Simple Summary
Although intramedullary nailing (IMN) is considered the standard of care for the surgical management of most femur metastatic diseases, the optimal treatment of metastatic humeral impending and/or pathologic fractures is still debatable. In this study, we explored the usage of cemented vs. uncemented IMN in treating both impending and pathologic fractures, secondary to metastatic disease or multiple myeloma, and compared the outcomes in terms of survival, function, blood loss, blood transfusions, and perioperative complications. Our findings demonstrated that both groups had comparable outcomes, except higher blood loss was found in the cemented group. Thus, intramedullary nailing, both with and without cement, is a relatively safe and effective therapeutic modality for metastatic humeral disease in select patients with similar clinical outcomes and acceptable complication rates.
Abstract
Although intramedullary nailing (IMN) is considered the standard of care for the surgical management of most femur metastatic diseases, the optimal treatment of metastatic humeral impending and/or pathologic fractures is still debatable. Moreover, the use of cemented humeral nails has not been thoroughly studied, and only a few small series have compared their results with uncemented nails. The purpose of this study was to compare the (1) survivorship, (2) functional outcomes, and (3) perioperative complications in patients receiving cemented versus uncemented humerus IMN for impending or complete pathologic fractures resulting from metastatic disease or multiple myeloma. We retrospectively reviewed 100 IMNs in 82 patients, of which 53 were cemented and 47 were uncemented. With a mean survival of 10 months (Cemented: 8.3 months vs. Uncemented: 11.6 months, p = 0.34), the mean Musculoskeletal Tumor Society (MSTS) scores increased from 42.4% preoperatively (Cemented: 40.2% vs. Uncemented: 66.7%, p = 0.01) to 89.2% at 3 months postoperatively (Cemented: 89.8% vs. Uncemented: 90.9%, p = 0.72) for the overall group (p < 0.001). Both cohorts yielded comparable complication rates (overall [22.6% vs. 19.1%)], surgical ([11.3% vs. 4.3%], and medical [13.2% vs. 14.9%], all p > 0.05), but estimated blood loss was significantly higher in the cemented group (203 mL vs. 126 mL, p = 0.003). Thus, intramedullary nailing, with and without cement augmentation in select patients, is a relatively safe and effective therapeutic modality for metastatic humeral disease with similar clinical outcomes and acceptable complication rates. While controlling for possible selection bias, larger-scale, higher-level studies are warranted to validate our results.
1. Introduction
The skeletal system represents the third most common site for metastases, following the lungs and liver, with the humerus being the second most affected long bone (after the femur), accounting for about 20% of all metastatic long bone lesions [1]. Bone metastases can present as impending or complete pathologic fractures that usually occur late in the course of the disease, with median survivorship falling below 1 year once diagnosed [2,3,4]. Given the unpredictable healing process of pathological fractures, the involvement of multiple bones, adjuvant therapy requirements, and early rehabilitation issues, non-operative treatments have yielded unsatisfactory results, and surgical stabilization has become the recommended therapeutic strategy [2,4,5,6,7,8].
Although intramedullary nailing (IMN) is widely considered the surgical standard of care for most occurrences of metastatic bone disease in the femur, there is still vast debate on the optimal treatment for the humerus [3,4,5,7,8]. Surgical options for the humerus include IMN, plate/screws, diaphyseal prostheses, and arthroplasty. The treatment is influenced by multiple factors, including lesion location and severity, bone quality, presence of complete fracture, and overall patient health [2,9,10,11]. Diaphyseal fractures are most frequently treated with IMN, plate–screw constructs, or diaphysis prostheses, whereas endo-prosthetic reconstruction or IMN with a supplemental plate are often used for periarticular humeral lesions [12,13,14,15]. In addition to protecting the entire humeral length, IMN is associated with less soft-tissue dissection and blood loss, shorter operative time, lower complication rates, and earlier rehabilitation [2,4,5,7,9,13,16,17,18]. However, concerns regarding IMN placement remain as complications, such as disease progression, neurovascular and rotator cuff damage, fat and tumor emboli, and fixation loss, are prevalent [10,19]. Additionally, limited open or closed IMN techniques often leave behind the tumor burden and preclude the use of the local adjuvant options of radio-resistant tumors, such as cryosurgery, argon beam, and phenol therapy. On the other hand, plating, prostheses, and arthroplasty may not protect the whole bone and may lead to more soft-tissue dissection, higher blood loss, increased frequency of complications, prolonged functional recovery, and delayed adjuvant chemotherapy or radiation [11,17].
The use of bone cement with the nail has been shown to promote fixation, provide greater construct stability, reduce local tumor mass, resume early adjuvant treatment, and slow disease progression [2,14,20,21]. However, some consider cemented IMN for humerus to be unnecessary due to increased complications, operative time, adequate stability with the nail alone, possible neurovascular damage secondary to the cement’s thermogenic effect and soft-tissue extrusion, and slower bony healing [2,16,22,23]. As of yet, the superiority of any of these modalities has not been established, and there is a paucity of studies that directly compare the survivorship, functional outcomes, and perioperative complications between patients undergoing cemented and uncemented humeral IMN (Supplement S1) [2,4,5,8,11,14,15,16,17,18,20,21,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Moreover, many of these studies have not distinguished if the bone cement was only used to fill the tumor cavity or the entire intramedullary canal (cemented IMN, as defined in our study).
Therefore, the current analysis aimed to study the effectiveness of IMN as a treatment for impending and pathologic humeral fractures in metastatic bone disease and multiple myeloma by assessing (1) survivorship, (2) functional outcomes, and (3) perioperative complication rates for the whole group, as well as by comparing the cemented IMN vs. uncemented IMN groups.
