The Role of Native T1 and T2 Mapping Times in Identifying PD-L1 Expression and the Histological Subtype of NSCLCs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Examination
2.3. Post-Processing of MRI Images
2.4. Statistical Analysis
3. Results
3.1. Patients’ Clinical Data
3.2. Correlation between T1 and T2 Mapping Values and PD-L1 Expression in NSCLC
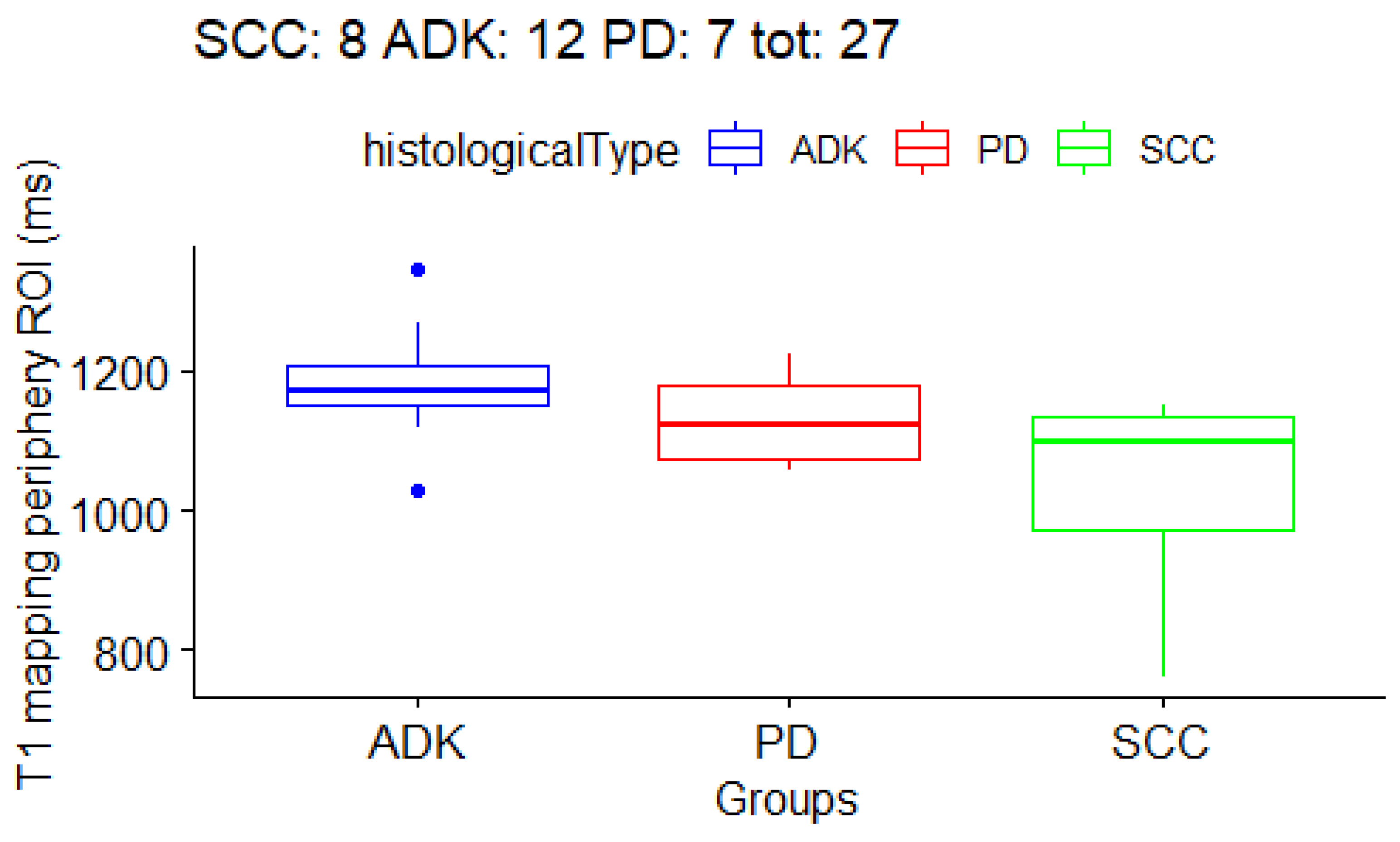
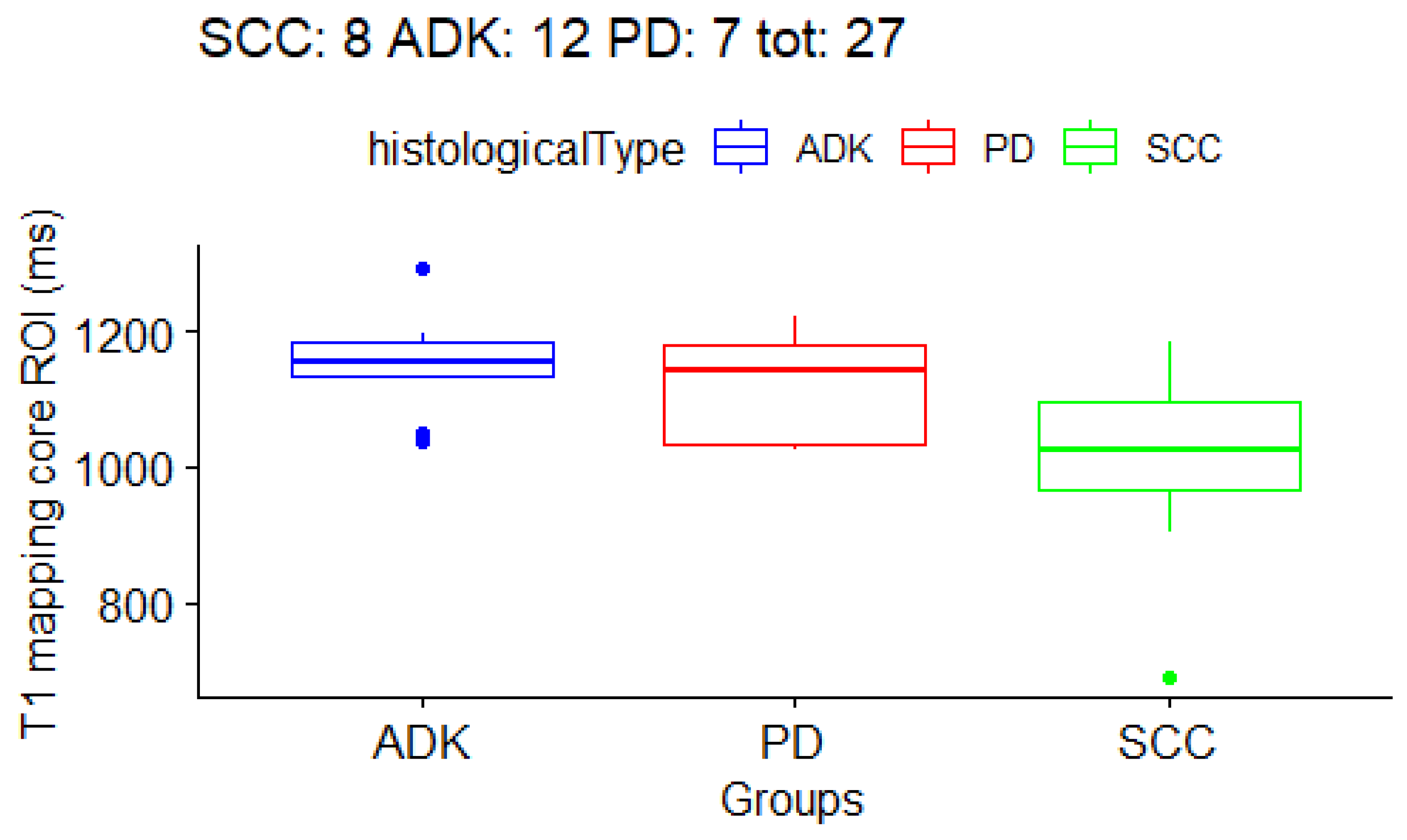
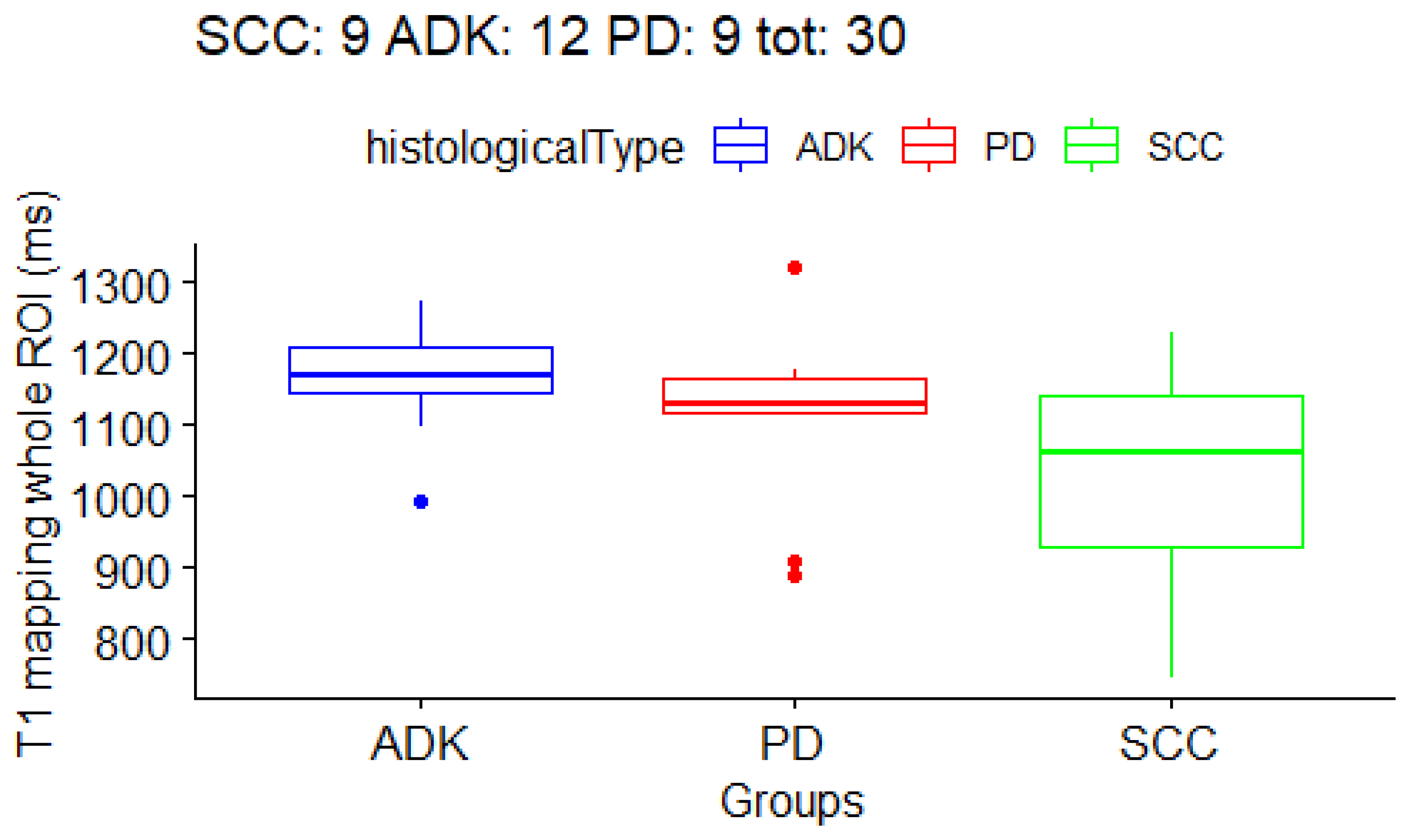
3.3. Correlation between T1 and T2 Mapping Values and Histological Subtype of NSCLC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Inamura, K. Lung cancer: Understanding its molecular pathology and the 2015 WHO classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Kocher, F.; Hilbe, W.; Seeber, A.; Pircher, A.; Schmid, T.; Greil, R.; Auberger, J.; Nevinny-Stickel, M.; Sterlacci, W.; Tzankov, A.; et al. Longitudinal analysis of 2293 NSCLC patients: A comprehensive study from the TYROL registry. Lung Cancer 2015, 87, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Shapiro, R. Lung Biopsy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Koyama, H.; Ohno, Y.; Seki, S.; Nishio, M.; Yoshikawa, T.; Matsumoto, S.; Sugimura, K. Magnetic resonance imaging for lung cancer. J. Thorac. Imaging 2013, 28, 138–150. [Google Scholar] [CrossRef]
- Bortolotto, C.; Stella, G.M.; Messana, G.; Lo Tito, A.; Podrecca, C.; Nicora, G.; Bellazzi, R.; Gerbasi, A.; Agustoni, F.; Grimm, R.; et al. Correlation between PD-L1 Expression of Non-Small Cell Lung Cancer and Data from IVIM-DWI Acquired during Magnetic Resonance of the Thorax: Preliminary Results. Cancers 2022, 14, 5634. [Google Scholar] [CrossRef]
