Management of Primary Uterine Cervix B-Cell Lymphoma Stage IE and Fertility Sparing Outcome: A Systematic Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
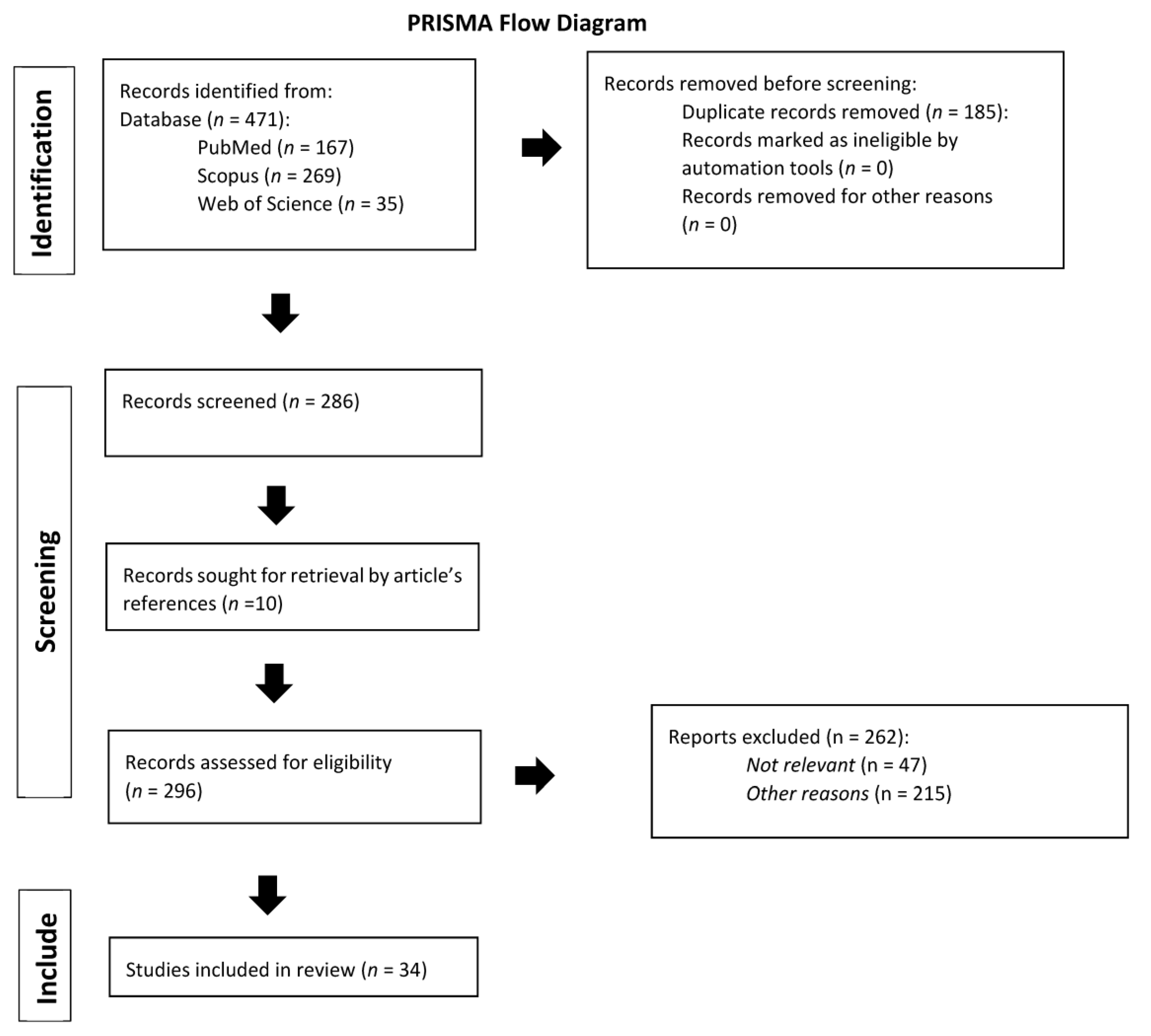
2. Materials and Methods
3. Results
4. Discussion
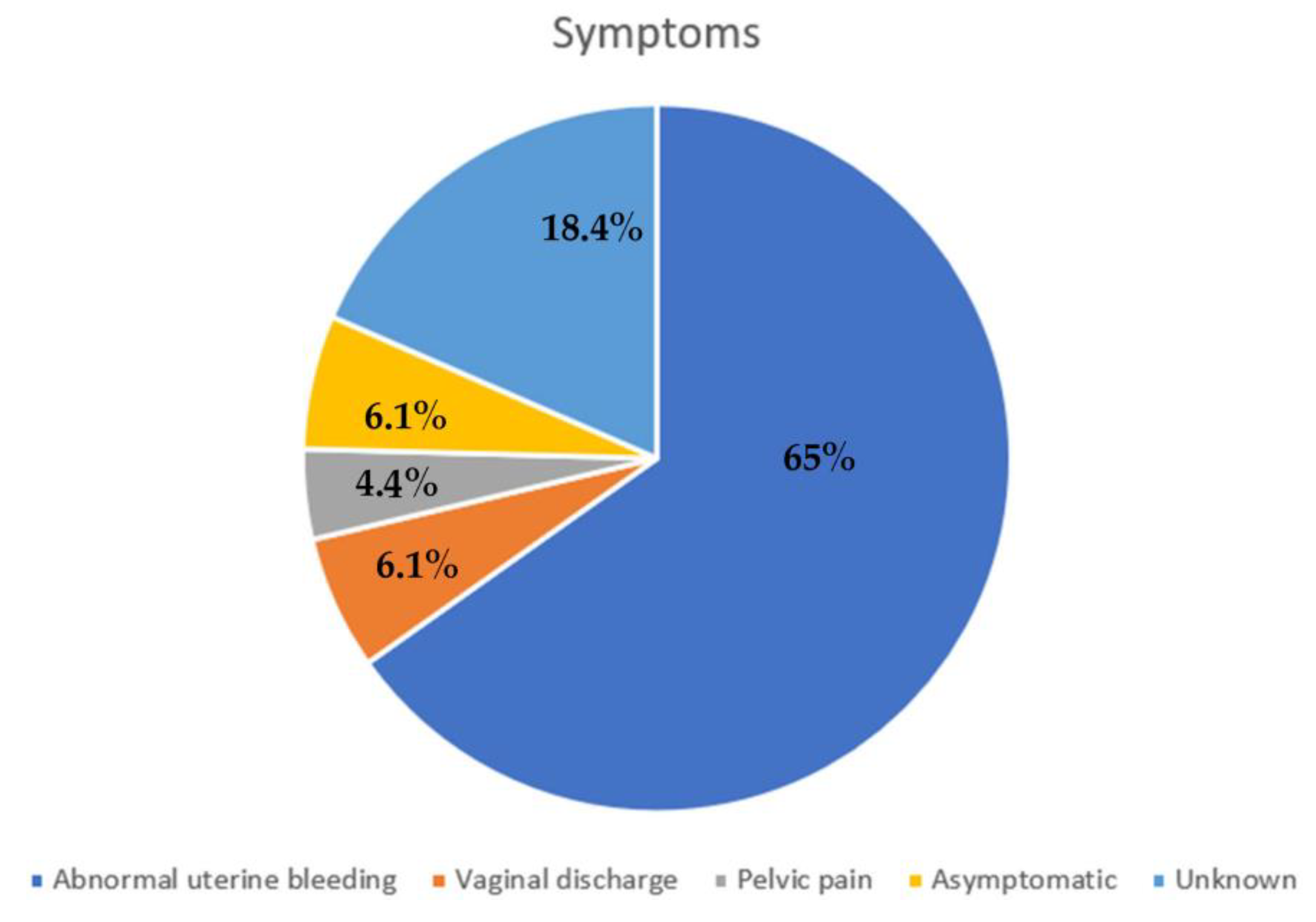
4.1. Symptoms and Diagnosis
4.2. Therapy
4.3. Fertility Sparing Treatment
4.4. Pregnancy Outcome after Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. JBI Checklist for Case Report and Case Series
| D1. | Were patient’s demographic characteristics clearly described? |
| D2. | Was the patient’s history clearly described and presented as a timeline? |
| D3. | Was the current clinical condition of the patient on presentation clearly described? |
| D4. | Were diagnostic tests or assessment methods and the results clearly described? |
| D5. | Was the intervention(s) or treatment procedure(s) clearly described? |
| D6. | Was the post-intervention clinical condition clearly described? |
| D7. | Were adverse events (harms) or unanticipated events identified and described? |
| D8. | Does the case report provide takeaway lessons? |
| D1. | Were there clear criteria for inclusion in the case series? |
| D2. | Was the condition measured in a standard, reliable way for all participants included in the case series? |
| D3. | Were valid methods used for identification of the condition for all participants included in the case series? |
| D4. | Did the case series have consecutive inclusion of participants? |
| D5. | Did the case series have complete inclusion of participants? |
| D6. | Was there clear reporting of the demographics of the participants in the study? |
| D7. | Was there clear reporting of clinical information of the participants? |
| D8. | Were the outcomes or follow up results of cases clearly reported? |
| D9. | Was there clear reporting of the presenting site(s)/clinic(s) demographic information? |
| D10. | Was statistical analysis appropriate? |
References
