Simple Summary
Nowadays, biosimilar drugs are numerous and widely used in many clinical fields, including oncology. However, skepticism remains towards these products among doctors and patients, particularly regarding their safety profile compared to the reference products. This prompted this comparative pharmacovigilance study using real-world clinical data. Consistent with the expected similarity in safety, our results reaffirm that biosimilars are comparable to the reference products in the real-world setting. This should further reassure and encourage their even greater use which, on the one hand, allows for all patients to be treated with the best available treatments and, on the other, frees up healthcare resources for innovative and more expensive drugs.
Abstract
In the last decades, the clinical management of oncology patients has been transformed by the introduction of biologics. The high costs associated with the development and production of biologics limit patient access to these therapies. The expiration of exclusive patents for biologics has led to the development and market introduction of biosimilars, offering the reduction of costs for cancer treatments. Biosimilars are highly similar to the reference products in terms of structure, biological activity, efficacy, safety, and immunogenicity. Therefore, the monitoring of biosimilars’ safety in real-world clinical practice though pharmacovigilance is essential. This study aimed to analyze the post-marketing pharmacovigilance data of biosimilar monoclonal antibodies used in oncology and compare them with respective reference products. Data of a 2-year period (1 January 2021–31 December 2022) were retrieved from EudraVigilance, and descriptive and comparative analysis were performed using the Reporting Odds Ratio to evaluate the distribution of medicine-reaction pairs related to biosimilars of three antitumor biological products and their corresponding reference products: bevacizumab, rituximab, and trastuzumab. The results showed that most frequently reported ADRs for biosimilars were non-serious and consistent with the safety profiles of reference products. These findings provide reassurance regarding safety equivalence of biosimilars and support their use as valid alternatives to originator biologics.
1. Introduction
In the last decades, the clinical management of oncologic patients has undergone substantial changes due to the introduction of biologic medicines, which may contribute to a positive clinical outcome. Since biologics presents a complex structure and are produced in living system under strictly controlled conditions, their development and manufacture require very high costs, and consequently, these high prices are hardly affordable by the health service structures. Following the expiry of the exclusive patents for these biologic medicines, several biopharmaceutical industries have developed and introduced into the market similar biologic products, named biosimilars, allowing for a significant price lowering with a cut costs for cancer treatments.
Biosimilar is defined by the European Medicines Agency (EMA) as “a biological medicine highly similar to another already approved biological medicine (the ‘reference medicine’). Biosimilars are approved according to the same standards of pharmaceutical quality, safety and efficacy that apply to all biological medicines” [1]. Other definitions of biosimilars according to major international regulatory organizations are reported in Table 1. The EMA also specifies that biosimilar agents are highly similar to the reference product in terms of structure, biological activity and efficacy, safety, and immunogenicity profile [1]. EMA like other regulatory agencies has delineated specific comparability pathways to ascertain the similarity between a biosimilar candidate and its reference product (RP) [2,3,4]. Biosimilars differ from the generic form of the chemical products, because in this case it is not possible to develop molecules identical to their reference products due to biologic natural variation and their heterogenic product process [5]. Therefore, it is fundamental that the safety profiles of biosimilars are similar to those of the original biologics. Rituximab, bevacizumab, and trastuzumab are monoclonal antibodies (mAbs) used in first- and second-line treatment regimens in combination with other anti-cancer therapies for a number of common malignant diseases [6,7,8]. A growing number of biosimilar versions of these agents are available on the market, and many others are in development. In these circumstances, as with any medication, the evaluation of the adverse drug reaction (ADR) profiles of biologics and biosimilars through post-marketing surveillance is essential.

Table 1.
Definition of biosimilars according to international regulatory organizations.
The aim of this study was to analyze the post-marketing pharmacovigilance data of biosimilar mAbs used in oncology and compare their safety information with the respective reference products.