2. Materials and Methods
2.1. Patient Selection and Demographics
The present study was a retrospective comparative analysis of a prospectively maintained institutional review board-approved single-surgeon database in an urban academic setting. Between August 2011 and July 2022, a total of 82 patients (41 [50%] males and 41 [50%] females) who underwent IMN for impending or complete pathologic humeral fractures, for metastatic disease (n = 28) or multiple myeloma (n = 54), were included. Some of these patients have been described, earlier, in a separate study that investigated the outcomes of single stage multiple nailing procedures [42]. Among these patients, 18 underwent bilateral IMN (8 cemented vs. 10 uncemented), bringing the total number of humeral nails placed to 100 (53 [53%] cemented and 47 [47%] uncemented) (Figure 1). There were 40 (40%) and 60 (60%) nails placed for impending and complete pathologic fractures, respectively (Table 1). Out of 82 patients, 33 (40.2%) (cemented: 26 [56.5%] vs. uncemented: 7 [19.4%]) had isolated the humeral nails placed, while 49 (59.8%) (cemented: 20 [43.5%] vs. uncemented: 29 [80.6%]) had more than 1 nail placed in other long bones in 1 or more settings. The most common combination was unilateral humerus and unilateral femur IMN (18; 8 cement and 10 uncemented), followed by unilateral humerus and bilateral femora IMN (9; 2 cement and 7 uncemented).
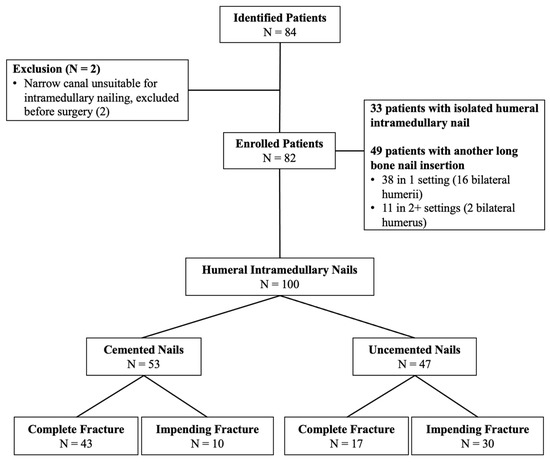
Figure 1.
Flow chart depicting the patient selection process adopted in the current investigation. There were 82 subjects enrolled, accounting for 100 intramedullary nailing procedures.

Table 1.
Demographic variables in the entire study cohort and in patients by cement usage.
The demographics, except for the following, were similar in both groups (Table 1). The uncemented group had more impending fractures than the cemented group, who had more completed pathologic fractures. The uncemented group had more diaphyseal lesions, and the cemented group had more proximal lesions. The uncemented group had more patients receiving multiple nails placed in one setting. Within the patients who received multiple nails, 38 (cemented: 15 [32.6%] vs. uncemented: 23 [63.9%]) were performed in one setting, while 11 (cemented: 5 [10.9%]) vs. uncemented: 6 [16.7%]) were performed in 2 or more settings.
2.2. Perioperative Protocols and after Care
All patients received coordinated care from a multidisciplinary team, ensuring medical optimization prior to surgery, including a hemoglobin level of at least 10 g/dL. Preoperative embolization of hyper-vascular metastatic tumors was performed in 2 cases (renal cell and hepatocellular carcinomas). Mirels’ criteria were mostly used for the prophylactic fixation of impending fractures [43]. The type of IMN (cemented vs. uncemented) predominantly depended on the extent of tumor involvement, bone quality, amount of bone loss, periarticular lesions, extensive and skip lesions, and fixation stability, and it was decided by the senior surgeon (AVM).
The surgical technique is detailed in Supplement S2 [44]. All surgeries were conducted with a minimally invasive technique, except for 7 pathologic fractures that required direct tumor site opening (curettage resection for radioresistant tumors and/or significant bone defects [5 cases], as well as failure to obtain acceptable fracture closed reduction [2 cases]). There were 3 patients who received plate fixation, in addition to intramedullary nailing, for extensive periarticular bone involvement. In addition, one patient in the cemented cohort required intraoperative conversion from a nail to a plate construct due to a proximal nail cutout of the lateral cortex. This patient was excluded from functional score analysis, but they were included in survival and complication analyses. The postoperative rehabilitation protocol was similar for all patients. An arm sling was given for initial postoperative comfort, and immediate range of motion, starting with pendulum exercises, was allowed. Weight bearing depended on bone involvement and fixation stability, but activities of daily living were allowed immediately. After discharge, outpatient visits were scheduled at 2 and 6 weeks postoperatively, every 3 months for the first year, every 6 months for the second year, and once a year afterwards. Radiographs were obtained immediate postoperatively, at 3 months, and at every subsequent visit (Figure 2 and Figure 3). In most patients, chemotherapy and/or radiation (3000–3500 Gy) were started/resumed 10 to 21 days after their index surgery, as deemed appropriate by the multidisciplinary team.