- Kosari, F.; Daneshbod, Y.; Parwaresch, R.; Krams, M.; Wacker, H.H. Lymphomas of the female genital tract: A study of 186 cases and review of the literature. Am. J. Surg. Pathol. 2005, 29, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhou, T.; Tao, Y.; Feng, Y.; Shen, X.; Mei, S. Exposure to organochlorine pesticides and non-Hodgkin lymphoma: A meta-analysis of observational studies. Sci. Rep. 2016, 6, 25768. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Kuo, H.C.; Chou, C.Y.; Chang, C.H.; Liu, M.T.; Tzeng, C.C.; Huang, K.E. Primary malignant lymphoma of the uterine cervix shows favorable response to neoadjuvant chemotherapy. Gynecol. Oncol. 1994, 52, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Baijal, G.; Vadiraja, B.M.; Fernandes, D.J.; Vidyasagar, M.S. Diffuse large B-cell lymphoma of the uterine cervix: A rare case managed novelly. J. Cancer Res. Ther. 2009, 5, 140–142. [Google Scholar]
- Cohn, D.E.; Resnick, K.E.; Eaton, L.A.; DeHart, J.; Zanagnolo, V. NonHodgkin’s lymphoma mimicking gynaecological malignancies of the vagina and cervix: A report of four cases. Int. J. Gynecol. Cancer 2007, 17, 254–293. [Google Scholar] [CrossRef]
- Korcum, A.F.; Karadogan, I.; Aksu, G.; Aralasmak, A.; Erdogan, G. Primary follicular lymphoma of the cervix uteri: A review. Ann. Hematol. 2007, 86, 623–630. [Google Scholar] [CrossRef]
- Cantu de Leon, D.; Perez Montiel, D.; Chanona Vilchis, J. Primary malignant lymphoma of uterine cervix. Int. J. Gynecol. Cancer 2006, 16, 923–927. [Google Scholar] [CrossRef]
- Ab Hamid, S.; Wastie, M.L. Primary non-Hodgkin’s lymphoma presenting as a uterine cervical mass. Singap. Med. J. 2008, 49, e73–e75. [Google Scholar]
- Stroh, E.L.; Besa, P.C.; Cox, J.D.; Fuller, L.M.; Cabanillas, F.F. Treatment of patients with lymphomas of the uterus or cervix with combination chemotherapy and radiation therapy. Cancer 1995, 75, 2392–2399. [Google Scholar] [CrossRef]
- Ferry, J.A.; Young, R.H. Malignant lymphoma, pseudolymphoma, and hematopoietic disorders of the female genital tract. Pathol. Annu. 1991, 26, 227–263. [Google Scholar]
- Rudders, R.A.; Ross, M.E.; De Lellis, R.A. Primary extranodal lymphoma: Response to treatment and factors influencing prognosis. Cancer 1978, 42, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wei, Z.; Zhou, D.; Zhang, Y.; Han, X.; Wang, W.; Zhang, L.; Yang, C.; Feng, J. Primary extra-nodal diffuse large B-ell lymphoma: A prognostic analysis of 141 patients. Oncol. Lett. 2018, 16, 1602–1614. [Google Scholar] [PubMed]
