, 13, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Abo, T.; Nonaka, T.; Nonaka, Y.; Morisaki, T.; Uehara, R.; Ohnita, K.; Fukuda, D.; Murakami, G.; Tou, K.; et al. Photodynamic therapy using talaporfin sodium (Laserphyrin®) for bile duct carcinoma: A preliminary clinical trial. Anticancer Res. 2012, 32, 4931–4938. [Google Scholar] [CrossRef] [PubMed]
- Murakami, G.; Nanashima, A.; Nonaka, T.; Tominaga, T.; Wakata, K.; Sumida, Y.; Akashi, H.; Okazaki, S.; Kataoka, H.; Nagayasu, T. Photodynamic Therapy Using Novel Glucose-conjugated Chlorin Increases Apoptosis of Cholangiocellular Carcinoma in Comparison with Talaporfin Sodium. Anticancer. Res. 2016, 36, 4493–4501. [Google Scholar] [CrossRef]
- Choi, J.H.; Oh, D.; Lee, J.H.; Park, J.H.; Kim, K.P.; Lee, S.S.; Lee, Y.J.; Lim, Y.S.; Song, T.J.; Lee, S.S.; et al. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy 2015, 47, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xia, J.; Gao, Y.; Chen, Z.; Wan, X. Chlorin A-mediated photodynamic therapy induced apoptosis in human cholangiocarcinoma cells via impaired autophagy flux. Am. J. Transl. Res. 2020, 12, 5080–5094. [Google Scholar] [PubMed]
- Gao, Y.H.; Li, M.Y.; Sajjad, F.; Wang, J.H.; Meharban, F.; Gadoora, M.A.; Yan, Y.J.; Nyokong, T.; Chen, Z.L. Synthesis and pharmacological evaluation of chlorin derivatives for photodynamic therapy of cholangiocarcinoma. Eur. J. Med. Chem. 2020, 189, 112049. [Google Scholar] [CrossRef]
- Stein, N.C.; Mulac, D.; Fabian, J.; Herrmann, F.C.; Langer, K. Nanoparticle albumin-bound mTHPC for photodynamic therapy: Preparation and comprehensive characterization of a promising drug delivery system. Int. J. Pharm. 2020, 582, 119347. [Google Scholar] [CrossRef]
- Dias, L.M.; de Keijzer, M.J.; Ernst, D.; Sharifi, F.; de Klerk, D.J.; Kleijn, T.G.; Desclos, E.; Kochan, J.A.; de Haan, L.R.; Franchi, L.P.; et al. Photodynamic Therapy Study Group. Metallated phthalocyanines and their hydrophilic derivatives for multi-targeted oncological photodynamic therapy. J. Photochem. Photobiol. B 2022, 234, 112500. [Google Scholar] [CrossRef]
- Whitehurst, C.; Byrne, K.; Moore, J.V. Development of an alternative light source to lasers for photodynamic therapy: 1. Comparative in vitro dose response characteristics. Lasers Med. Sci. 1993, 8, 259–267. [Google Scholar] [CrossRef]
- Matsumoto, J.; Suzuki, K.; Yasuda, M.; Yamaguchi, Y.; Hishikawa, Y.; Imamura, N.; Nanashima, A. Photodynamic therapy of human biliary cancer cell line using combination of phosphorus porphyrins and light emitting diode. Bioorg. Med. Chem. 2017, 25, 6536–6541. [Google Scholar] [CrossRef]
- Mai, N.N.H.; Yamaguchi, Y.; Choijookhuu, N.; Matsumoto, J.; Nanashima, A.; Takagi, H.; Sato, K.; Tuan, L.Q.; Hishikawa, Y. Photodynamic Therapy Using a Novel Phosphorus Tetraphenylporphyrin Induces an Anticancer Effect via Bax/Bcl-xL-related Mitochondrial Apoptosis in Biliary Cancer Cells. Acta Histochem. Cytochem. 2020, 53, 61–72. [Google Scholar] [CrossRef]
- Shi, X.; Yin, H.; Dong, X.; Li, H.; Li, Y. Photodynamic therapy with light-emitting diode arrays producing different light fields induces apoptosis and necrosis in gastrointestinal cancer. Front. Oncol. 2022, 12, 1062666. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Hiyoshi, M.; Imamura, N.; Yano, K.; Hamada, T.; Kai, K. Recent Advances in Photodynamic Imaging and Therapy in Hepatobiliary Malignancies: Clinical and Experimental Aspects. Curr. Oncol. 2021, 28, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- McCaughan JSJr Mertens, B.F.; Cho, C.; Barabash, R.D.; Payton, H.W. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch. Surg. 1991, 126, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Abulafi, A.M.; Allardice, J.T.; Williams, N.S.; van Someren, N.; Swain, C.P.; Ainley, C. Photodynamic therapy for malignant tumours of the ampulla of Vater. Gut 1995, 36, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, F.L.; Gerhardt, T.; Fuchs, S.; Scheurlen, C.; Neubrand, M.; Layer, G.; Sauerbruch, T. Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma. Gastrointest. Endosc. 2003, 57, 860–867. [Google Scholar] [CrossRef]
- Cheon, Y.K.; Lee, T.Y.; Lee, S.M.; Yoon, J.Y.; Shim, C.S. Longterm outcome of photodynamic therapy compared with biliary stenting alone in patients with advanced hilar cholangiocarcinoma. HPB 2012, 14, 185–193. [Google Scholar] [CrossRef]
- Lee, T.Y.; Cheon, Y.K.; Shim, C.S.; Cho, Y.D. Photodynamic therapy prolongs metal stent patency in patients with unresectable hilar cholangiocarcinoma. World J. Gastroenterol. 2012, 18, 5589–5594. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Xiao, H.; Chen, S.; Zhu, W.; Lu, H.; Cao, L.; Xue, P.; Li, H.; Zhang, D. Long-term results of ERCP- or PTCS-directed photodynamic therapy for unresectable hilar cholangiocarcinoma. Surg. Endosc. 2021, 35, 5655–5664. [Google Scholar] [CrossRef]
- Quyn, A.J.; Ziyaie, D.; Polignano, F.M.; Tait, I.S. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB 2009, 11, 570–577. [Google Scholar] [CrossRef]
- Witzigmann, H.; Berr, F.; Ringel, U.; Caca, K.; Uhlmann, D.; Schoppmeyer, K.; Tannapfel, A.; Wittekind, C.; Mossner, J.; Hauss, J.; et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: Palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann. Surg. 2006, 244, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, T.; Shi, P.; Shen, J.; Feng, Q.; Su, J. Benefits and safety of photodynamic therapy in patients with hilar cholangiocarcinoma: A meta-analysis. Photodiagnosis Photodyn. Ther. 2022, 37, 102712. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An Emerging Treatment Option for Solid Tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, N.; Wang, Y.; Shi, Q.; Yu, R.; Gu, B.; Maswikiti, E.P.; Chen, H. Photodynamic therapy combined with systemic chemotherapy for unresectable extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2023, 41, 103318. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Yoneda, M. Recent Updates on Local Ablative Therapy Combined with Chemotherapy for Extrahepatic Cholangiocarcinoma: Photodynamic Therapy and Radiofrequency Ablation. Curr. Oncol. 2023, 30, 2159–2168. [Google Scholar] [CrossRef]
- Hirata, H.; Kuwatani, M.; Nakajima, K.; Kodama, Y.; Yoshikawa, Y.; Ogawa, M.; Sakamoto, N. Near-infrared photoimmunotherapy (NIR-PIT) on cholangiocarcinoma using a novel catheter device with light emitting diodes. Cancer Sci. 2021, 112, 828–838. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Pantarat, N.; Suzuki, T.; Evdokiou, A. Near-Infrared Photoimmunotherapy Using a Small Protein Mimetic for HER2-Overexpressing Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5835. [Google Scholar] [CrossRef]