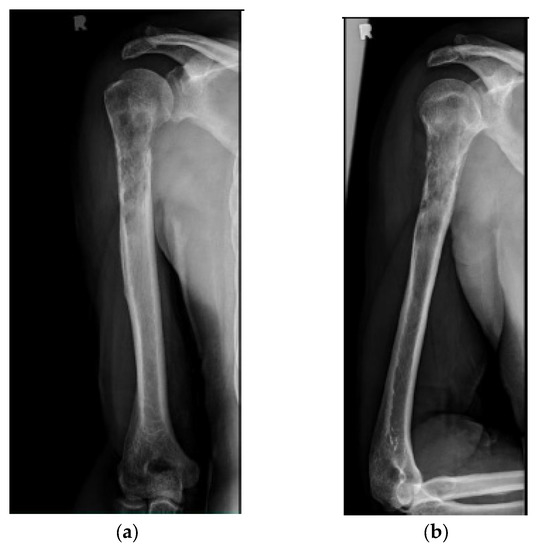
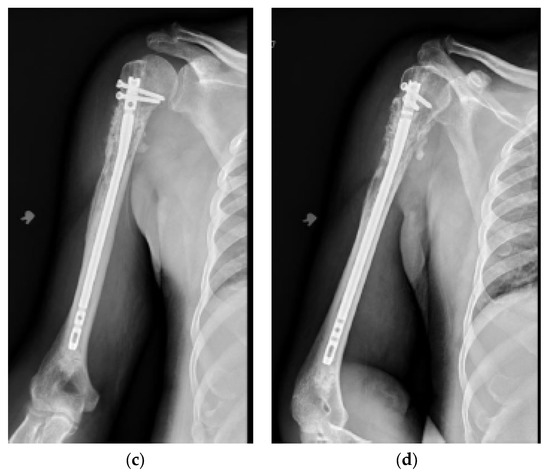
Figure 2.
(a) AP and (b) lateral right humerus radiographs of a 67-year-old male with a mixed lytic sclerotic lesion in the proximal meta-diaphyseal region, with a pathologic fracture from a newly diagnosed metastatic prostate cancer. This was treated by a bone biopsy, followed by a cemented IMN with two proximal inter-locking screws and no distal screw, as shown in the (c) AP and (d) lateral humerus radiographs. Cement was used for augmentation, due to poor proximal humerus bone quality, to support the nail and the inter-locking screws.
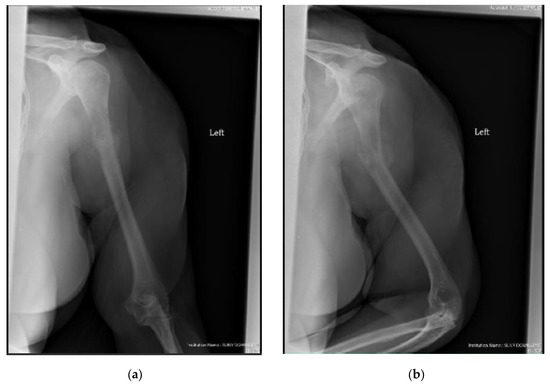
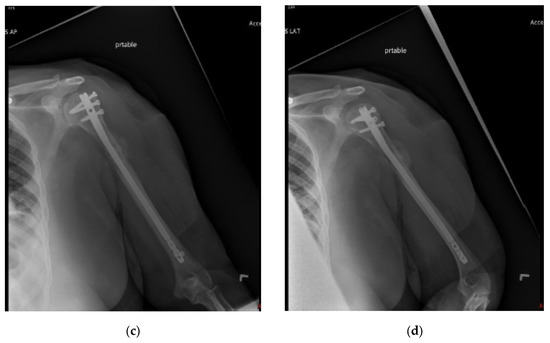
Figure 3.
(a) AP and (b) oblique left humerus radiographs of a 47-year-old female with mixed lytic sclerotic lesion in the proximal meta-diaphyseal region, with a pathologic fracture and periosteal reaction in a patient with established metastatic breast cancer. This was treated by an uncemented IMN, with three proximal locking screws and one distal screw, as shown in (c) AP and (d) oblique humerus radiographs. No cement was used, as there was enough proximal and distal bone to support the nail and the inter-locking screws, and some healing changes were already evident.
2.3. Collected Variables
Extracted variables included patient demographics (age, sex, body mass index [BMI]), primary malignancy diagnosis, fracture type (impending or complete pathologic), lesion location, cement use, concomitant procedures (e.g., other long bone IMN in the same or different setting), intraoperative blood loss, and blood transfusion volume (up to 24 h postoperatively, 1-unit PRBC = 325 mL). Perioperative complications and the return to the operating room were recorded. Complications were labeled as “surgical” if they were directly related to the nailing procedure, and they included infection, wound dehiscence, reoperations, and implant-related mechanical complications. Some cement extrusion to soft tissues was common in pathologic fracture cases, but it was only classified as a complication if there were subsequent mechanical issues, neurovascular damage, and/or a need for additional intervention. Medical complications were primarily recorded for that admission and cardiopulmonary, including pulmonary embolism, pneumonia, hypotension, myocardial infarction, and respiratory distress. Other recorded complications included gastrointestinal bleeding, Clostridium difficile infection, coagulopathies such as disseminated intravascular coagulation, deep vein thrombosis, and thrombocytopenia, sepsis, urinary tract infection, as well as transaminitis with hyperbilirubinemia. Intraoperative estimated blood loss (EBL) was estimated by quantifying the number of laparotomy sponges utilized along with the total blood in the suction canister [45]. Functional outcomes were recorded using the Musculoskeletal Tumor Society (MSTS) upper extremity scoring system at the initial presentation and 3 months postoperatively [46]. Due to death, loss to follow-up, or an inability to have a direct follow-up visit, especially during the COVID-19 pandemic, functional score was not available for the majority of patients beyond 3 months, and thus, the MSTS score was reported for 3 months. Oncologic outcomes were evaluated with patient survivorship and calculated by tracking patients from surgery until death. Several of our patients were foreign citizens traveling to and from their home country to receive treatment, making data collection difficult for certain variables. Patient death was documented from medical chart review and direct communication with their families and other medical providers [47]. When mortality was not able to be confirmed by available means and no further communication with the patient or their family members was identified in the patient’s chart, individuals who missed two consecutive visits without rescheduling were presumed to be lost to follow-up at the date of the last documented visit to the clinic or hospital, and they were subsequently excluded from analysis of survivorship and functional outcomes. By a 3 month postoperative period, 18 (22.0%) patients died and 18 (22.0%) were lost to follow-up. By a 6 month postoperative period, 23 (28.0%) patients died and 25 (30.5%) were lost to follow-up. By a 12 month postoperative period, 27 (32.9%) patients had died and 29 (35.4%) were lost to follow-up, and thus, analysis was done based on available patients at each time point.