- Fox, H.; Langley, F.A.; Govan, A.D.; Hill, A.S.; Bennett, M.H. Malignant lymphoma presenting as an ovarian tumour: Clinicopathological analysis of 34 cases. Br. J. Obstet. Gynaecol. 1988, 95, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Kasai, M.; Ichimura, T.; Murakami, M.; Matsuda, M.; Kawamura, N.; Sumi, T. Two cases of utrine malignant lymphoma diagnosed by needle biopsy. J. Obstet. Gynaecol. Res. 2015, 41, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Kampaktsis, P.N.; Frey, M.; Witkin, S.S.; Holcomb, K. Primary lymphoma of the female genital tract: An analysis of 697 cases. Gynecol. Oncol. 2017, 145, 305–309. [Google Scholar] [CrossRef]
- Bode, M.K.; Tikkakoski, T.; Jhansson, J.; Johannson, K.; Kariniemi, J.; Apaja-Sarkkinen, M. Lymphoma of the cervix: Imaging and transcatheter arterial embolization. Acta Radiol. 2002, 43, 431–432. [Google Scholar] [CrossRef]
- Abbas, M.A.; Birdwell, R.; Katz, D.S.; Chang, H.; Ostrow, K. Primary Lymphoma of the cervix in a Heart Transplant Patient. Am. J. Roentgenol. 1996, 167, 1136–1138. [Google Scholar] [CrossRef]
- Gupta, S.; Sachdev, L.; Gupta, R.; Beotra, A. Non-Hodgkin Lymphoma of the Uterine Cervix. Ann. Saudi Med. 1995, 15, 187–188. [Google Scholar] [CrossRef]
- Stabile, G.; Zinicola, G.; Romano, F.; Laganà, A.S.; Dal Pozzolo, C.; Ricci, G. Pelvic mass, ascites, hydrothorax: A malignant or benign condition? Meigs syndrome with high levels of CA 125. Prz. Menopauzalny 2021, 20, 103–107. [Google Scholar] [CrossRef]
- Goda, J.S.; Gaikwad, U.; Narayan, A.; Kurkure, D.; Yadav, S.; Khanna, N.; Jain, H.; Bagal, B.; Epari, S.; Singh, P.; et al. Primary diffuse large B cell lymphoma of Uterine Cervix: Treatment outcomes of a rare entity with literature review. Cancer Rep. 2020, 3, e1264. [Google Scholar] [CrossRef]
- Chan, J.K.; Loizzi, V.; Magistris, A. Clinicopathologic features of six cases of primary cervical lymphoma. Am. J. Obstet. Gynecol. 2005, 193, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Vardiman, J.W. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2001. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2008. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Prospero International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 3 December 2022).
