Peripherally Inserted Central Venous Catheter (PICC) Related Bloodstream Infection in Cancer Patients Treated with Chemotherapy Compared with Noncancer Patients: A Propensity-Score-Matched Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Patients
2.3. Data Collection
2.4. Microbiology
2.5. Study Definitions
2.6. Statistical Analysis
3. Results
3.1. Patients and Peripherally Inserted Central Venous Catheters
3.2. Incidence of PICC-Related Complications
3.3. Microbiology
3.4. Incidence of PICC-Related Complications after Propensity Score Matching
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrigah-Benissan, K.; Ory, J.; Simon, C.; Loubet, P.; Martin, A.; Beregi, J.-P.; Lavigne, J.-P.; Sotto, A.; Larcher, R. Clinical Factors Associated with Peripherally Inserted Central Catheters (PICC) Related Bloodstream Infections: A Single Centre Retrospective Cohort. Antimicrob. Resist. Infect. Control 2023, 12, 5. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; Saint, S.; Woller, S.C.; O’Grady, N.P.; Safdar, N.; Trerotola, S.O.; Saran, R.; Moureau, N.; Wiseman, S.; et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann. Intern. Med. 2015, 163, S1–S40. [Google Scholar] [CrossRef]
- Herc, E.; Patel, P.; Washer, L.L.; Conlon, A.; Flanders, S.A.; Chopra, V. A Model to Predict Central-Line-Associated Bloodstream Infection Among Patients with Peripherally Inserted Central Catheters: The MPC Score. Infect. Control Hosp. Epidemiol. 2017, 38, 1155–1166. [Google Scholar] [CrossRef]
- Piredda, A.; Radice, D.; Zencovich, C.; Cerri, M.; Aventino, L.; Naccarato, F.; Magon, G.; Biffi, R. Safe Use of Peripherally Inserted Central Catheters for Chemotherapy of Solid Malignancies in Adult Patients: A 1-Year Monocentric, Prospectively-Assessed, Unselected Cohort of 482 Patients. J. Vasc. Access 2021, 22, 873–881. [Google Scholar] [CrossRef]
- Vidal, V.; Muller, C.; Jacquier, A.; Giorgi, R.; Le Corroller, T.; Gaubert, J.Y.; Champsaur, P.; Bartoli, J.M.; Moulin, G. Évaluation prospective des complications des PICCs. J. Radiol. 2008, 89, 495–498. [Google Scholar] [CrossRef]
- Al-Asadi, O.; Almusarhed, M.; Eldeeb, H. Predictive Risk Factors of Venous Thromboembolism (VTE) Associated with Peripherally Inserted Central Catheters (PICC) in Ambulant Solid Cancer Patients: Retrospective Single Centre Cohort Study. Thromb. J. 2019, 17, 2. [Google Scholar] [CrossRef]
- Madabhavi, I.; Patel, A.; Sarkar, M.; Kataria, P.; Kadakol, N.; Anand, A. A Study of the Use of Peripherally Inserted Central Catheters in Cancer Patients: A Single-Center Experience. J. Vasc. Nurs. 2018, 36, 149–156. [Google Scholar] [CrossRef]
- Nakaya, Y.; Imasaki, M.; Shirano, M.; Shimizu, K.; Yagi, N.; Tsutsumi, M.; Yoshida, M.; Yoshimura, T.; Hayashi, Y.; Nakao, T.; et al. Peripherally Inserted Central Venous Catheters Decrease Central Line-Associated Bloodstream Infections and Change Microbiological Epidemiology in Adult Hematology Unit: A Propensity Score-Adjusted Analysis. Ann. Hematol. 2022, 101, 2069–2077. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, X.; Lan, Y.; Xia, Q.; Chen, F.; Wu, J.; Li, W.; Cao, H.; Xie, C.; Li, L.; et al. Peripherally Inserted Central Catheters Have a Protective Role and the Effect of Fluctuation Curve Feature in the Risk of Bloodstream Infection Compared with Central Venous Catheters: A Propensity-Adjusted Analysis. BMC Infect. Dis. 2022, 22, 289. [Google Scholar] [CrossRef]
- Pitiriga, V.; Bakalis, J.; Theodoridou, K.; Kanellopoulos, P.; Saroglou, G.; Tsakris, A. Lower Risk of Bloodstream Infections for Peripherally Inserted Central Catheters Compared to Central Venous Catheters in Critically Ill Patients. Antimicrob. Resist. Infect. Control 2022, 11, 137. [Google Scholar] [CrossRef]
- Böll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central Venous Catheter–Related Infections in Hematology and Oncology: 2020 Updated Guidelines on Diagnosis, Management, and Prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2020, 100, 239–259. [Google Scholar] [CrossRef]