2.4. Statistical Analyses
Descriptive analyses were performed to summarize patient demographics, operative variables, and patient outcomes. Statistical analyses included two-sided Fisher’s exact and Student’s t-tests to compare categorical and continuous variables, respectively. Patient survivorship was reported using Kaplan–Meier estimates with the log–rank test. All analyses were performed in R Statistical Software using a p-value of <0.05 as the threshold for statistical significance.
3. Results
A total of 82 patients with 100 nails were analyzed (53 [53.0%] cemented and 47 [47.0%] uncemented).
3.1. Survivorship
Mean survivorship for all available patients was 10.0 ± 14.3 (range, 0–86) months. No significant differences in mean survivorship were detected between the two cohorts (cemented: 8.3 ± 9.3 [range, 0–35] vs. uncemented: 11.6 ± 17.7 [range, 0–86] months, p = 0.34) (Table 2). Kaplan–Meier median survivorship was estimated at 6.0 months for the overall patient population. No significant differences in the Kaplan–Meier median survivorship were detected between the two cohorts (cemented: 6.0 vs. uncemented: 8.0 months, p = 0.30) (Figure 4). There was no intraoperative death. There were 2 patients in the cemented group who died during the same admission, at 4 weeks, compared to none in the uncemented group (p = 0.22; Table 2 and Table 3).

Table 2.
Complications, overall survival, and functional outcomes in study patients.
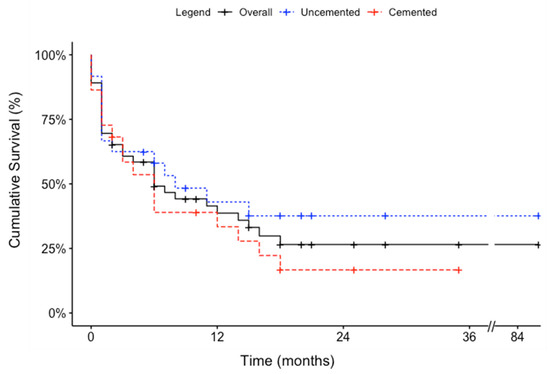
Figure 4.
Kaplan–Meier curves depicting the survival of patients who underwent humeral intramedullary nail fixation with and without cement.

Table 3.
List of patients undergoing humeral intramedullary nailing who exhibited surgical and medical complications.
3.2. Functional Outcomes
Mean MSTS scores for all available patients increased from 42.4% ± 8.4% (range, 28–60%) preoperatively to 89.2% ± 5.5% (range, 76–96%) at 3 months postoperatively (p < 0.001). Patients with cemented IMN had lower preoperative MSTS scores (40.2% ± 9.6% [range, 16–64%]) than those without cement (66.7% ± 28.5% [range, 20–100%], p = 0.01). However, postoperative MSTS scores were comparable between both groups (cemented: 89.8% ± 7.0% [range, 80–100%]) vs. uncemented: 90.9% ± 1.9% [range, 90–100%]) (p = 0.72) (Table 2).
3.3. Perioperative Outcomes and Complications
The details of the complications are summarized in Table 2 and Table 3. There was a total of 21 (21.0%) perioperative complications in 100 surgeries, of which 14 (14.0%) and 8 (8.0%) were medical and surgical, respectively. There was one cemented IMN patient who had both medical and surgical complications. In the cemented IMN cohort, there were 12 (22.6%) perioperative complications in 53 surgeries, of which 7 (13.2%) were medical and 6 (11.3%) were surgical. In the uncemented IMN cohort, there were 9 (19.1%) perioperative complications in 47 surgeries, of which 7 (14.9%) were medical and 2 (4.3%) were surgical. Overall (22.6% vs. 19.1%; p = 1.00), medical (13.2% vs. 14.9%; p = 0.76), and surgical (11.3% vs. 4.3%; p = 0.47) complication rates were similar between patients undergoing cemented and uncemented humeral IMN, respectively. There were no cases of surgical site infections, wound dehiscence, or implant breakage requiring returns to the operating room.
There were two returns to the operating room in the entire study. From the cemented group, 1 patient developed quadriparesis from cervical spine tumor involvement and instability, requiring urgent neurosurgical decompression and fixation 2 days after nailing procedure. Another individual from the cemented group was taken back to the operating room, 2 years after their index surgery, for a symptomatic prominent backed out proximal locking screw that was removed.
In the entire study cohort, there was a mean EBL of 166 ± 138 (range, 0–1000) mL (Table 2). EBL was significantly higher in patients with cement-augmented IMN (203 ± 179 [range, 0–1000] mL) compared to patients with uncemented IMN (126 ± 49 [range, 50–300] mL) (p = 0.003). There was a mean perioperative transfusion of 236 ± 394 (range, 0–1950) mL for both study cohorts (Table 2). There were no significant differences in perioperative transfusion volumes between the cemented (204 ± 318 [range, 0–975] mL) and uncemented (271 ± 462 [range, 0–1950] mL).