2. Materials and Methods
Data were retrieved from the European Union’s post-marketing surveillance database EudraVigilance (EV), using the online interface adrreports.eu [11]. EV is a public spontaneous reporting system maintained by the European Medicines Agency on behalf of the European Union (EU), which receives Individual Case Safety Reports (ICSRs) of suspected adverse drug reactions within the European Economic Area (EEA) [12].
We retrieved all reports related to biosimilars of three antitumor biological products reported as suspected drugs bevacizumab, rituximab, and trastuzumab, and we compared their safety profile to the corresponding reference products (Avastin®, MabThera®, and Herceptin®, respectively). We considered the EU-licenced biosimilars which have been authorized before the year 2021: three different biosimilar bevacizumabs (Aybintio®, Mvasi®, Zirabev®), five biosimilar rituximabs (Blitzima®, Rixathon®, Riximyo®, Ruxience®, Truxima®), and six biosimilar trastuzumabs (Herzuma®, Kanjinti®, Ogivri®, Ontruzant®, Trazimera®, Zercepac®). Table 2 lists these medicine products with their respective dates of approval and the patent expiry dates for the corresponding reference products.

Table 2.
Bevacizumab, rituximab, and trastuzumab: reference products and biosimilar agents approved in the European Union considered in our study.
We performed our retrospective analysis on biosimilars, which have more time on the market, and take into account that two of them received a marketing authorization valid throughout the European Union in 2020 (Ruxience® on 1 April 2020 and Aybintio® on 19 August 2020); we considered the 2-year period between 1 January 2021 and 31 December 2022 in order to analyze and compare as many biosimilars as possible over an equal period of time.
2.1. Descriptive Analysis
The extracted reports were identified by a unique EU Local Number, reporting information on the report type (spontaneous or from clinical studies), primary source qualification (healthcare professional or non-healthcare professional), EV gateway receipt date, patient sex and age group, MedDRA preferred terms (PT), seriousness criteria, and suspect and concomitant drugs. All ADRs are categorized using the Medical Dictionary for Regulatory Activities (MedDRA), a specific standardized medical terminology to facilitate sharing of regulatory information internationally for medical products used by humans. MedDRA terms are arranged in a five-tiered multi-axial hierarchy, which provides increasing specificity as one descends it [13]. At the top-level of hierarchy there are 27 System Organ Classes (SOC), which incorporate at the lower levels High Level Group Terms (HLGTs) and High Level Terms (HLTs). Each member of the next level, Preferred Terms (PTs), is a distinct descriptor (single medical concept) for a symptom, sign, disease diagnosis, therapeutic indication, investigation, surgical or medical procedure, and medical social or family history characteristic. Finally, one or more Lower Level Terms (LLTs) correspond to each PT; the LLTs are effectively entry terms that include synonyms and lexical variants [14,15,16]. One or more symptoms can be reported for each EV report. We analyzed all the reports related to the reference drugs and their biosimilars included in this study performing a descriptive analysis. For each drug, the notoriety of the adverse reactions was ascertained by checking if the most frequently reported ADRs were listed in the corresponding Summary of the Product Characteristics (SPCs) [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
2.2. Statistical Analysis
We performed comparative analysis using the Reporting Odds Ratio (ROR) with 95% confidence interval as statistical parameter to evaluate medicine-reaction pairs distribution. ROR allows for a quantitative approach using 2 × 2 contingency tables, comparing the frequency of a drug-reaction pair with all the others in the database. An increased frequency for the drug-reaction pair can be assumed if ROR is >1. The biosimilars and their respective original products were analyzed separately. A disproportionality analysis was carried out between the ADRs associated to biosimilars and each respective reference product. The EMA provides guidance on signal detection and management in pharmacovigilance to define a signal of disproportionate reporting in the EV system, and these criteria were used in this present study. The following criteria were applied: the lower bound of the 95% confidence interval greater than one; the number of individual cases greater than or equal to three for active substances contained in medicinal products included in the additional monitoring list in accordance with REG Art 23 (see GVP Module X), unless the sole reason for inclusion on the list is the request of a post-authorization safety study (PASS); five for the other active substances; and the event belongs to the Important Medical Event list [34]. (https://www.ema.europa.eu/en/documents/other/screening-adverse-reactions-eudravigilance_en.pdf. Accessed on 31 March 2023)
3. Results
3.1. Descriptive Analysis
Figure 1 describes the number of reports for bevacizumab, trastuzumab, and rituximab biologics and their respective biosimilars. Table 3, Table 4 and Table 5 summarize the characteristics of each report by patients’ sex (female, male, and unknown), age range (0–17 years, 18–64 years, 65–85 years, over 85 years, and unknown), and type of reporter (healthcare professional, and non-healthcare professional) for all medicine products in study. A total of 13,306 reports were collected for these products: 9806 reports (74%) referred to the original products and 3500 (26%) to biosimilars.