- Pu, Y.-L.; Li, Z.-S.; Zhi, X.-X.; Shi, Y.-A.; Meng, A.-F.; Cheng, F.; Ali, A.; Li, C.; Fang, H.; Wang, C. Complications and Costs of Peripherally Inserted Central Venous Catheters Compared with Implantable Port Catheters for Cancer Patients: A Meta-Analysis. Cancer Nurs. 2020, 43, 455–467. [Google Scholar] [CrossRef]
- Cotogni, P.; Mussa, B.; Degiorgis, C.; De Francesco, A.; Pittiruti, M. Comparative Complication Rates of 854 Central Venous Access Devices for Home Parenteral Nutrition in Cancer Patients: A Prospective Study of Over 169,000 Catheter-Days. J. Parenter. Enter. Nutr. 2021, 45, 768–776. [Google Scholar] [CrossRef]
- Picardi, M.; Della Pepa, R.; Cerchione, C.; Pugliese, N.; Mortaruolo, C.; Trastulli, F.; Giordano, C.; Grimaldi, F.; Zacheo, I.; Raimondo, M.; et al. A Frontline Approach with Peripherally Inserted Versus Centrally Inserted Central Venous Catheters for Remission Induction Chemotherapy Phase of Acute Myeloid Leukemia: A Randomized Comparison. Clin. Lymphoma Myeloma Leuk. 2019, 19, e184–e194. [Google Scholar] [CrossRef]
- Mitbander, U.B.; Geer, M.J.; Taxbro, K.; Horowitz, J.K.; Zhang, Q.; O’Malley, M.E.; Ramnath, N.; Chopra, V. Patterns of Use and Outcomes of Peripherally Inserted Central Catheters in Hospitalized Patients with Solid Tumors: A Multicenter Study. Cancer 2022, 128, 3681–3690. [Google Scholar] [CrossRef]
- Yuen, H.L.A.; Zhao, J.; Tran, H.; Chunilal, S.D. Development of a Risk Score to Predict Peripherally Inserted Central Catheter Thrombosis in Active Cancer. Intern. Med. J. 2022, 52, 1733–1740. [Google Scholar] [CrossRef]
- Taxbro, K.; Hammarskjöld, F.; Thelin, B.; Lewin, F.; Hagman, H.; Hanberger, H.; Berg, S. Clinical Impact of Peripherally Inserted Central Catheters vs Implanted Port Catheters in Patients with Cancer: An Open-Label, Randomised, Two-Centre Trial. Br. J. Anaesth. 2019, 122, 734–741. [Google Scholar] [CrossRef]
- Scrivens, N.; Sabri, E.; Bredeson, C.; McDiarmid, S. Comparison of Complication Rates and Incidences Associated with Different Peripherally Inserted Central Catheters (PICC) in Patients with Hematological Malignancies: A Retrospective Cohort Study. Leuk. Lymphoma 2020, 61, 156–164. [Google Scholar] [CrossRef]
- Sapkota, S.; Sannur, R.; Naik, R. Analysis of Peripherally Inserted Central Catheter Line in Cancer Patients: A Single-Center Experience. South Asian J. Cancer 2020, 9, 253–256. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hosoda, R.; Omura, H.; Tanaka, T. Catheter-Related Bloodstream Infection Associated with Multiple Insertions of the Peripherally Inserted Central Catheter in Patients with Hematological Disorders. Sci. Rep. 2021, 11, 12209. [Google Scholar] [CrossRef]
- Mollee, P.; Okano, S.; Looke, D.; Kennedy, G.; Harper, J.; Clouston, J.; Van Kuilenburg, R.; Geary, A.; Joubert, W.; Eastgate, M.; et al. Catheter-Associated Bloodstream Infections (CA-BSI) in Adults with Cancer: A Prospective Randomized Controlled Trial. Blood 2017, 130, 4729. [Google Scholar] [CrossRef]
- Caris, M.G.; de Jonge, N.A.; Punt, H.J.; Salet, D.M.; de Jong, V.M.T.; Lissenberg-Witte, B.I.; Zweegman, S.; Vandenbroucke-Grauls, C.M.J.E.; van Agtmael, M.A.; Janssen, J.J.W.M. Indwelling Time of Peripherally Inserted Central Catheters and Incidence of Bloodstream Infections in Haematology Patients: A Cohort Study. Antimicrob. Resist. Infect. Control 2022, 11, 37. [Google Scholar] [CrossRef]
- Swaminathan, L.; Flanders, S.; Horowitz, J.; Zhang, Q.; O’Malley, M.; Chopra, V. Safety and Outcomes of Midline Catheters vs Peripherally Inserted Central Catheters for Patients with Short-Term Indications: A Multicenter Study. JAMA Intern. Med. 2022, 182, 50. [Google Scholar] [CrossRef]
- Safdar, N.; Maki, D.G. Risk of Catheter-Related Bloodstream Infection with Peripherally Inserted Central Venous Catheters Used in Hospitalized Patients. Chest 2005, 128, 489–495. [Google Scholar] [CrossRef]
- Société Française d’hygiène Hospitalière (SF2H). Recommendations from a Formalized Expert Consensus: Good Practice and Risk Management for the Use of PICC. December 2013. Available online: https://sf2h.net/wp-content/uploads/2016/04/SF2H_Recos_PICC_Def.pdf (accessed on 23 April 2023).