- Cheng, H.-L.M.; Stikov, N.; Ghugre, N.R.; Wright, G.A. Practical medical applications of quantitative MR relaxometry. J. Magn. Reson. Imaging 2012, 36, 805–824. [Google Scholar] [CrossRef]
- Germain, P.; El Ghannudi, S.; Jeung, M.-Y.; Ohlmann, P.; Epailly, E.; Roy, C.; Gangi, A. Native T1 mapping of the heart—A pictorial review. Clin. Med. Insights Cardiol. 2014, 8, CMC.S19005. [Google Scholar] [CrossRef]
- Alamidi, D.F.; Morgan, A.R.; Hubbard Cristinacce, P.L.; Nordenmark, L.H.; Hockings, P.D.; Lagerstrand, K.M.; Young, S.S.; Naish, J.H.; Waterton, J.C.; Maguire, N.C.; et al. COPD patients have short lung magnetic resonance T1 relaxation time. COPD 2016, 13, 153–159. [Google Scholar] [CrossRef]
- Morgan, A.R.; Parker, G.J.M.; Roberts, C.; Buonaccorsi, G.A.; Maguire, N.C.; Hubbard Cristinacce, P.L.; Singh, D.; Vestbo, J.; Bjermer, L.; Jögi, J.; et al. Feasibility assessment of using oxygen-enhanced magnetic resonance imaging for evaluating the effect of pharmacological treatment in COPD. Eur. J. Radiol. 2014, 83, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shan, F.; Yan, Q.; Shen, J.; Ye, P.; Zhang, Z.; Shi, Y.; Zhang, R. A pilot study of native T1-mapping for focal pulmonary lesions in 3.0 T magnetic resonance imaging: Size estimation and differential diagnosis. J. Thorac. Dis. 2020, 12, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cui, L.; Xiao, Y.; Zhou, X.; Fu, Y.; Xu, G.; Shao, W.; Chen, W.; Hu, S.; Hu, C.; et al. B1 -Corrected T1 Mapping in Lung Cancer: Repeatability, Reproducibility, and