- Quaresima, P.; Saccone, G.; Zuccalà, V.; Guarascio, G.; Leo, L.; Amendola, G.; Zullo, F.; Morelli, M.; Venturella, R. Successful Vaginal Delivery after Induction of Labour in a Patient Treated for Non-Hodgkin’s Lymphoma of the Cervix: A Case Report and Literature Review. Case Rep. Obstet. Gynecol. 2022, 2022, 3541046. [Google Scholar] [CrossRef]
- Sorrentino, F.; De Feo, V.; Stabile, G.; Tinelli, R.; D’Alterio, M.N.; Ricci, G.; Angioni, S.; Nappi, L. Cesarean Scar Pregnancy Treated by Artery Embolization Combined with Diode Laser: A Novel Approach for a Rare Disease. Medicina 2021, 57, 411. [Google Scholar] [CrossRef] [PubMed]
- Capsa, C.; Calustian, L.A.; Antoniu, S.A.; Bratucu, E.; Simion, L.; Prunoiu, V.M. Primary Non-Hodgkin Uterine Lymphoma of the Cervix: A Literature Review. Medicina 2022, 58, 106. [Google Scholar] [CrossRef]
- Akkour, K.; Alhulwah, M.; Alhalal, H.; Alqahtani, N.; Arafah, M. Primary extranodal diffuse large B-cell lymphoma of the uterine cervix. Malays J. Pathol. 2021, 43, 327–331. [Google Scholar]
- Gui, W.; Li, J.; Zhang, Z.; Wang, L.; Zhao, J.; Ma, L.; Su, L. Primary hematological malignancy of the uterine cervix: A case report. Oncol. Lett. 2019, 18, 3337–3341. [Google Scholar] [CrossRef]
- Boussios, S.; Zerdes, I.; Vassou, A.; Bareta, E.; Seraj, E.; Papoudou-Bai, A.; Pavlidis, N.; Batistatou, A.; Pentheroudakis, G. Extranodal diffuse large B-cell lymphomas: A retrospective case series and review of the literature. Hematol. Rep. 2018, 10, 7070. [Google Scholar] [CrossRef]
- Roberts, M.B.; Cottrill, H.M. A case of primary lymphoma in a patient with abnormal uterine bleeding. Gynecol. Oncol. Rep. 2018, 26, 105–107. [Google Scholar] [CrossRef]
- Cubo, A.M.; Soto, Z.M.; Cruz, M.Á.; Doyague, M.J.; Sancho, V.; Fraino, A.; Blanco, Ó.; Puig, N.; Alcoceba, M.; González, M.; et al. Primary diffuse large B cell lymphoma of the uterine cervix successfully treated by combined chemotherapy alone: A case report. Medicine 2017, 96, e6846. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, É.; Nagy, K.; Marton, I.; Bánfalvi, A.; Kocsis, L.; Cserni, G. Incidentally discovered diffuse large B-cell lymphoma limited to the endocervical mucosa in a young female patient. Gynecol. Obstet. Investig. 2015, 80, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.X.; Li, J.; Zhang, W.; Duan, M.H.; Shen, T.; Zhou, D.B. Patients with primary diffuse large B-cell lymphoma of female genital tract have high risk of central nervous system relapse. Ann. Hematol. 2014, 93, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Mouhajir, N.; Diakité, A.; Toulba, A.; Hemmich, M.; Saadi, I. Primary Non-Hodgkin’s Lymphoma of the Uterine Cervix: Case Report of Long-Term Survival Patient. J. Obstet. Gynecol. India 2014, 64, S145–S147. [Google Scholar] [CrossRef] [PubMed]
- Binesh, F.; Karimi Zarchi, M.; Vahedian, H.; Rajabzadeh, Y. Primary malignant lymphoma of the uterine cervix. BMJ Case Rep. 2012. [Google Scholar] [CrossRef]
- Parnis, J.; Camilleri, D.J.; Babic, D.; Degaetano, J.; Savona-Ventura, C. Lymphoma of the cervix. Case Rep Hematol. 2012, 2012, 326127. [Google Scholar] [CrossRef]
- Vasudev, D.S.; Kaler, A.K. Non-Hodgkin’s Lymphoma of the Uterine Cervix. Online J. Health Allied Sci. 2012, 11, 13. [Google Scholar]
- Parva, M.; Lamb, K.; Savior, D.C.; Gilman, P.; Belden, M. Full-term pregnancy and vaginal delivery after treatment for non-Hodgkin’s lymphoma of the cervix and lower uterine segment: A case report. J. Obstet. Gynaecol. Can. 2011, 33, 620–624. [Google Scholar] [CrossRef]
- Upanal, N.; Enjeti, A. Primary lymphoma of the uterus and cervix: Two case reports and review of the literature. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 559–562. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Verona, C.; Bolognesi, A.; Taccagni, G.; Ponzoni, M.; Ferrari, S. Successful pregnancy after chemo-immuno-radiation therapy for aggressive lymphoma of the uterus. Br. J. Haematol. 2008, 142, 141–143. [Google Scholar] [CrossRef]