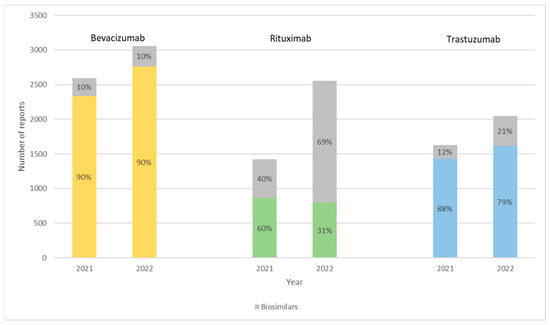
Figure 1.
Number of reports for bevacizumab, rituximab, and trastuzumab biologics in 2021 and 2022 divided between the originators and the respective biosimilars, expressed in percentages. Yellow for Avastin®, green for MabThera®, and blue for Herceptin®.

Table 3.
Demographic characteristics and reporter type for reports of rituximabs.

Table 4.
Demographic characteristics and reporter type for reports of bevacizumabs.

Table 5.
Demographic characteristics and reporter type for reports of trastuzumabs.
We analyzed a total of 36,200 reported PTs; 23,592 (65%) PTs were identified from reports for original products and 12,608 (35%) PTs for biosimilars. The proportions of reports from females were higher than from males for both categories: 58.5% vs. 36.2% for original products, and 57.6% vs. 26% for biosimilars. However, in 16.4% reports for the biosimilars, the information on sex was missing. Most of the reports referred to patients aged 18–64 years and a slightly lower percentage to those of the age group 65–85 years. Roughly 20% of the reports concerning originator biologics and biosimilars were missing information about patients’ age. Approximately 90% of reports were submitted by healthcare professionals.
3.2. Statistical Analysis
For the disproportionality analysis, all 13,306 safety reports had been examined, corresponding to 36,200 drug-reaction pairs. Reported ADRs referring to incorrect product storage, routine laboratory tests or incorrect administration, were not considered because they were not pertinent to our investigation. Overall, almost all of the most frequently reported and statistically significant ADRs for biosimilars were non-serious and listed in the corresponding SPCs. Bevacizumab biosimilars vs. Avastin®: asthenia n = 32 reactions, ROR 2.63 [CI 95% 1.81–3.82], neuropathy peripheral n = 29, ROR 2.44 [CI 95% 1.65–3.61], abdominal pain n = 28 ROR 3.92 [CI 95% 2.58–5.94]. Rituximab biosimilars vs. MabThera®: COVID-19 n = 169 reactions, ROR 1.73 [CI 95% 1.42–2.09], pruritus n = 163, ROR 1.96 [CI 95% 1.6–2.41], throat irritation n = 135 ROR 4.9 [CI 95% 3.55–6.74]. Trastuzumab biosimilars vs. Herceptin®: diarrhea n = 78 reactions, ROR 1.55 [CI 95% 1.23–1.95], chills n = 68, ROR 2.07 [CI 95% 1.61–2.66], nausea n = 57 ROR 1.6 [CI 95% 