Identification of Histological Types. J. Magn. Reson. Imaging 2021, 54, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, R.; Zhu, M.; Du, M.; Zhu, J.; Sun, Z.; Liu, K.; Li, Y. Native T1-mapping and diffusion-weighted imaging (DWI) can be used to identify lung cancer pathological types and their correlation with Ki-67 expression. J. Thorac. Dis. 2022, 14, 443–454. [Google Scholar] [CrossRef]
- Salerno, M.; Kramer, C.M. Advances in parametric mapping with CMR imaging. JACC Cardiovasc. Imaging 2013, 6, 806–822. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Piechnik, S.K.; Robson, M.D.; Neubauer, S.; Karamitsos, T.D. Myocardial tissue characterization by magnetic resonance imaging: Novel applications of T1 and T2 mapping. J. Thorac. Imaging 2014, 29, 147–154. [Google Scholar] [CrossRef]
- Lee, C.H. Quantitative T2-mapping using MRI for detection of prostate malignancy: A systematic review of the literature. Acta Radiol. 2019, 60, 1181–1189. [Google Scholar] [CrossRef]
- Meng, T.; He, N.; He, H.; Liu, K.; Ke, L.; Liu, H.; Zhong, L.; Huang, C.; Yang, A.; Zhou, C.; et al. The diagnostic performance of quantitative mapping in breast cancer patients: A preliminary study using synthetic MRI. Cancer Imaging 2020, 20, 88. [Google Scholar] [CrossRef]
- Kaggie, J.D.; Deen, S.; Kessler, D.A.; McLean, M.A.; Buonincontri, G.; Schulte, R.F.; Addley, H.; Sala, E.; Brenton, J.; Graves, M.J.; et al. Feasibility of quantitative magnetic resonance fingerprinting in ovarian tumors for T1 and T2 mapping in a PET/MR setting. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 509–515. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, W.; Wang, F.; Wang, Y.; Wu, P.-Y.; Zhou, J.; Liu, H. Study of T2 mapping in quantifying and discriminating uterine lesions under different magnetic field strengths: 1.5 T vs. 3.0 T. BMC Med. Imaging 2023, 23, 1. [Google Scholar] [CrossRef]
- Mittal, S.; Pradhan, G.; Singh, S.; Batra, R. T1 and T2 mapping of articular cartilage and menisci in early osteoarthritis of the knee using 3-Tesla magnetic resonance imaging. Pol. J. Radiol. 2019, 84, e549–e564. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 mapping: Basic techniques and clinical applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Piechnik, S.K.; Banypersad, S.M.; Fontana, M.; Ntusi, N.B.; Ferreira, V.M.; Whelan, C.J.; Myerson, S.G.; Robson, M.D.; Hawkins, P.N.; et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc. Imaging 2013, 6, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Ugander, M.; Bagi, P.S.; Oki, A.J.; Chen, B.; Hsu, L.-Y.; Aletras, A.H.; Shah, S.; Greiser, A.; Kellman, P.; Arai, A.E. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Naba, A.; Bhutkar, A.; Guardia, T.; Miller, K.M.; Li, C.M.-C.; Dayton, T.L.; Sanchez-Rivera, F.J.; Kim-Kiselak, C.; Jailkhani, N.; et al. Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc. Natl. Acad. Sci. USA 2017, 114, E5625–E5634. [Google Scholar] [CrossRef]
- Parker, A.L.; Cox, T.R. The role of the ECM in lung cancer dormancy and outgrowth. Front. Oncol. 2020, 10, 1766. [Google Scholar] [CrossRef]
- Lim, S.B.; Tan, S.J.; Lim, W.-T.; Lim, C.T. An extracellular matrix-related prognostic and predictive indicator for early-stage non-small cell lung cancer. Nat. Commun. 2017, 8, 1734. [Google Scholar] [CrossRef]