- Timsit, J.-F.; Baleine, J.; Bernard, L.; Calvino-Gunther, S.; Darmon, M.; Dellamonica, J.; Desruennes, E.; Leone, M.; Lepape, A.; Leroy, O.; et al. Expert Consensus-Based Clinical Practice Guidelines Management of Intravascular Catheters in the Intensive Care Unit. Ann. Intensive Care 2020, 10, 118. [Google Scholar] [CrossRef]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 1 March 2023).
- Zhang, S.; Sun, X.; Lei, Y. The Microbiological Characteristics and Risk Factors for PICC-Related Bloodstream Infections in Intensive Care Unit. Sci. Rep. 2017, 7, 15074. [Google Scholar] [CrossRef]
- Park, S.; Moon, S.; Pai, H.; Kim, B. Appropriate Duration of Peripherally Inserted Central Catheter Maintenance to Prevent Central Line-Associated Bloodstream Infection. PLoS ONE 2020, 15, e0234966. [Google Scholar] [CrossRef]
- Liu, X.; Tao, S.; Ji, H.; Chen, S.; Gu, Y.; Jin, X. Risk Factors for Peripherally Inserted Central Catheter (PICC)-Associated Infections in Patients Receiving Chemotherapy and the Preventive Effect of a Self-Efficacy Intervention Program: A Randomized Controlled Trial. Ann. Palliat. Med. 2021, 10, 9398–9405. [Google Scholar] [CrossRef]
- Kim, Y.O.; Chung, C.R.; Gil, E.; Park, C.-M.; Suh, G.Y.; Ryu, J.-A. Safety and Feasibility of Ultrasound-Guided Placement of Peripherally Inserted Central Catheter Performed by Neurointensivist in Neurosurgery Intensive Care Unit. PLoS ONE 2019, 14, e0217641. [Google Scholar] [CrossRef]
- Yoon, K.W.; Wi, W.; Choi, M.S.; Gil, E.; Park, C.-M.; Yoo, K. Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit. J. Pers. Med. 2023, 13, 863. [Google Scholar] [CrossRef]
- Laporte-Amargos, J.; Sastre, E.; Bergas, A.; Pomares, H.; Paviglianiti, A.; Rodriguez-Arias, M.; Pallares, N.; Badia-Tejero, A.M.; Pons-Oltra, P.; Carratalà, J.; et al. Increasing Gram-Negative Catheter-Related Bloodstream Infection in Cancer Patients. Pathogens 2023, 12, 228. [Google Scholar] [CrossRef]
- Chaftari, A.M.; Hachem, R.; Jiang, Y.; Shah, P.; Hussain, A.; Hamal, Z.A.; Yousif, A.; Jordan, M.; Michael, M.; Raad, I. Changing Epidemiology of Catheter-Related Bloodstream Infections in Cancer Patients. Infect. Control Hosp. Epidemiol. 2018, 39, 727–729. [Google Scholar] [CrossRef]