- Wilson, B.C.; Patterson, M.S. The determination of light fluence distributions in photodynamic therapy. In Photodynamic Therapy of Neoplastic Disease; Kessel, D., Ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume 1, pp. 129–144. [Google Scholar]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Huang, X.; Ding, J.; Bi, A.; Wang, S.; Chen, F.; Zeng, W. NIR-I Dye-Based Probe: A New Window for Bimodal Tumor Theranostics. Front. Chem. 2022, 10, 859948. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, T.M.; Zhang, C.; Sheng, W.; Al-Rawe, M.; Zeppernick, F.; Meinhold-Heerlein, I.; Hussain, A.F. Near Infrared Photoimmunotherapy: A Review of Recent Progress and Their Target Molecules for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2655. [Google Scholar] [CrossRef]
- Nakajima, K.; Miyazaki, F.; Terada, K.; Takakura, H.; Suzuki, M.; Ogawa, M. Comparison of Low-Molecular-Weight Ligand and Whole Antibody in Prostate-Specific Membrane Antigen Targeted near-Infrared Photoimmunotherapy. Int. J. Pharm. 2021, 609, 121135. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Oda, T.; Kiyoi, K.; Yusuke, O.; Kimura, S.; Kurimori, K.; Miyazaki, Y.; Yu, Y.; Furuya, K.; Akashi, Y.; et al. Carcinoembryonic antigen as a specific glycoprotein ligand of rBC2LCN lectin on pancreatic ductal adenocarcinoma cells. Cancer Sci. 2021, 112, 3722–3731. [Google Scholar] [CrossRef]
- Kuroda, Y.; Oda, T.; Shimomura, O.; Hashimoto, S.; Akashi, Y.; Miyazaki, Y.; Furuya, K.; Furuta, T.; Nakahashi, H.; Louphrasitthiphol, P.; et al. Lectin-based phototherapy targeting cell surface glycans for pancreatic cancer. Int. J. Cancer 2023, 152, 1425–1437. [Google Scholar] [CrossRef]
- Nishimura, T.; Mitsunaga, M.; Sawada, R.; Saruta, M.; Kobayashi, H.; Matsumoto, N.; Kanke, T.; Yanai, H.; Nakamura, K. Photoimmunotherapy targeting biliary-pancreatic cancer with humanized anti-TROP2 antibody. Cancer Med. 2019, 8, 7781–7792. [Google Scholar] [CrossRef]
- Nishikawa, D.; Suzuki, H.; Beppu, S.; Terada, H.; Sawabe, M.; Hanai, N. Near-Infrared Photoimmunotherapy for Oropharyngeal Cancer. Cancers 2022, 14, 5662. [Google Scholar] [CrossRef]
- Urman, J.M.; Herranz, J.M.; Uriarte, I.; Rullán, M.; Oyón, D.; González, B.; Fernandez-Urién, I.; Carrascosa, J.; Bolado, F.; Zabalza, L.; et al. Pilot Multi-Omic Analysis of Human Bile from Benign and Malignant Biliary Strictures: A Machine-Learning Approach. Cancers 2020, 12, 1644. [Google Scholar] [CrossRef]
| Preclinical Study | Clinical Study | |||||
|---|---|---|---|---|---|---|
| Name | Type | ADC | ADC-Payload | PIT | ADC | ADC-Payload |
| HER2 | Receptor | ✓ | Emtansine (DM1) | ✓ | ✓ | Emtansine (DM1) Deruxtecan (DXd) |
| EGFR | Receptor | ✓ | ||||
| FGFR2 | Receptor | ✓ | Ixadotin | |||
| MUC1 | Secretion (mucin) | ✓ | ||||
| Glypican-1 (GPC1) | Secretion (proteoglycan) | ✓ | Monomethyl auristatin F (MMAF) | |||
| CD133/ prominin-1 | Secretion (glycoprotein) | ✓ | Maleimidocaproyl-valine-citrulline-p-aminobenzoyl-MMAF (vcMMAF) | |||
| TROP2 | Secretion (glycoprotein) | ✓ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwatani, M.; Sakamoto, N. Promising Highly Targeted Therapies for Cholangiocarcinoma: A Review and Future Perspectives. Cancers 2023, 15, 3686. https://doi.org/10.3390/cancers15143686
Kuwatani M, Sakamoto N. Promising Highly Targeted Therapies for Cholangiocarcinoma: A Review and Future Perspectives. Cancers. 2023; 15(14):3686. https://doi.org/10.3390/cancers15143686
Chicago/Turabian StyleKuwatani, Masaki, and Naoya Sakamoto. 2023. "Promising Highly Targeted Therapies for Cholangiocarcinoma: A Review and Future Perspectives" Cancers 15, no. 14: 3686. https://doi.org/10.3390/cancers15143686
APA StyleKuwatani, M., & Sakamoto, N. (2023). Promising Highly Targeted Therapies for Cholangiocarcinoma: A Review and Future Perspectives. Cancers, 15(14), 3686. https://doi.org/10.3390/cancers15143686