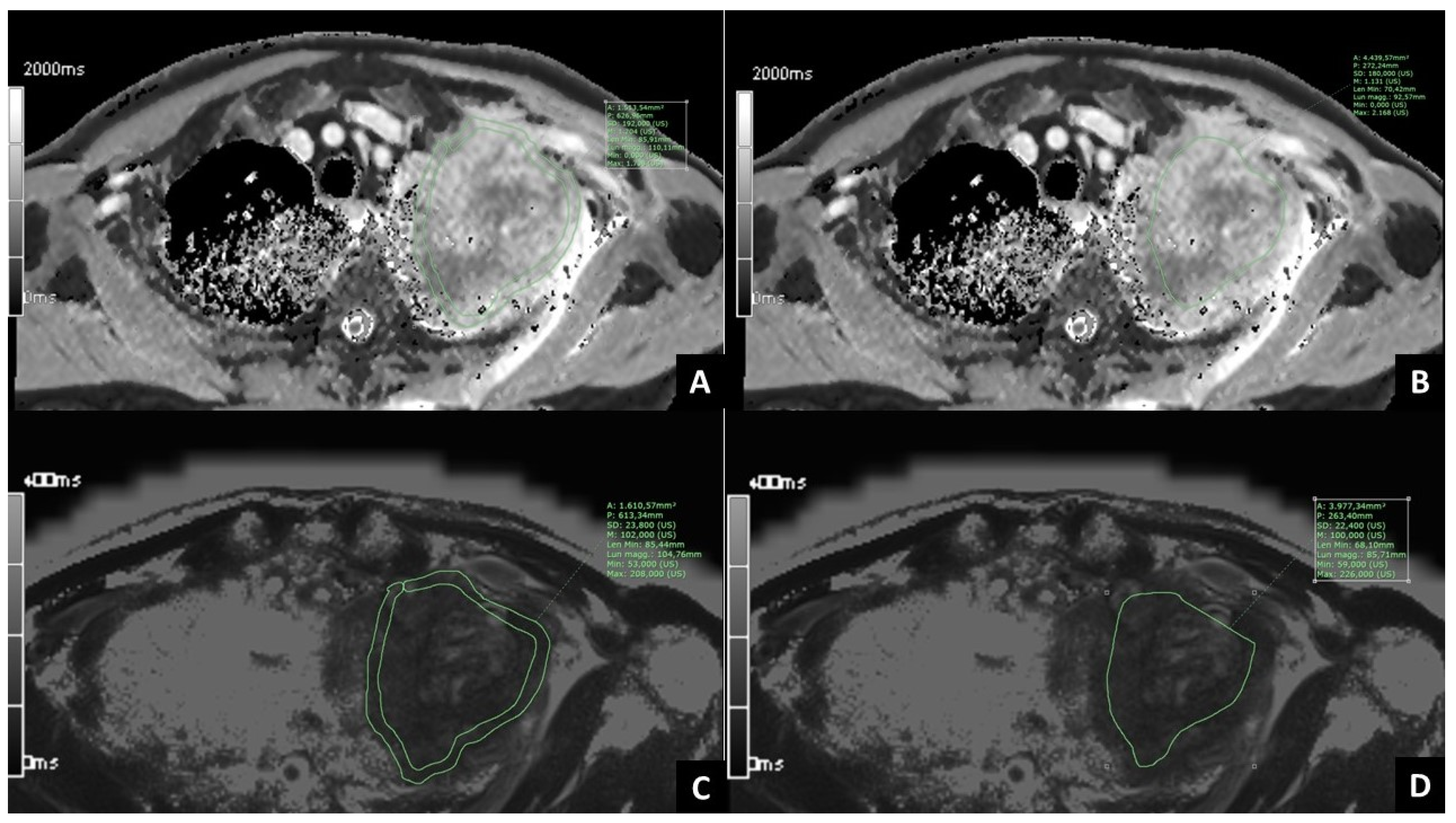
| Main Patients’ Characteristics | |
|---|---|
| Characteristic | Value |
| Age (n = 35) | |
| Median | 68 |
| Range | 49–84 |
| Sex (n = 35) | |
| Females | 26% (9) |
| Males | 74% (26) |
| NSCLC histotype (n = 33) | |
| Adenocarcinoma | 40% (13) |
| Squamocellular carcinoma | 33% (11) |
| Poorly-differentiated NSCLC | 27% (9) |
| PD-L1 expression (n = 30) | |
| PD-L1 ≥ 1 | 60% (18) |
| PD-L1 < 1 | 40% (12) |
| μ ± σ T1 Mapping | μ ± σ T2 Mapping | |||
|---|---|---|---|---|
| PD-L1 < 1% | PD-L1 ≥ 1% | PD-L1 < 1% | PD-L1 ≥ 1% | |
| whole tumor | 1109 ± 120 ms | 1108.2 ± 150 ms | 108.7 ± 28 ms | 105.6 ± 28 ms |
| periphery | 1150.5 ± 102 ms | 1100.1 ± 133 ms | 104.9 ± 24 ms | 106 ± 31 ms |
| core | 1106.8 ± 73 ms | 1099.7 ± 148 ms | 96.6 ± 24 ms | 105 ± 31 ms |
| microenv. 3 mm | 1014.1 ± 145 ms | 934.9 ± 73 ms | 180.4 ± 47 ms | 180 ± 49 ms |
| microenv. 6 mm | 938.9 ± 77 ms | 912.9 ± 81 ms | 189.6 ± 48 ms | 186.6 ± 51 ms |
| normal lung | 897.9 ± 51 ms | 856.1 ± 83 ms | 237.4 ± 25 ms | 231.7 ± 27 ms |
| μ ± σ T1 Mapping | μ ± σ T2 Mapping | |||
|---|---|---|---|---|
| SCC | ADK | PD | SCC | |
| whole tumor | 1032.9 ± 154 ms | 1169.2 ± 76 ms | 1107.1 ± 133 ms | 101 ± 27 ms |
| periphery | 1029 ± 150 ms | 1180.5 ± 79 ms | 1129.1 ± 68 ms | 103.5 ± 31 ms |
| core | 1005.3 ± 156 ms | 1153.4 ± 68 ms | 1115.9 ± 84 ms | 100.6 ± 28 ms |
| micro env. 3 mm | 928.3 ± 50 ms | 978.3 ± 92 ms | 1020.5 ± 188 ms | 175.8 ± 57 ms |