1.22–2.1]. Table 6, Table 7 and Table 8 show the most frequently reported and statistically significant ADRs for biosimilars compared to their original products by number of ADRs. Among these drug-reaction pairs we identified (on the basis of the Important Medical Event terms list [34]), serious reactions are listed below. Bevacizumab biosimilars vs. Avastin ®: neuropathy peripheral n = 29 reactions, ROR 2.44 [CI 95% 1.65–3.61], neutropenia n = 24 reactions, ROR 1.75 [CI 95% 1.14–2.67], thrombocytopenia n = 18 ROR 2 [CI 95% 1.22–3.3], seizure n = 13 ROR 4.42 [CI 95% 2.3–8.5]. Rituximab biosimilars vs. MabThera ®: rheumatoid arthritis n = 69 reactions, ROR 4.62 [CI 95% 2.76–7.74], immune thrombocytopenia n = 59 reactions, ROR 9.22 [CI 95% 3.71–22.88], mantle cell lymphoma n = 34 ROR 15.9 [CI 95% 1.6–158.05], systemic lupus erythematosus n = 34 ROR 7.95 [CI 95% 2.13–29.65]. Trastuzumab biosimilars vs. Herceptin ®: neutropenia n = 32 reactions, ROR 3.29 [CI 95% 2.23–4.87], thrombocytopenia n = 16 ROR 2.59 [CI 95% 1.48–4.53], hypokalaemia n = 7 reactions, ROR 2.52 [CI 95% 1.03–6.16]. Furthermore, we analyzed the drug-reaction pairs with a higher and statistically significant ROR, which are listed as follows. Bevacizumab biosimilars vs. Avastin®: weight increased n = 14 reactions, ROR 8.58 [CI 95% 4.17–17.65], general physical deterioration n = 12 reactions, ROR 8.16 [CI 95% 3.72–17.9], chills n = 12 ROR 5.65 [CI 95% 2.76–11.58]. Rituximab biosimilars vs. MabThera®: blood pressure fluctuation n = 116 reactions, ROR 27.36 [CI 95% 8.55–87.51], heart rate irregular n = 48 reactions, ROR 22.48 [CI 95% 2.38–211.89], ear pruritus n = 39 ROR 18.24 [CI 95% 1.88–177.4]. Trastuzumab biosimilars vs. Herceptin®: hypertransaminasaemia n = 8 reactions, ROR 18.77 [CI 95% 3.52–100.17], bronchospasm n = 4 ROR 9.36 [CI 95% 1.32–66.58], paraesthesia n = 34, ROR 7.35 [CI 95% 4.8–11.26].

Table 6.
The most frequent and statistically significant ADRs related to Rituximab biosimilars compared to MabThera® reported in EudraVigilance.

Table 7.
The most frequent and statistically significant ADRs related to Bevacizumab biosimilars compared to Avastin® reported in EudraVigilance.

Table 8.
The most frequent and statistically significant ADRs related to Trastuzumab biosimilars compared to Herceptin® reported in EudraVigilance.