- Signorelli, M.; Maneo, A.; Cammarota, S.; Isimbaldi, G.; Garcia Parra, R.; Perego, P.; Maria Pogliani, E.; Mangioni, C. Conservative management in primary genital lymphomas: The role of chemotherapy. Gynecol. Oncol. 2007, 104, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, R.; Kumar, L.; Bhatla, D.M.; Kukreja, M.; Papaiah, S. Primary uterine lymphoma: Report of 2 cases and review of literature. Am. J. Obstet. Gynecol. 2006, 195, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Semczuk, A.; Skomra, D.; Korobowicz, E.; Balon, B.; Rechberger, T. Primary non-Hodgkin’s lymphoma of the uterine cervix mimicking leiomyoma: Case report and review of the literature. Pathol. Res. Pract. 2006, 202, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Dursun, P.; Gultekin, M.; Bozdag, G.; Usubutun, A.; Under, A.; Celik, N.Y.; Yuce, K.; Ayhan, A. Primary cervical lymphoma: Report of two cases and review of the literature. Gynecol. Oncol. 2005, 98, 484–489. [Google Scholar] [CrossRef]
- Lee, K.M.; Seah, E.S.; Sethi, V.K. Primary non-Hodgkin’s lymphoma of the uterine cervix: Case report of long-term survival of two patients treated with surgery and radiotherapy. Australas. Radiol. 1998, 42, 126–127. [Google Scholar] [CrossRef]
- Fratoni, S.; Abruzzese, E.; Trawinska, M.M.; Niscola, P.; de Fabritiis, P.; Santeusanio, G. Primitive “Spindle Cell Variant” (Sarcomatoid Variant) Diffuse Large B-Cell Lymphoma of the Uterine Cervix: Description and Outcome of a Rare Case. Int. J. Gynecol. Pathol. 2016, 35, 593–597. [Google Scholar] [CrossRef]
- Wuntakal, R.; Janga, D.; Satyanarayana, D.; Reynolds, K.; Hollingworth, A. An unusual cause of postmenopausal bleeding. J. Low. Genit. Tract. Dis. 2008, 12, 130–133. [Google Scholar] [CrossRef]
- Lorusso, D.; Ferrandina, G.; Pagano, L.; Gagliardi, M.L.; Scambia, G. Successful pregnancy in stage IE primary non-Hodgkin’s lymphoma of uterine cervix treated with neoadjuvant chemotherapy and conservative surgery. Oncology 2007, 72, 261–264. [Google Scholar] [CrossRef]
- González-Cejudo, C.; Martínez-Maestre, M.A.; Peregrín-Álvarez, I.; Daza-Manzano, C. Primary lymphoma of the cervix: Unusual location for a common disease. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 268–269. [Google Scholar] [CrossRef]
- Chandy, L.; Kumar, L.; Dawar, R. Non-Hodgkin’s Lymphoma Presenting as a Primary Lesion in Uterine Cervix: Case Report. J. Obstet. Gynaecol. Res. 1998, 24, 183–187. [Google Scholar] [CrossRef]
- Stabile, G.; Sancin, L.; Boschian Bailo, P.; Ripepi, C.; Romano, A.; Carlucci, S.; Ricci, G. Hysteroscopic Resection Followed by Adjuvant Radiotherapy: Report of a New Therapeutic Approach to Primary Cervical Diffuse Large B-Cell Lymphoma. Int. J. Environ. Res. Public. Health 2022, 19, 11779. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Yoshimatsu, J.; Urata, K.; Miyakawa, I. A case of primary non-Hodgkin’s lymphoma of the uterine cervix treated by combination chemotherapy (THP-COP). J. Obstet. Gynaecol. Res. 1998, 24, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, J.; Illidge, T.; Specht, L.; Hoppe, R.T.; Li, Y.-X.; Tsang, R.; Wirth, A. Modern Radiation Therapy for Extranodal Lymphomas: Field and Dose Guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.; Farrant, M.; McCarthy, K.; Harper, P.; Wiltshaw, E. Lymphomas of the cervix and upper vagina: A report of five cases and a review of the literature. Gynecol. Oncol. 1992, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 |
|---|---|---|---|---|---|---|---|---|
| Stabile et al., 2022 [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Quaresima et al., 2022 [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Capsa et al., 2022 [29] | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| Akkour et al., 2021 [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Gui et al., 2019 [31] | Yes | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes |
| Boussios et al., 2018 [32] | Unclear | No | Unclear | No | Yes | No | Unclear | Yes |