- Lendak, D.; Puerta-Alcalde, P.; Moreno-García, E.; Chumbita, M.; García-Pouton, N.; Cardozo, C.; Morata, L.; Suárez-Lledó, M.; Hernández-Meneses, M.; Ghiglione, L.; et al. Changing Epidemiology of Catheter-Related Bloodstream Infections in Neutropenic Oncohematological Patients. PLoS ONE 2021, 16, e0251010. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Lederman, H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016, 4, 1–43. [Google Scholar] [CrossRef]
- Braun, E.; Hussein, K.; Geffen, Y.; Rabino, G.; Bar-Lavie, Y.; Paul, M. Predominance of Gram-Negative Bacilli among Patients with Catheter-Related Bloodstream Infections. Clin. Microbiol. Infect. 2014, 20, O627–O629. [Google Scholar] [CrossRef]
- Badia-Cebada, L.; Peñafiel, J.; Saliba, P.; Andrés, M.; Càmara, J.; Domenech, D.; Jiménez-Martínez, E.; Marrón, A.; Moreno, E.; Pomar, V.; et al. Trends in the Epidemiology of Catheter-Related Bloodstream Infections; towards a Paradigm Shift, Spain, 2007 to 2019. Eurosurveillance 2022, 27, 2100610. [Google Scholar] [CrossRef]
- Ripa, M.; Morata, L.; Rodríguez-Núñez, O.; Cardozo, C.; Puerta-Alcalde, P.; Hernández-Meneses, M.; Ambrosioni, J.; Linares, L.; Bodro, M.; Valcárcel, A.; et al. Short-Term Peripheral Venous Catheter-Related Bloodstream Infections: Evidence for Increasing Prevalence of Gram-Negative Microorganisms from a 25-Year Prospective Observational Study. Antimicrob. Agents Chemother. 2018, 62, e00892-18. [Google Scholar] [CrossRef]
- Mielke, D.; Wittig, A.; Teichgräber, U. Peripherally Inserted Central Venous Catheter (PICC) in Outpatient and Inpatient Oncological Treatment. Support. Care Cancer 2020, 28, 4753–4760. [Google Scholar] [CrossRef]
- Cotogni, P.; Barbero, C.; Garrino, C.; Degiorgis, C.; Mussa, B.; De Francesco, A.; Pittiruti, M. Peripherally Inserted Central Catheters in Non-Hospitalized Cancer Patients: 5-Year Results of a Prospective Study. Support. Care Cancer 2015, 23, 403–409. [Google Scholar] [CrossRef]
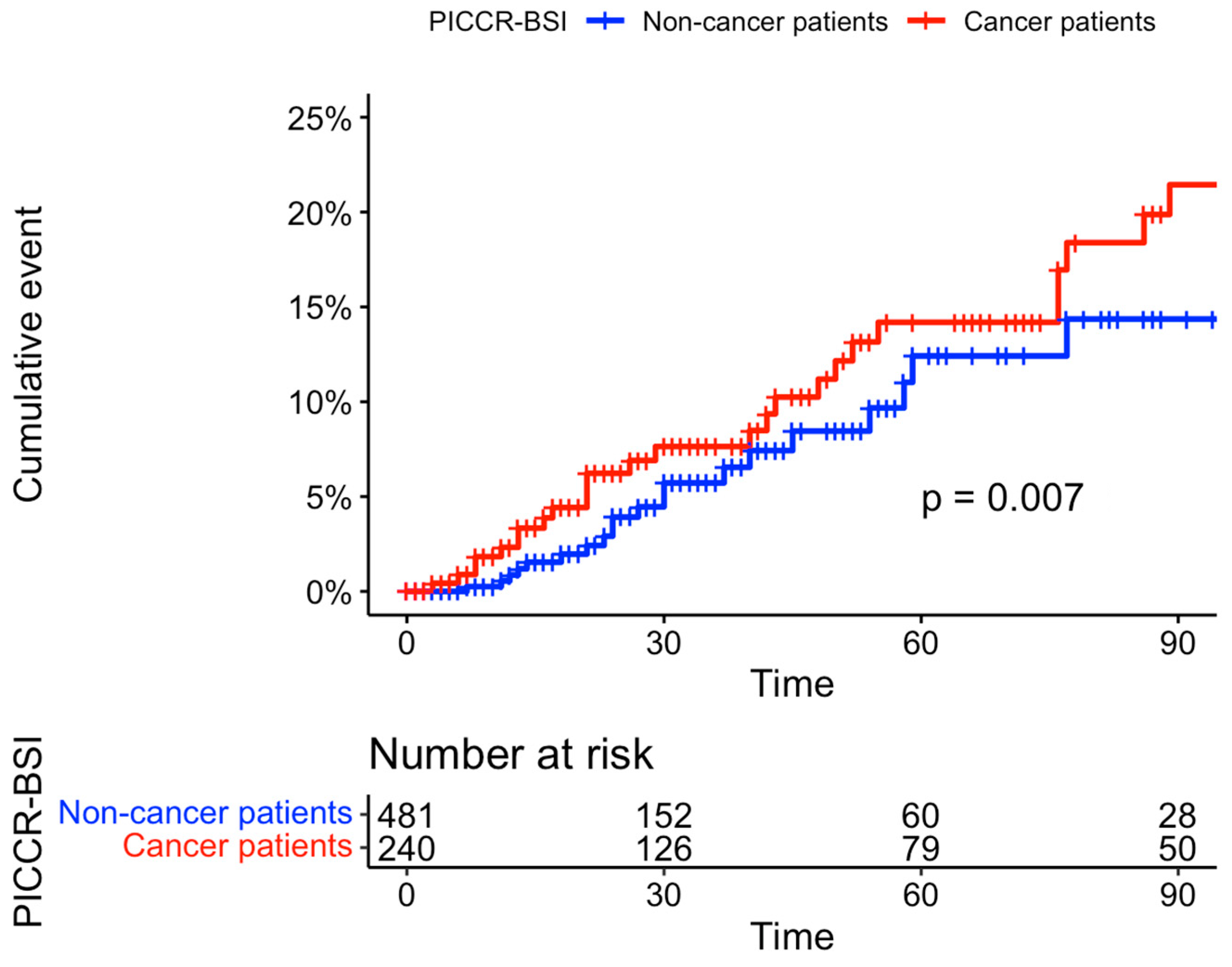
| Characteristics | Overall n = 721 1 | Cancer Patients n = 240 1 | Noncancer Patients n = 481 1 | p-Value 2 |
|---|---|---|---|---|
| Demographics: | ||||