4. Discussion
Biosimilars require the submission of a Risk Management Plan, including further safety study, for their approval to be granted. Their safety profile is also monitored through pharmacovigilance activities once they are on the market, in the same way as the other medicines [1,3]. The spontaneous reporting system remains a cornerstone of pharmacovigilance since it allows the early detection of possible safety signals and the continuous monitoring and evaluation of potential safety issues in relation to reported ADRs [35]. In particular, it is crucial to identify rare or long-term ADRs that may not have been captured during the limited duration and controlled settings of premarketing clinical trials [36,37,38]. Spontaneous reporting system represents an important tool for identifying previously unknown ADRs and emerging safety issues since the data are collected from a wide range of healthcare providers, including physicians, pharmacists, and also patients. Moreover, post marketing data encompass a broader patient population compared to those involved in strictly controlled clinical trials settings, including, for instance, those with co-morbidities, concomitant medications, and vulnerable groups. This allows the system to reflect data on general population, real-world clinical practice, and patient experiences. However, pharmacovigilance studies such as this present one, based on spontaneous reporting system, have some limitations. First, the lack of denominator data, such as the number of patients exposed to a particular drug, does not allow for the accurate calculation of incidence rates, or determining the true risk associated with the use of a specific medication. Secondly, the information contained in the reports may be incomplete and inaccurate, may lack essential details, have inconsistencies, or be subjected to data entry errors. The quality and completeness of the reported data can affect the reliability and validity of the data. In addition, the lack of comprehensive information on the patient’s medical history, the usage of concomitant medications, and other relevant factors make it difficult to assess a causal relationship between the medication and reported ADR. Finally, and above all, the system is affected by the drawback of underreporting, owing to various factors such as lack of awareness, uncertainty about causality, lack of time or perception that reporting is burdensome [39,40,41,42]. This last limitation can affect the validity and accuracy of ROR estimates since its calculations are based on the assumption that the reporting rate is constant across all medications and ADRs. Furthermore, Reporting Odds Ratio does not intend to establish a causality relation between a drug and a given adverse reaction but simply to detect a safety signal. To overcome these limitations, it is crucial to integrate ROR with other pharmacovigilance methods, such as signal detection algorithms, disproportionality analyses, and additional observational or experimental studies. These complementary approaches can provide a more comprehensive understanding of the safety profile of a drug and minimize the impact of limitations.
This study provided post-marketing pharmacovigilance evidence in ADR reporting of originator biologics and corresponding biosimilars marketed in the EU, focusing on reported ADRs and the detection of disproportionality. Overall, during the study period the number of reports in EV for reference products has slightly increased in 2022 compared to the previous year, meanwhile, the number of reports for biosimilars has noticeably raised. This trend is evident for biosimilars of rituximab, most of reports referred to Ruxience® and Truxima®, even though the number of reports for the reference product MabThera® was a bit lower in 2022. The increasing number of reports for biosimilars in EV may reflect the significant increase in biosimilar usage in the EU in response to incentive programs instituted by individual member states, health authorities, and payers over the last few years [43,44]. In this contest, it is important to mention that the EU is leading the way with regard to the approvals, utilization, and realization of cost savings of biosimilars [45,46].
Almost all of the most frequently reported ADRs for analyzed biologics were non-serious, listed in the corresponding SPCs and in line with the studies by Giezen et al. [47,48,49]; thus, the adverse reactions to biologics that are included in their safety profiles are mostly related to their pharmacologic actions and immunologic reactions, e.g., immunogenicity and injection site reactions. Among serious, not listed in SPCs, and statistically significant ADRs, we focused on three adverse reactions of rituximab biosimilars: rheumatoid arthritis, mantle cell lymphoma, and systemic lupus erythematosus. Specifically, the signal of disproportionate reporting for rheumatoid arthritis referred to Ruxience® and Truxima®, while mantle cell lymphoma and systemic lupus erythematosus only to Truxima®. We observed that in almost all reports that had those three ADRs, Ruxience®, and Truxima® were used for rheumatoid arthritis, while Truxima® for mantle cell lymphoma and systemic lupus erythematosus. In particular, Truxima® was used off-label for the last two indications. These three reported ADRs lead to different interpretations. It can be argued that it is simply a writing mistake of the reporters, with rheumatoid arthritis, mantle cell lymphoma, and systemic lupus erythematosus being the target disease for the molecules in study rather than an adverse reaction. From another side, it cannot be excluded that the mention of the target disease in the field of adverse reactions may be understood as a statement of the disease progression due to therapeutic ineffectiveness. The high number of these PTs and their statistically significant ROR in our data makes this second interpretation preferable. These may be investigated by future pharmacoepidemiology studies. However, it is essential to acknowledge that ADR reporting can be influenced by several factors, such as drug usage patterns, and the underreporting is an important limit of spontaneous reporting databases like EV.