| Roberts et al., 2018 [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cubo et al., 2017 [34] | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| Fratoni et al., 2016 [35] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Posfai et al., 2015 [36] | Yes | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes |
| Mouhajir et al., 2014 [37] | Yes | No | Yes | Unclear | Yes | Yes | No | Yes |
| Binesh et al., 2012 [38] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Parnis et al., 2012 [39] | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| Vasudev et al., 2012 [40] | Yes | No | Yes | Yes | Yes | Yes | Unclear | Yes |
| Parva et al., 2011 [41] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Upanal et al., 2011 [42] | Yes | No | Yes | Yes | Yes | Yes | Unclear | Yes |
| Baijal et al., 2009 [3] | Yes | No | Yes | Unclear | Yes | Yes | Unclear | No |
| Ab Hamid et al., 2008 [7] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ferreri et al., 2008 [43] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wuntakal et al., 2008 [44] | Yes | Unclear | Yes | Yes | Yes | Yes | Unclear | Yes |
| Lorusso et al., 2007 [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Semczuk et al., 2006 [46] | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes |
| Gonzalez-Cejudo et al., 2006 [47] | Unclear | No | Unclear | No | Yes | No | Unclear | Unclear |
| Dursun et al., 2005 [48] | Yes | No | Yes | Yes | Yes | Yes | Unclear | Yes |
| Chandy et al., 1998 [49] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Nasu et al., 1998 [50] | Yes | No | Unclear | No | Yes | No | Unclear | Unclear |
| Author, Year | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Goda et al., 2020 [18] | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear |
| Cao et al., 2014 [51] | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear |
| Signorelli et al., 2007 [52] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Hariprasad et al., 2006 [53] | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear |
| Chan et al., 2005 [19] | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear |
| Lee et al., 1998 [54] | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear |
| Makarewicz et al., 1995 [55] | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear |
| Stroh et al., 1995 [8] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Age | Menopause | Presence of “B” Symptoms | DLBCL Subtype | Immuno-Phenotype | Macroscopic Appearance /Dimension | PAP Smear | Diagnosis | Treatment | Outcome/Follow-Up (Months) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 83 | Yes | No | GCB | Cervical mass/4 cm | - | Surgical specimen | Hysteroscopic resection + RT () | DF/ | Stabile et al., 2022 [28] | |
| 30 | No | No | - | CD20+, CD10+, BCL6+, PAX5+ | Cervical mass/5 cm | Normal | Hysteroscopi c biopsy and D&C | CT (R-CHOP ×8) + ovarian suppression | DF/- | Quaresima et al., 2022 [56] |
| 75 | Yes | No | - | CD20+, CD10-, CD23-, CD3-, CD5-, Ki 67 50% | Cervical mass extended to upper vagina/4 cm | - | Biopsy | CT (CHOP) + RT (45 Gy) | DF/29 | Capsa et al., 2022 [30] |
| 54 | Yes | No | ABC | CD45+, CD20+, BCL2+, MUM1+, BCL6+ | Exophytic mass /9 × 8 × 8 cm | Normal | Biopsy | Surgery (radical trachelectomy in patient with previous subtotal hysterectomy + BSO) + CT (R- CHOP ×6 + RT (20 Gy) | DF/24 | Akkour et al., 2021 [31] |
| 52 | Yes | No | GCB | CD20+ CD10+B CL-6- | Cervical mass/6 cm | - | Biopsy | CT (R-CHOP ×6) + RT (45Gy) | DF/18 | Goda et al., 2020 [20] |
| 50 | Yes | No | GCB | CD20+ CD10+ BCL-6- | Cervical mass/3 cm | - | Biopsy | CT (R-CHOP ×6) + RT (45Gy) | DF/43 | |
| 39 | No | No | ABC | CD20+ CD10- BCL-6- | Bulky cervical mass extending to upper third of vagina and lower uterine segment/8 cm | - | Biopsy | CT (R-CHOP ×6) + RT (45Gy) | DF/8 | |
| 36 | No | No | - | LCA+ CD20+ | Cervical mass/4 cm | - | Biopsy | CT (CHOP ×6) | DF/73 | Gui et al., 2019 [32] |
| 60 | Yes | No | - | - | - | - | - | CT (R-CHOP ×6) + RT | DF/- | Boussios et al., 2018 [33] |