4. Discussion
Patients presenting with impending or complete pathologic humeral fractures, secondary to metastatic bone disease or myeloma, may be treated with multiple modalities. Although IMN fixation is commonly used, the procedure has been associated with various complications [2,4,5,7,9,10,13,16,17,18,19]. Furthermore, the benefit of IMN, with or without cement, has not been adequately addressed in the literature, and most studies have not compared clinical outcomes [2,4,5,8,11,14,15,16,17,18,20,21,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
In our study population, median and 1 year survival were comparable to those previously reported by other studies (6.0 vs. 6.4–10.6 months and 38.7% vs. 26.7–49%, respectively), although there may be a difference in calculation methodology and follow-ups between studies, impeding direct comparison [2,14,17,21,27,49,50]. In our study, there was no difference in the survival of patients in the cemented vs. uncemented group. With the numbers available, this may signify that survival after metastasis to the humerus has a similar prognosis, irrespective of the extent of the disease. Thus, surgical management mainly stays a part of palliative care, improving pain, function, and quality of life.
Functional outcomes of patients with metastatic humeral disease improved following IMN insertion, as evidenced by a two-fold overall mean MSTS score improvement at 3 month follow-up. Coupled with the relatively low surgical complication rate and adequate functional gain, our study found IMN fixation to be an effective operative modality for our patients. Patients undergoing cemented humeral IMN exhibited significantly lower preoperative MSTS scores (40.2%) than patients with uncemented nail insertion (66.7%) (p = 0.01). While this was likely due to selection bias, both groups were similar with respect to postoperative MSTS scores at 3 months (89.8% and 90.9%), justifying the use of cement in this group. Given that individuals with larger tumor burdens—and resultant poorer bone stock—are more likely to receive cement augmentation (over 70% of pathologic fractures in the current analysis), bone cement may promote functional gain in more advanced humeral diseases. This finding corroborates previous studies that endorse cement for the improvement of fixation stability, pain mitigation, and function restoration, especially in the short term [10,20,51,52,53].
Perioperative complications occurred in 21.0% of procedures, with medical (14.0%) and surgical (8.0%) complication rates comparable to those reported in the literature (0–26.0% and 0–28.6%, respectively) [Table 2 and Table 3, Supplement S1]. The variation in complication rates between studies could be explained by discrepancies in defining adverse events. For instance, while some analyses placed emphasis, mostly, on cardiopulmonary complications, including thromboembolism, others also included gastrointestinal bleeding and postoperative pneumonia as complications [17,18]. Embolic events are notable in IMN fixation because reaming, nail insertion, and cement augmentation may cause an intramedullary pressure surge, potentially increasing the risk of tumor and bone marrow fat liberation, subsequently leading to an intraoperative cardiopulmonary event [28,54]. However, this has, mostly, been studied in the femur and not the humerus. In this study, only 1 (1.0%) case of symptomatic pulmonary embolism was noted, which is consistent with a previously reported incidence of fat (0.3%) and pulmonary (1.3%) emboli following humeral nail insertion [17]. In our patients undergoing cemented IMN, cement was used in a relatively more liquid state without pressurization, which, combined with predominant use of unreamed or minimally reamed smaller diameter (8 mm) nails and the presence of a fracture acting as a natural vent site, could have contributed to the overall low rate of embolic events.
Patients undergoing cemented and uncemented humeral IMN displayed comparable rates of overall (22.6% vs. 19.1%), medical (13.2% vs. 14.9%), and surgical (11.3% vs. 4.3%) complications. Intraoperative surgical complications (4 [4.0%] cases) primarily occurred in individuals treated with cemented IMN, reflecting the higher proportion of pathologic fractures in this patient population, more extensive disease with relatively poorer bone stock, and the procedure’s technical complexity. Although our cementing technique increased the probability of extrusion at the fracture sites, many of these at the fracture site had no clinical significance and required no intervention, possibly due to an intact protective soft-tissue sleeve, as most of these fractures are usually low-energy events. When needed, the cement was removed in the same setting by using either the same or a different incision. Adding to the literature, most surgical complications encountered with cemented nails were technical, which were recognized and managed appropriately with no impact on patient outcomes [21,29]. Furthermore, intraoperative blood loss was also higher in subjects with cemented IMN, which usually required taking additional surgical steps, increasing canal reaming, and, possibly, lengthening the operative time as a result, although this extra time was slightly offset by not using distal interlocking screws.
The current study has several limitations. Given its retrospective and complex nature, no true matched control group was included. As such, there were certain confounders of survivorship, functional outcomes, and perioperative complications that could not be accounted and controlled. Several patients underwent multiple long bone nailing procedures, either in a single stage or multiple staged fashion, which can influence overall outcomes. Moreover, there was a selection bias for the usage of cement based on the surgeon’s assessment. Long bones requiring prophylactic fixation have relatively better quality, so uncemented intramedullary nails were, more often, inserted for diaphyseal lesions and impending fractures. Diaphyseal lesions also have better proximal and distal bones remaining for fixation and interlocking screw purchasing, thus precluding the need for cement augmentation. Patients with uncemented nails also underwent significantly more than one IMN procedure in multiple long bones in the same setting, creating another bias for complications, as anticipated complexity, surgical time, and blood loss was less. In contrast, complete pathologic fractures often stem from more advanced disease, and as a result, they exhibit inferior bone quality, warranting cement augmentation for better fixation. The lower preoperative MSTS score in subjects who underwent cemented nailing could have mirrored the higher percentage of complete pathologic fractures documented in this patient population. Despite evaluating one of the largest groups of a detailed humeral IMN reported to date (Supplement S1), the sample size included in the current analysis is still relatively small, and it may be limited by insufficient statistical power for the comparison of the two cohorts. Moreover, several patients were lost to follow-up, and they were excluded from the analysis. This study also did not compare operative time directly, as overall surgical time was influenced by several independent factors such as fluoroscopy, perioperative additional anesthesia preparation, and multiple single-stage nailing [42]. Likewise, we did not compare the length of hospital stays and returns to definitive adjuvant therapy in this patient population due to several factors listed above. In addition, most subjects had multiple myeloma as their primary malignancy, due to the disease’s high prevalence in our community. Nevertheless, there was no significant difference (p > 0.05) in perioperative complications or survivorship between those with multiple myeloma and those with metastatic disease. Despite similar orthopedic management, metastatic tumors and multiple myeloma may differ regarding disease history and prognosis, potentially skewing the study’s findings.