| Age | 69 (57, 79) | 66 (54, 74) | 72 (60, 82) | <0.001 |
| Male | 399 (55%) | 129 (54%) | 270 (56%) | 0.5 |
| Body Mass Index (BMI) | 24 (21, 29) | 23 (21, 27) | 25 (21, 30) | <0.001 |
| Charlson comorbidity index | 6 (3, 8) | 6 (4, 9) | 5 (3, 7) | <0.001 |
| Cancer type: | ||||
| Solid tumor | 161 (22%) | 161 (67%) | - | |
| Localized solid tumor | 55 (7.6%) | 55 (23%) | - | |
| Metastatic solid tumor | 106 (15%) | 106 (44%) | - | |
| Hematological malignancies | 79 (11%) | 79 (33%) | - | |
| Leukemia | 43 (6.0%) | 43 (18%) | - | |
| Lymphoma | 18 (2.5%) | 18 (7.5%) | - | |
| Myeloma | 18 (2.5%) | 18 (7.5%) | - | |
| Main reason for PICC placement: | ||||
| Cancer chemotherapy | 240 (33%) | 240 (100%) | - | |
| Antimicrobial therapy | 306 (42%) | - | 306 (64%) | |
| Limited peripheral access | 109 (15%) | - | 109 (23%) | |
| Long-term venous access | 31 (4.3%) | - | 31 (6.4%) | |
| Parenteral nutrition | 35 (4.9%) | - | 35 (7.3%) | |
| Double-lumen PICC | 155 (21%) | 104 (43%) | 51 (11%) | <0.001 |
| Right side PICC insertion site | 167 (23%) | 51 (21%) | 116 (24%) | 0.4 |
| Reason for PICC removal: | ||||
| End of intravenous therapy | 426 (59%) | 89 (37%) | 337 (70%) | <0.001 |
| Port implantation | 23 (3.2%) | 18 (7.5%) | 5 (1.0%) | <0.001 |
| Mechanical complication | 67 (9.3%) | 20 (9.8%) | 47 (9.8%) | >0.9 |
| PICCRI 3 (suspected or confirmed) | 123 (17%) | 77 (32%) | 46 (9.6%) | <0.001 |
| Death | 82 (11%) | 36 (15%) | 46 (9.6%) | 0.03 |
| PICC duration (days) | 21 (10, 46) | 32 (15, 76) | 17 (8, 35) | <0.001 |
| Number of catheter days | 31,831 | 15,108 | 16,723 |
| PICC Related Complications | Overall 1 n = 721 | Cancer Patients 1 n = 240 | Noncancer Patients 1 n = 481 | p-Value 2 |
|---|---|---|---|---|
| Accidental removal (rate) | 47 (6.5%) | 10 (4.2%) | 37 (7.7%) | 0.071 |
| Accidental removal per 1000 catheter days | 1.5 | 0.7 | 2.2 | |
| Vein thrombosis (rate) | 14 (1.9%) | 8 (3.3%) | 6 (1.2%) | 0.082 |
| Vein thrombosis per 1000 catheter days | 0.4 | 0.5 | 0.4 | |
| Catheter dysfunction (rate) | 6 (0.8%) | 2 (0.8%) | 4 (0.8%) | >0.9 |
| Catheter dysfunction per 1000 catheter days | 0.2 | 0.1 | 0.2 | |
| PICC colonization (rate) | 33 (4.6%) | 15 (6.2%) | 18 (3.7%) | 0.13 |