| 55 | Yes | No | - | - | Bulky endocervical mass/10 cm | - | Biopsy, D&C | CT (R-CHOP ×3) + LAVH | DF/36 | Roberts et al., 2018 [34] |
| 51 | Yes | No | GCB | CD20+, CD5+, BCL2+, BCL6+, CD45, CD23, CD43, CD10- , EBER-, Cyclin D1- | Bulky exophytic cervical mass/10 cm | Normal | Biopsy | CT (R-CHOP ×6) | DF/24 | Cubo et al.2017 [35] |
| 39 | No | No | Spindle cell or sarcomatoi d variant, GCB | LCA+, CD20+, CD45+, BCL6+, | Bulky endocervical mass/- | - | Biopsy | Surgery (TH) + CT (R- CHOP ×3) | DF/48 | Fratoni et al., 2016 [36] |
| 27 | No | No | ABC | CD20+ MUM1+ | Mass between uterus and bladder/6.5 × 2.3 cm | LSIL HPV - | LLETZ | CT (R-CHOP ×6) | DF/49 | Posfai et al., 2015 [51] |
| 20 | No | No | - | - | - | - | Biopsy | CT (CHOP ×7) | DF/84 | Cao et 2014 [37] al. |
| 58 | Yes | No | - | - | Bulky mass/- | - | Biopsy | CT (CHOP x6) + RT | CR + CNS relapse/47 alive/56 | |
| 49 | No | No | - | CD20+ CD45+ | Bulky exophitic cervical mass/ | - | Biopsy | CT (CHOP ×6) + RT (46Gy) | DF/192 | Mouhajir et al., 2014 [38] |
| 85 | Yes | No | - | CD20+ | Bulky, exophytic cervical mass/7 × 4 cm | - | Biopsy | CT (R-CHOP ×3) | PR, refused other treatments /5 | Binesh et al., 2012 [39] |
| 54 | Yes | No | - | LCA+ CD20+ BCL2 - | Friable cervical mass extending to low uterine segment/- | - | Biopsy | CT (R-CHOP ×6) + RT (35Gy) | DF/17 | Parnis et al., 2012 [40] |
| 52 | Yes | No | - | CD20+ CD45+ CD3- | endocervical polyp /5 × 3 cm | Normal | Surgical specimen | Surgery (TH BSO) | DF/20 | Vasudev et al., 2012 [41] |
| 21 | No | No | - | LCA+ CD45+ CD20+ CD79A+ | Enlarged cervix/- | Normal | Biopsy | CT (R-CHOP ×6) + previous cryopreservation of embryos + ovarian suppression | DF/72 | Parva et al., 2011 [42] |
| 49 | - | No | - | CD20+ | Enlarged cervix/8 × 4.5 × 6 cm | - | Biopsy | CT (R-CHOP ×6) + RT (30 Gy) | DF/20 | Upanal et al., 2011 [43] |
| 44 | No | No | - | LCA+, CD20+ | Cervical mass/7 × 7 cm | - | Biopsy | CT (R-CHOP ×3) + RT (46Gy) | DF/15 | Baijal et al., 2009 [5] |
| 43 | No | No | - | L26/CD20+ | Bulky exophytic cervical mass/5 × 7.8 × 3 cm | Normal | Biopsy | CT (CHOP ×6) | DF/- | Ab Hamid et al., 2008 [9] |
| 29 | No | No | - | CD20+ CD79a+ BCL6+ CD30- CD3- CD10- | Cervical mass infiltrating upper vagina/3 cm | Normal | Biopsy | Ovarian transposition + CT (CHOP ×6) + RT (30Gy) | DF/54 | Ferreri et al., 2008 [44] |
| 60 | Yes | No | - | CD20+ CD3+ CD5+ CD21+ CD23+ P53+ MUM1+ | Cervical mass extending to upper vagina and left parametrium, bilateral hydronephrosis/7 cm | - | Biopsy | CT (CHOP ×6) | DF/- | Wuntakal et al., 2008 [45] |
| 29 | No | No | - | CD20+ LCA+ CD30- CD45- | Bulky cervical mass extending to upper vagina/5 cm | Normal | Biopsy | CT (CHOP ×3) + surgery (cold-knife conization) + CT (CHOP ×3) | DF/48 | Lorusso et al., 2007 [52] |
| 32 | No | - | - | - | - | - | - | CT (CHOP ×6) | DF/91 | Signorelli et al., 2007 [53] |
| 45 | - | - | - | - | Cervical mass/6 × 5 cm | - | - | CT (CHOP ×6) + surgery (TH + BSO + pelvic nodal sampling) | DF/38 | |
| 58 | - | - | - | - | Cervical mass/2 × 1 cm | - | - | Surgery (TH + BSO) + CT (CHOP ×4) | DF/228 | |
| 54 | - | - | - | - | - | - | - | Surgery (TH + BSO) | DF/118 | |
| 47 | No | No | - | LCA+ CD20+ | Cervical mass extending to parametrium bilaterally, uterus, upper vagina and with bilateral vesicoureteral junction obstruction/- | - | Biopsy | CT (CHOP ×3 + COP ×3 with Adriamicin omitted because of cardiotoxicity ) + RT (45Gy) | DF/13 | Hariprasad et al., 2006 [46] |
| 80 | Yes | No | - | - | Necrotic, bleeding cervical growth extending to upper vagina, left parametrium and uterus/- | - | Biopsy | CT (CHOP ×6), RT omitted because of old age | DF/12 | |
| 43 | Yes | - | - | CD20+ CD45+ BCL6+ BCL2- CD30- CD3- | Bulky cervical mass mimicking leiomyoma/10 cm | Normal | Surgical specimen | Surgery (TH) + CT (CHOP ×6) | DF/10 | Semczuk et al. 2006 [47] |
| 26 | No | No | - | CD20+ CD3+ CD30+ | Cervical mass/6.5 cm | - | Surgical specimen | Surgery (TH) + CT (R- CHOP) | DF/12 | Gonzalez- Cejudo et al., 2006 [48] |