| micro env. 6 mm | 905.8 ± 61 ms | 955.9 ± 73 ms | 922 ± 130 ms | 179.3 ± 57 ms |
| normal lung | 885 ± 70 ms | 877.8 ± 71 ms | 865.7 ± 87 ms | 233.9 ± 22 ms |
| Missing Values | Histotype: 2/35 (5%) → Tot 33 | PD-L1: 5/35 (14%) → Tot 30 |
|---|---|---|
| T1 whole tumor | 3/33 (9%) | 3/30 (10%) |
| T1 periphery | 6/33 (18%) | 5/30 (17%) |
| T1 core | 6/33 (18%) | 5/30 (17%) |
| T1 micro env. 3 mm | 7/33 (21%) | 6/30 (20%) |
| T1 micro env. 6 mm | 7/33 (21%) | 6/30 (20%) |
| T1 normal lung | 6/33 (18%) | 5/30 (17%) |
| T2 whole | 1/33 (3%) | 1/30 (3%) |
| T2 periphery | 2/33 (6%) | 1/30 (3%) |
| T2 core | 2/33 (6%) | 1/30 (3%) |
| T2 micro env. 3 mm | 3/33 (9%) | 2/30 (7%) |
| T2 micro env. 6 mm | 3/33 (9%) | 2/30 (7%) |
| T2 normal lung | 2/33 (6%) | 1/30 (3%) |
| ROI | p-Value | FDR |
|---|---|---|
| T1 whole tumor | 0.063 | 0.176 |
| T1 periphery | 0.013 | 0.116 |
| T1 core | 0.046 | 0.176 |
| T1 micro env. 3 mm | 0.297 | 0.612 |
| T1 micro env. 6 mm | 0.314 | 0.612 |
| T1 normal lung | 0.946 | 0.946 |
| T2 whole | 0.350 | 0.612 |
| T2 periphery | 0.690 | 0.878 |
| T2 core | 0.937 | 0.946 |
| T2 micro env. 3 mm | 0.558 | 0.781 |
| T2 micro env. 6 mm | 0.555 | 0.781 |
| T2 normal lung | 0.871 | 0.946 |
| SCC | ADK | PD | p-Value | |
|---|---|---|---|---|
| T1 periphery | 1029 ± 150 ms | 1180.5 ± 79 ms | 1129.1 ± 68 ms | 0.01 |
| ADK vs. SCC | - | - | - | 0.004 |
| ADK vs. PD | - | - | - | 0.13 |
| SCC vs. PD | - | - | - | 0.26 |
| T1 core | 1005.3 ± 156 ms | 1153.4 ± 68 ms | 1115.9 ± 84 ms | 0.04 |
| ADK vs. SCC | - | - | - | 0.01 |
| ADK vs. PD | - | - | - | 0.41 |
| SCC vs. PD | - | - | - | 0.15 |