The results of this study show an overall comparability in safety profiles between the biosimilars and original biologics. Our findings are also in line with those of Kurki et al. [50], who investigated data on post-marketing safety of biosimilar mAbs up to 7 years post-approval, and observed no significant differences between the safety profiles of biosimilars and their reference products, no new or unexpected adverse reactions and no differences in the ADRs’ severity. The evidence acquired over 10 years of clinical experience shows that biosimilars approved through EMA can be used as safely and effectively in all their approved indications as other biological medicines [1]. Biosimilars have the potential to create a more sustainable healthcare system since the number of biosimilars in development in oncology is rising, especially biosimilars of bevacizumab, rituximab, and trastuzumab [51]. Copies of original biologics and biosimilars have lowered the costs and increased access to biologicals throughout the EU. Savings from the impact of biosimilar competition continues to grow: as of 2022, the cumulative savings at list prices from the impact of biosimilar competition in Europe reached over EUR 30 billion [52]. Therefore, biosimilars are key in significant economic savings of healthcare systems, allowing more patients to access modern therapies while offering comparable safety and efficacy profiles.
5. Conclusions
Biological therapies are a cost-effective alternative that have revolutionized the treatment of oncologic diseases. Based on the analysis of ADR reports from EudraVigilance, there were no significant differences in the safety profiles between bevacizumab, trastuzumab, and rituximab biosimilars and their respective originators in Europe. These findings provide reassurance regarding the safety equivalence of biosimilars and support their use as viable alternatives to originator biologics. As with any medication, constant pharmacovigilance monitoring is essential to ensure the ongoing safety of these products.
Author Contributions
Substantial contributions to conception or design of the work (D.M., V.N., G.S.L. and N.M.), or the acquisition (V.N.), analysis (V.N. and D.M.) or interpretation of data for the work (V.N., G.S.L., D.M. and N.M.). Drafting of the work (V.N. and D.M.) or revising it critically for important intellectual content (N.M. and G.S.L.). All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors report no conflict of interest.
Abbreviations
| ADR | Adverse Drug Reaction |
| EEA | European Economic Area |
| EMA | European Medicines Agency |
| EU | European Union |
| EV | EudraVigilance |
| FDA | United States Food and Drug Administration agency |
| mAb | monoclonal antibody |
| MedDRA | Medical Dictionary for Regulatory Activities |
| PT | Preferred term |
| RP | reference product |
| ROR | Reporting Odds Ratio |
| SPC | Summary of the Product Characteristic |
References
- The European Medicines Agency. Human Regulatory. Biosimilar Medicines: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview (accessed on 31 March 2023).
- European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues. Available online: www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf (accessed on 31 March 2023).
- The European Medicines Agency. The European Medicines Agency Committee for Medicinal Products for Human Use. Guideline on Similar Biological Medicinal Products Containing Monoclonal Antibodies—Non-clinical and Clinical Issues EMA/CHMP/BMWP/403543/2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies-non-clinical_en.pdf (accessed on 31 March 2023).
- Kirchhoff, C.F.; Wang, X.-Z.M.; Conlon, H.D.; Anderson, S.; Ryan, A.M.; Bose, A. Biosimilars: Key regulatory considerations and similarity assessment tools. Biotechnol. Bioeng. 2017, 114, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Ogura, M.; Kwon, H.-C.; Yoon, S.W. An introduction to biosimilar cancer therapeutics: Definitions, rationale for development and regulatory requirements. Futur. Oncol. 2017, 13, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Maximiano, S.; Magalhães, P.; Guerreiro, M.P.; Morgado, M. Trastuzumab in the treatment of breast cancer. BioDrugs 2016, 30, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Jacobs, I.A.; Burkes, R.L. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target. Oncol. 2017, 12, 599–610. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Food and Drug Administration. Biosimilars. Available online: https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars (accessed on 31 March 2023).
- The World Health Organization. Biosimilars. Guidelines on Evaluation of Biosimilars Replacement of Annex 2 of WHO Technical Report Series, No. 977. Available online: https://cdn.who.int/media/docs/default-source/biologicals/who_trs_1043_annex-3_biosimilars_tk.pdf?sfvrsn=998a85d_1&download=true (accessed on 31 March 2023).