| 51 | - | No | - | LCA+, CD20+ | Cervical mass/4 cm | HSIL | LEEP | Surgery (TH + BSO + pelvic and paraaortic LMP) + CT (CHOP ×6) | DF/19 | Dursun et al., 2005 [54] |
| 76 | Yes | No | - | - | Cervical mass/3 cm | ASCUS | Biopsy | Surgery (TH + BSO + pelvic limphadenect | DF/14 | Chan et al., 2005 [21] |
| 67 | Yes | No | - | - | Cervical polyp | - | Biopsy (polypectom y) | Surgery (TH + BSO + pelvic nodes dissection) + RT (44 Gy) | DF/120 | Lee et al., 1998 [49] |
| 65 | Yes | No | - | - | Bulky exophytic cervical mass/- | - | Biopsy | Surgery (TH + BSO + pelvic nodes dissection) + RT (44 Gy) | DF/120 | |
| 50 | Yes | No | - | CD45+ | Firm multiple nodules involving cervix and upper vagina/10 cm | - | Biopsy | CT (CHOP ×4) + RT (46Gy) + CT (CHOP ×2) | DF/17 | Chandy et al., 1998 [50] |
| 64 | Yes | No | - | LCA+ L26+ MB1+ | Hards cervical mass/10 cm | - | Biopsy | CT (THP- COP x10) | DF/18 | Nasu et al., 1998 [55] |
| 37 | No | No | Centroblast ic (Kiel classificati on) | - | Exophitic cervical mass extending to upper vagina and left parametrium | - | - | CT (cyclophospham ide, vincristine, prednisone ×3) + RT (45Gy) | DF/96 | Makarewicz et al., 1995 [57] |
| 33 | No | No | Centroblast ic centrocytic | - | Cervical mass extending to upper third of vagina/- | Normal 1 year before | Surgical specimen | Surgery (TH+BSO+pelvi c limph node dissection) | Relapse after 6 months with a 6 cm tumour of upper vagina treated with CT (CHOP ×6), after it DF/42 | |
| 53 | Yes | No | - | - | Cervical mass extending to upper vagina/ 4 × 10 cm | - | Biopsy | CT (CHOP + bleomycin) + RT (40 Gy) + CT (as previously) | DF/173 | Stroh et al 1995 [10] |
| 64 | Yes | No | - | - | Cervical mass/8 Cm | - | Biopsy | RT (60 Gy) + CT (CHOP + bleomycin) + RT + CT | DF/165 | |
| 66 | Yes | No | - | - | Cervical mass/3 Cm | - | Biopsy | CT (CHOP) + RT (40 Gy) + CT | DF/60 | |
| 67 | Yes | No | - | - | Cervical mass/- | - | Biopsy | CT (ASAP, MBACOS, MINE) + RT (40 Gy) | PR/18 |
| Reference | Age | Treatment | Timing Post-Treatment | Mode of Delivery | Complications |
|---|---|---|---|---|---|
| Quaresima et al., 2022 [29] | 30 | CHOP + Rituximab + ovarian suppression (Leuprolide) | 1 year | Induction of labour at 41 gw—Vaginal delivery | None |
| Posfai et al., 2015 [37] | 27 | CHOP + Rituximab | 14 months | - | Miscarriage 8th gw |
| Parva et al., 2011 [43] | 21 | CHOP + Rituximab | 5.5 years | Vaginal delivery | None |
| Ferreri et al., 2008 [44] | 29 | CHOP + Rituximab + (ovarian transposition) + RT | 3 years | Vaginal delivery | None |
| Lorusso et al., 2007 [52] | 29 | CHOP + cold knife conization | 3 years | Planned CS at 38 gw | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabile, G.; Ripepi, C.; Sancin, L.; Restaino, S.; Mangino, F.P.; Nappi, L.; Ricci, G. Management of Primary Uterine Cervix B-Cell Lymphoma Stage IE and Fertility Sparing Outcome: A Systematic Review of the Literature. Cancers 2023, 15, 3679. https://doi.org/10.3390/cancers15143679
Stabile G, Ripepi C, Sancin L, Restaino S, Mangino FP, Nappi L, Ricci G. Management of Primary Uterine Cervix B-Cell Lymphoma Stage IE and Fertility Sparing Outcome: A Systematic Review of the Literature. Cancers. 2023; 15(14):3679. https://doi.org/10.3390/cancers15143679
Chicago/Turabian StyleStabile, Guglielmo, Chiara Ripepi, Lara Sancin, Stefano Restaino, Francesco Paolo Mangino, Luigi Nappi, and Giuseppe Ricci. 2023. "Management of Primary Uterine Cervix B-Cell Lymphoma Stage IE and Fertility Sparing Outcome: A Systematic Review of the Literature" Cancers 15, no. 14: 3679. https://doi.org/10.3390/cancers15143679
APA StyleStabile, G., Ripepi, C., Sancin, L., Restaino, S., Mangino, F. P., Nappi, L., & Ricci, G. (2023). Management of Primary Uterine Cervix B-Cell Lymphoma Stage IE and Fertility Sparing Outcome: A Systematic Review of the Literature. Cancers, 15(14), 3679. https://doi.org/10.3390/cancers15143679