| T1 whole | 1032.9 ± 154 ms | 1169.2 ± 76 ms | 1107.1 ± 133 ms | 0.06 |
| ADK vs. SCC | - | - | - | 0.02 |
| ADK vs. PD | - | - | - | 0.15 |
| SCC vs. PD | - | - | - | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolotto, C.; Messana, G.; Lo Tito, A.; Stella, G.M.; Pinto, A.; Podrecca, C.; Bellazzi, R.; Gerbasi, A.; Agustoni, F.; Han, F.; et al. The Role of Native T1 and T2 Mapping Times in Identifying PD-L1 Expression and the Histological Subtype of NSCLCs. Cancers 2023, 15, 3252. https://doi.org/10.3390/cancers15123252
Bortolotto C, Messana G, Lo Tito A, Stella GM, Pinto A, Podrecca C, Bellazzi R, Gerbasi A, Agustoni F, Han F, et al. The Role of Native T1 and T2 Mapping Times in Identifying PD-L1 Expression and the Histological Subtype of NSCLCs. Cancers. 2023; 15(12):3252. https://doi.org/10.3390/cancers15123252
Chicago/Turabian StyleBortolotto, Chandra, Gaia Messana, Antonio Lo Tito, Giulia Maria Stella, Alessandra Pinto, Chiara Podrecca, Riccardo Bellazzi, Alessia Gerbasi, Francesco Agustoni, Fei Han, and et al. 2023. "The Role of Native T1 and T2 Mapping Times in Identifying PD-L1 Expression and the Histological Subtype of NSCLCs" Cancers 15, no. 12: 3252. https://doi.org/10.3390/cancers15123252
APA StyleBortolotto, C., Messana, G., Lo Tito, A., Stella, G. M., Pinto, A., Podrecca, C., Bellazzi, R., Gerbasi, A., Agustoni, F., Han, F., Nickel, M. D., Zacà, D., Filippi, A. R., Bottinelli, O. M., & Preda, L. (2023). The Role of Native T1 and T2 Mapping Times in Identifying PD-L1 Expression and the Histological Subtype of NSCLCs. Cancers, 15(12), 3252. https://doi.org/10.3390/cancers15123252