To the best of our knowledge, this study is the largest series of its kind, despite these limitations, and it is the first to compare the survivorship, functional outcomes, and perioperative complications of cemented and uncemented IMN in order to assess their effectiveness as treatments for impending and complete pathologic humeral fractures. Future plans may include a higher-powered analysis, an in-depth analysis of differences between patients with multiple myeloma and metastatic disease, as well as a way to account for other variables and confounders.
5. Conclusions
Intramedullary nailing, both with and without cement, is a relatively safe and effective therapeutic modality for impending and pathologic humeral fractures, resulting in similarly acceptable clinical outcomes and complication rates. The use of bone cement is often based on several clinical factors and, thus, induces selection bias. Nevertheless, the outcomes were similar between both cemented and uncemented groups, justifying the use of cement in this select group. Most intraoperative surgical complications resulted from technical errors stemming from bone cement use, and they could be minimized with awareness, meticulous attention to surgical technique, and more abundant experience. While controlling for possible selection bias, larger-scale higher-level studies are warranted to validate these results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15143601/s1, Supplement S1: Literature review of the last 30 years summarizing complication rates and mean survivorship in patients undergoing intramedullary nailing for metastatic humeral disease. Supplement S2: Surgical technique.
Author Contributions
Conceptualization, A.V.M. and A.K.; methodology, A.V.M., A.K., P.N. and J.B.; validation, A.V.M., A.K., P.N. and J.B.; formal analysis, A.K., J.B. and T.L.L.; data curation, A.K., P.N., J.B. and T.L.L.; writing—original draft preparation, A.K. and P.N.; writing—review and editing, A.V.M., A.K., P.N. and J.B.; visualization, J.B.; supervision, A.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of SUNY Downstate Medical Center (study number: 372416-3, last amended: 22 January 2018).
Informed Consent Statement
Patient consent was waived due to retrospective study design with deidentified patient information.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical considerations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef]
- Laitinen, M.; Nieminen, J.; Pakarinen, T.-K. Treatment of Pathological Humerus Shaft Fractures with Intramedullary Nails with or without Cement Fixation. Arch. Orthop. Trauma Surg. 2011, 131, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ratasvuori, M.; Wedin, R.; Keller, J.; Nottrott, M.; Zaikova, O.; Bergh, P.; Kalen, A.; Nilsson, J.; Jonsson, H.; Laitinen, M. Insight Opinion to Surgically Treated Metastatic Bone Disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry Report of 1195 Operated Skeletal Metastasis. Surg. Oncol. 2013, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Sarahrudi, K.; Wolf, H.; Funovics, P.; Pajenda, G.; Hausmann, J.T.; Vécsei, V. Surgical Treatment of Pathological Fractures of the Shaft of the Humerus. J. Trauma Inj. Infect. Crit. Care 2009, 66, 789–794. [Google Scholar] [CrossRef]
- Dijkstra, S.; Stapert, J.; Boxma, H.; Wiggers, T. Treatment of Pathological Fractures of the Humeral Shaft Due to Bone Metastases: A Comparison of Intramedullary Locking Nail and Plate Osteosynthesis with Adjunctive Bone Cement. Eur. J. Surg. Oncol. 1996, 22, 621–626. [Google Scholar] [CrossRef]
- Flemming, J.; Beals, R. Pathologic Fracture of the Humerus. Clin. Orthop. Relat. Res. 1986, 203, 258–260. [Google Scholar] [CrossRef]
- Frassica, F.J.; Frassica, D.A. Evaluation and Treatment of Metastases to the Humerus. Clin. Orthop. Relat. Res. 2003, 415, S212–S218. [Google Scholar] [CrossRef]
- Piccioli, A.; Maccauro, G.; Rossi, B.; Scaramuzzo, L.; Frenos, F.; Capanna, R. Surgical Treatment of Pathologic Fractures of Humerus. Injury 2010, 41, 1112–1116. [Google Scholar] [CrossRef]
- Bryson, D.J.; Wicks, L.; Ashford, R.U. The Investigation and Management of Suspected Malignant Pathological Fractures: A Review for the General Orthopaedic Surgeon. Injury 2015, 46, 1891–1899. [Google Scholar] [CrossRef]
- Weiss, K.R.; Bhumbra, R.; Biau, D.J.; Griffin, A.M.; Deheshi, B.; Wunder, J.S.; Ferguson, P.C. Fixation of Pathological Humeral Fractures by the Cemented Plate Technique. J. Bone Jt. Surg. Br. 2011, 93-B, 1093–1097. [Google Scholar] [CrossRef]
- Camnasio, F.; Scotti, C.; Peretti, G.M.; Fontana, F.; Fraschini, G. Prosthetic Joint Replacement for Long Bone Metastases: Analysis of 154 Cases. Arch. Orthop. Trauma Surg. 2008, 128, 787–793. [Google Scholar] [CrossRef] [PubMed]