- Eudravigilance—European Database of Suspected Adverse Drug Reaction Reports. Available online: http://www.adrreports.eu/ (accessed on 31 March 2023).
- The European Medicines Agency. Human Regulatory. EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory/researchdevelopment/pharmacovigilance/eudravigilance (accessed on 31 March 2023).
- Brown, E.G.; Wood, L.; Wood, S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999, 20, 109–117. [Google Scholar] [CrossRef]
- The Medical Dictionary for Regulatory Activities-MedDRA. Available online: https://www.meddra.org/how-to-use/basics/hierarchy (accessed on 31 March 2023).
- Richesson, R.L.; Fung, K.W.; Krischer, J.P. Heterogeneous but “standard” coding systems for adverse events: Issues in achieving interoperability between apples and oranges. Contemp. Clin. Trials 2008, 29, 635–645. [Google Scholar] [CrossRef]
- Introductory Guide MedDRA Version 26. March 2023. Available online: https://admin.meddra.org/sites/default/files/guidance/file/intguide_26_0_English.pdf (accessed on 31 March 2023).
- Avastin®. INN-Bevacizumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf (accessed on 31 March 2023).
- Aybintio®. INN-Bevacizumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/aybintio-epar-product-information_en.pdf (accessed on 31 March 2023).
- Mvasi®. INN-Bevacizumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/mvasi-epar-product-information_en.pdf (accessed on 31 March 2023).
- Zirabev®. INN-Bevacizumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/zirabev-epar-product-information_en.pdf (accessed on 31 March 2023).
- MabThera®. INN-Rituximab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf (accessed on 31 March 2023).
- Blitzima®. INN-Rituximab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/blitzima-epar-product-information_en.pdf (accessed on 31 March 2023).
- Rixathon®. INN-Rituximab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/rixathon-epar-product-information_en.pdf (accessed on 31 March 2023).
- Riximyo®. INN-Rituximab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/riximyo-epar-product-information_en.pdf (accessed on 31 March 2023).
- Ruxience®. INN-Rituximab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ruxience-epar-product-information_en.pdf (accessed on 31 March 2023).
- Truxima®. INN-Rituximab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/truxima-epar-product-information_en.pdf (accessed on 31 March 2023).
- Herceptin®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf (accessed on 31 March 2023).
- Herzuma®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/herzuma-epar-product-information_en.pdf (accessed on 31 March 2023).
- Kanjinti®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/kanjinti-epar-product-information_en.pdf (accessed on 31 March 2023).
- Ogivri®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ogivri-epar-product-information_en.pdf (accessed on 31 March 2023).
- Ontruzant®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ontruzant-epar-product-information_en.pdf (accessed on 31 March 2023).
- Trazimera®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/trazimera-epar-product-information_en.pdf (accessed on 31 March 2023).
- Zercepac®. INN-Trastuzumab-European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/zercepac-epar-product-information_en.pdf (accessed on 31 March 2023).
- The Medical Dictionary for Regulatory Activities-MedDRA. Important Medical Event terms. Available online: https://www.ema.europa.eu/en/documents/other/meddra-important-medical-event-terms-list-version-260_en.xlsx (accessed on 31 March 2023).