- El Beaino, M.; Liu, J.; Lewis, V.O.; Lin, P.P. Do Early Results of Proximal Humeral Allograft-Prosthetic Composite Reconstructions Persist at 5-Year Followup? Clin. Orthop. Relat. Res. 2019, 477, 758–765. [Google Scholar] [CrossRef]
- Janssen, S.J.; Teunis, T.; Hornicek, F.J.; Bramer, J.A.M.; Schwab, J.H. Outcome of Operative Treatment of Metastatic Fractures of the Humerus: A Systematic Review of Twenty Three Clinical Studies. Int. Orthop. 2015, 39, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Thai, D.M.; Kitagawa, Y.; Choong, P.F. Outcome of Surgical Management of Bony Metastases to the Humerus and Shoulder Girdle: A Retrospective Analysis of 93 Patients. Int. Semin. Surg. Oncol. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wedin, R.; Hansen, B.H.; Laitinen, M.; Trovik, C.; Zaikova, O.; Bergh, P.; Kalén, A.; Schwarz-Lausten, G.; Vult von Steyern, F.; Walloe, A.; et al. Complications and Survival after Surgical Treatment of 214 Metastatic Lesions of the Humerus. J. Shoulder Elbow. Surg. 2012, 21, 1049–1055. [Google Scholar] [CrossRef]
- Hunt, K.J.; Gollogly, S.; Randall, R.L. Surgical Fixation of Pathologic Fractures: An Evaluation of Evolving Treatment Methods. Bull. Hosp. Jt. Dis. 2006, 63, 77–82. [Google Scholar]
- Janssen, S.J.; van Dijke, M.; Lozano-Calderón, S.A.; Ready, J.E.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; Schwab, J.H. Complications after Surgery for Metastatic Humeral Lesions. J. Shoulder Elbow. Surg. 2016, 25, 207–215. [Google Scholar] [CrossRef]
- Pretell, J.; Rodriguez, J.; Blanco, D.; Zafra, A.; Resines, C. Treatment of Pathological Humeral Shaft Fractures with Intramedullary Nailing. A Retrospective Study. Int. Orthop. 2010, 34, 559–563. [Google Scholar] [CrossRef]
- Noger, M.; Berli, M.C.; Fasel, J.H.D.; Hoffmeyer, P.J. The Risk of Injury to Neurovascular Structures from Distal Locking Screws of the Unreamed Humeral Nail (UHN): A Cadaveric Study. Injury 2007, 38, 954–957. [Google Scholar] [CrossRef]
- Atesok, K.; Liebergall, M.; Sucher, E.; Temper, M.; Mosheiff, R.; Peyser, A. Treatment of Pathological Humeral Shaft Fractures with Unreamed Humeral Nail. Ann. Surg. Oncol. 2007, 14, 1493–1498. [Google Scholar] [CrossRef]
- Choi, E.-S.; Han, I.; Cho, H.S.; Park, I.W.; Park, J.W.; Kim, H.-S. Intramedullary Nailing for Pathological Fractures of the Proximal Humerus. Clin. Orthop. Surg. 2016, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Barker, M.E.; Hamlin, B.H. Thermal Effects of Acrylic Cementation at Bone Tumour Sites. Int. J. Hyperth. 1997, 13, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.J.; Lopez-Ben, R.; Mann, J.P.; Ponce, B.A. Pathological Fractures of the Proximal Humerus Treated with a Proximal Humeral Locking Plate and Bone Cement. J. Bone Jt. Surg. Br. 2010, 92-B, 707–712. [Google Scholar] [CrossRef]
- Bauze, A.; Clayer, M. Treatment of Pathological Fractures of the Humerus with a Locked Intramedullary Nail. J. Orthop. Surg. 2003, 11, 34–37. [Google Scholar] [CrossRef]
- Ingman, A.M.; Waters, D.A. Locked Intramedullary Nailing of Humeral Shaft Fractures. Implant Design, Surgical Technique, and Clinical Results. J. Bone Jt. Surg. Br. 1994, 76, 23–29. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, H.G.; Kim, J.H.; Kim, S.; Lin, P.P.; Kim, H.S. Closed Intramedullary Nailing with Percutaneous Cement Augmentation for Long Bone Metastases. Bone Jt. J. 2016, 98-B, 703–709. [Google Scholar] [CrossRef]
- Moura, D.L.; Alves, F.; Fonseca, R.; Freitas, J.; Casanova, J. Tratamento de Fraturas Patológicas Tumorais Diafisárias do Úmero Com Haste Intramedular Rígida Bloqueada Estática—Experiência de 22 Anos. Rev. Bras. Ortop. 2019, 54, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ofluoglu, O.; Erol, B.; Ozgen, Z.; Yildiz, M. Minimally Invasive Treatment of Pathological Fractures of the Humeral Shaft. Int. Orthop. 2009, 33, 707–712. [Google Scholar] [CrossRef]
- Pizzo, R.A.; Hoskins, T.; Patel, J.N.; Miller, J.M.; Goyette, D.; Mazzei, C.; Wittig, J.C. Distally Unlocked Intramedullary Nailing With Cement Fixation for Impending and Actual Pathologic Humerus Fractures: A Retrospective Case Series. JAAOS Glob. Res. Rev. 2020, 4, e20.00090. [Google Scholar] [CrossRef]
- Redmond, B.J.; Biermann, J.S.; Blasier, R.B. Interlocking Intramedullary Nailing of Pathological Fractures of the Shaft of the Humerus. J. Bone Jt. Surg. 1996, 78, 891–896. [Google Scholar] [CrossRef]
- Rommens, P.M.; Blum, J. Retrograde Locked Nailing of Humeral Shaft Fractures Using the Unreamed Humeral Nail (UHN). Orthop. Traumatol. 1999, 7, 251–259. [Google Scholar] [CrossRef]
- Spencer, S.J.; Holt, G.; Clarke, J.V.; Mohammed, A.; Leach, W.J.; Roberts, J.L.B. Locked Intramedullary Nailing of Symptomatic Metastases in the Humerus. J. Bone Jt. Surg. Br. 2010, 92-B, 142–145. [Google Scholar] [CrossRef]
- Tomé, J.; Carsi, B.; García-Fernández, C.; Marco, F.; López-Durán Stern, L. Treatment of Pathologic Fractures of the Humerus with Seidel Nailing. Clin. Orthop. Relat. Res. 1998, 350, 51–55. [Google Scholar]
- Vandeweyer, E.; Gebhart, M. Treatment of Humeral Pathological Fractures by Internal Fixation and Methylmetacrylate Injection. Eur. J. Surg. Oncol. (EJSO) 1997, 23, 238–242. [Google Scholar] [CrossRef]
- Gebhart, M.; Dequanter, D.; Vandeweyer, E. Metastatic Involvement of the Humerus: A Retrospective Study of 51 Cases. Acta Orthop. Belg. 2001, 67, 456–463. [Google Scholar]
- Bickels, J.; Kollender, Y.; Wittig, J.C.; Meller, I.; Malawer, M.M. Function after Resection of Humeral Metastases. Clin. Orthop. Relat. Res. 2005, 437, 201–208. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, H.G.; Kim, J.R.; Lin, P.P.; Kim, H.S. Minimally Invasive Surgery of Humeral Metastasis Using Flexible Nails and Cement in High-Risk Patients with Advanced Cancer. Surg. Oncol. 2011, 20, e32–e37. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yeh, T.-T.; Pan, R.-Y.; Wu, C.-C. Ante-Grade Intramedullary Nailing for the Treatment of Humeral Shaft Metastatic Bone Tumor. J. Med. Sci. 2014, 34, 247. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.; Kang, H.G.; Kim, J.H.; Kim, H.S. Joint-Preserving Palliative Surgery Using Self-Locking Screws of Intramedullary Nail and Percutaneous Cementoplasty for Proximal Humeral Metastasis in the Advanced Cancer Patients. World J. Surg. Oncol. 2018, 16, 93. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.; Kang, H.G.; Kim, J.H.; Kim, H.S. Preliminary Results: Use of Multi-Hole Injection Nails for Intramedullary Nailing with Simultaneous Bone Cement Injection in Long-Bone Metastasis. Skeletal. Radiol. 2019, 48, 219–225. [Google Scholar] [CrossRef]
- Ricard, M.-A.M.; Stavropoulos, N.A.; Nooh, A.; Ste-Marie, N.; Goulding, K.; Turcotte, R. Intramedullary Nailing Versus Plate Osteosynthesis for Humeral Shaft Metastatic Lesions. Cureus 2021, 13, e13788. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.V.; Kobryn, A.; Alam, J.S.; Tretiakov, M. Single-Stage versus Multi-Stage Intramedullary Nailing for Multiple Synchronous Long Bone Impending and Pathologic Fractures in Metastatic Bone Disease and Multiple Myeloma. Cancers 2023, 15, 1227. [Google Scholar] [CrossRef] [PubMed]
- Mirels, H. Metastatic Disease in Long Bones. A Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 1989, 249, 256–264. [Google Scholar] [CrossRef]
- Beickert, R.; Buckle, R.; Buhren, V.; Mason, M.D. T2 Humeral Nailing System. In Shoulder and Elbow Trauma and Its Complications; Woodhead Publishing: Sawston, UK, 2010; pp. 1–52. [Google Scholar]
- Cai, D.F.; Fan, Q.H.; Zhong, H.H.; Peng, S.; Song, H. The Effects of Tourniquet Use on Blood Loss in Primary Total Knee Arthroplasty for Patients with Osteoarthritis: A Meta-Analysis. J. Orthop. Surg. Res. 2019, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A System for the Functional Evaluation of Reconstructive Procedures after Surgical Treatment of Tumors of the Musculoskeletal System. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- National Center for Health Statistics. How to Use the National Death Index: Steps in the Process. Available online: https://www.cdc.gov/nchs/ndi/portal.htm (accessed on 1 June 2023).
- Riddoch, G.; Rowley Bristow, W.; Cairns, H.W.B.; Carmichael, E.A.; Critchley, M.; Greenfield, J.G.; Learmonth, J.R.; Platt, H.; Seddon, H.J.; Symonds, C.P.; et al. Aids to the examination of the peripheral nervous system. Med. Res. Counc.–Nerves Inj. Comm. 1943, 45, 1–62. [Google Scholar]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.-M.; Smith, M.; Coleman, R. Pathologic Fractures Correlate with Reduced Survival in Patients with Malignant Bone Disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef]
- Galán-Olleros, M.; Marco, J.; Oteo, D.; Cristóbal-Bilbao, R.; Manrique, E.; García-Maroto, R.; Marco, F.; Cebrián-Parra, J.L. Orthopedic Surgical Treatment and Perioperative Complications in Multiple Myeloma Bone Disease: Analysis of a Series (2009–2018). Ann. Surg. Oncol. 2021, 28, 1158–1166. [Google Scholar] [CrossRef]
- Harrington, K.D.; Johnston, J.O.; Turner, R.H.; Green, D.L. The Use of Methylmethacrylate as an Adjunct in the Internal Fixation of Malignant Neoplastic Fractures. J. Bone Jt. Surg. Am. 1972, 54, 1665–1676. [Google Scholar] [CrossRef]
- Harrington, K.D. The Use of Methylmethacrylate as an Adjunct in the Internal Fixation of Unstable Comminuted Intertrochanteric Fractures in Osteoporotic Patients. J. Bone Jt. Surg. Am. 1975, 57, 744–750. [Google Scholar] [CrossRef]
- Sim, F.H.; Kruse, R.L.; Johnson, E.W. Problems in Pathologic Fractures. Minn. Med. 1974, 57, 266–269. [Google Scholar]
- Johnson, J.A.; Berkshire, A.; Leighton, R.K.; Gross, M.; Chess, D.G.; Petrie, D. Some Basic Biomechanical Characteristics of Medullary Pressure Generation during Reaming of the Femur. Injury 1995, 26, 451–454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).