- Pacurariu, A.C.; Coloma, P.M.; Van Haren, A.; Genov, G.; Sturkenboom, M.C.J.M.; Straus, S.M.J.M. A Description of Signals During the First 18 Months of the EMA Pharmacovigilance Risk Assessment Committee. Drug Saf. 2014, 37, 1059–1066. [Google Scholar] [CrossRef]
- Spelsberg, A.; Prugger, C.; Doshi, P.; Ostrowski, K.; Witte, T.; Hüsgen, D.; Keil, U. Contribution of industry funded post-marketing studies to drug safety: Survey of notifications submitted to regulatory agencies. BMJ 2017, 356, j337. [Google Scholar] [CrossRef]
- Pierce, C.E.; Bouri, K.; Pamer, C.; Proestel, S.; Rodriguez, H.W.; Van Le, H.; Freifeld, C.C.; Brownstein, J.S.; Walderhaug, M.; Edwards, I.R.; et al. Evaluation of Facebook and Twitter Monitoring to Detect Safety Signals for Medical Products: An Analysis of Recent FDA Safety Alerts. Drug Saf. 2017, 40, 317–331. [Google Scholar] [CrossRef]
- Vlahović-Palčevski, V.; Mentzer, D. Postmarketing Surveillance. Handb. Exp. Pharmacol. 2011, 205, 339–351. [Google Scholar] [CrossRef]
- AlOmar, M.; Tawfiq, A.; Hassan, N.; Palaian, S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: Current status, challenges and the future. Ther. Adv. Drug Saf. 2020, 11, 2042098620938595. [Google Scholar] [CrossRef]
- Vallano, A.; Cereza, G.; Pedròs, C.; Agustí, A.; Danés, I.; Aguilera, C.; Arnau, J.M. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br. J. Clin. Pharmacol. 2005, 60, 653–658. [Google Scholar] [CrossRef]
- Hasford, J.; Goettler, M.; Munter, K.-H.; Müller-Oerlinghausen, B. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J. Clin. Epidemiol. 2002, 55, 945–950. [Google Scholar] [CrossRef]
- Varallo, F.R.; Guimarães, S.D.O.P.; Abjaude, S.A.R.; Mastroianni, P.D.C. Causes for the underreporting of adverse drug events by health professionals: A systematic review. Rev. Esc. Enferm. USP 2014, 48, 739–747. [Google Scholar] [CrossRef]
- Brasington, R.; Strand, V. New Treatments in Rheumatology: Biosimilars. Curr. Treat. Options Rheumatol. 2020, 6, 325–336. [Google Scholar] [CrossRef]
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals; European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Phelps, G.; Wang, J.; Schwab, C.; Li, M.S. Barriers Impeding the Availability and Uptake of Biosimilars in the US. Value Health 2018, 21, S94. [Google Scholar] [CrossRef]
- Harston, A. How the U.S. compares to Europe on biosimilar approvals and products in the pipeline. Mondaq Business Briefing, 16 May 2022. [Google Scholar]
- Giezen, T.J.; Schneider, C.K. Safety assessment of biosimilars in Europe: A regulatory perspective. Generics Biosimilars Initiat. J. 2014, 3, 180–183. [Google Scholar] [CrossRef]
- Giezen, T.J.; Mantel-Teeuwisse, A.K.; Straus, S.M.J.M.; Schellekens, H.; Leufkens, H.G.M.; Egberts, A.C.G. Safety-Related Regulatory Actions for Biologicals Approved in the United States and the European Union. JAMA 2008, 300, 1887–1896. [Google Scholar] [CrossRef]
- Giezen, T.J.; Mantel-Teeuwisse, A.K.; Meyboom, R.H.; Straus, S.M.; Leufkens, H.G.; Egberts, T.C. Mapping the safety profile of biologicals: A disproportionality analysis using the WHO adverse drug reaction database. VigiBase. Drug Saf. 2010, 33, 865–878. [Google Scholar] [CrossRef]
- Kurki, P.; Barry, S.; Bourges, I.; Tsantili, P.; Wolff-Holz, E. Safety, Immunogenicity and Interchangeability of Biosimilar Monoclonal Antibodies and Fusion Proteins: A Regulatory Perspective. Drugs 2021, 81, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.P.; Millán, S.; Chaban, N.; del Campo, A.; Spitzer, E. Current state and comparison of the clinical development of bevacizumab, rituximab and trastuzumab biosimilars. Futur. Oncol. 2021, 17, 2529–2544. [Google Scholar] [CrossRef] [PubMed]
- Troein, P.; Newton, M.; Stoddart, K.; Arias, A. The Impact of Biosimilar Competition in Europe. 2022. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/the-impact-of-biosimilar-competition-in-europe-2022.pdf (accessed on 31 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).