| PICC colonization per 1000 catheter days | 1.0 | 0.9 | 1.1 | |
| NB-PICCRI 3 (rate) | 11 (1.5%) | 5 (2.1%) | 6 (1.2%) | 0.5 |
| NB-PICCRI per 1000 catheter days | 0.3 | 0.3 | 0.4 | |
| PICCR-BSI 4 (rate) | 58 (8.0%) | 40 (17%) | 18 (3.7%) | <0.001 |
| PICCR-BSI per 1000 catheter days | 1.8 | 2.6 | 1.1 |
| Bacterial Species | Overall 1 n = 58 | Cancer Patients 1 n = 40 | Noncancer Patients 1 n = 18 |
|---|---|---|---|
| Gram-negative bacteria | 38 (66%) | 31 (78%) | 7 (39%) |
| Enterobacterales 2 | 29 (50%) | 22 (55%) 2 | 7 (39%) |
| Escherichia coli | 8 (14%) | 5 (13%) | 3 (17%) |
| Enterobacter cloacae | 7 (12%) | 6 (15%) | 1 (6%) |
| Klebsiella pneumoniae | 5 (9%) | 4 (10%) | 1 (6%) |
| Klebsiella oxytoca | 3 (5%) | 3 (8%) | 0 (0%) |
| Serratia marcescens | 2 (3%) | 1 (3%) | 1 (6%) |
| Citrobacter koseri | 1 (2%) | 0 (0%) | 1 (6%) |
| Hafnia alvei | 1 (2%) | 1 (3%) | 0 (0%) |
| Klebsiella aerogenes | 1 (2%) | 1 (3%) | 0 (0%) |
| Proteus mirabilis | 1 (2%) | 1 (3%) | 0 (0%) |
| Nonfermenters | 9 (16%) | 9 (23%) | 0 (0%) |
| Acinetobacter baumannii | 3 (5%) | 3 (8%) | 0 (0%) |
| Acinetobacter ursingii | 1 (2%) | 1 (3%) | 0 (0%) |
| Stenotrophomonas maltophilia | 2 (3%) | 2 (5%) | 0 (0%) |
| Achromobacter xylosoxidans | 1 (2%) | 1 (3%) | 0 (0%) |
| Pseudomonas aeruginosa | 1 (2%) | 1 (3%) | 0 (0%) |
| Rhizobium radiobacter | 1 (2%) | 1 (3%) | 0 (0%) |
| Gram-positive bacteria | 26 (45%) | 17 (43%) | 9 (50%) |
| Staphylococcus epidermidis | 13 (22%) | 7 (18%) | 6 (30%) |
| Staphylococcus aureus | 4 (7%) | 4 (10%) | 0 (0%) |
| Staphylococcus haemolyticus | 1 (2%) | 0 (0%) | 1 (6%) |
| Streptococcus pasteurianus | 3 (5%) | 2 (5%) | 1 (6%) |
| Streptococcus mitis | 1 (2%) | 1 (3%) | 0 (0%) |
| Enterococcus faecium | 2 (3%) | 1 (3%) | 1 (6%) |
| Enterococcus faecalis | 1 (2%) | 1 (3%) | 0 (0%) |
| Bacillus licheniformis | 1 (2%) | 1 (3%) | 0 (0%) |
| Fungi | 4 (8%) | 0 (0%) | 4 (22%) |
| Candida glabrata | 2 (3%) | 0 (0%) | 2 (11%) |
| Candida parapsilosis | 2 (3%) | 0 (0%) | 2 (11%) |
| Characteristics | Overall n = 480 1 | Cancer Patients n = 240 1 | Noncancer Patients n = 240 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 68 (54, 77) | 66 (54, 74) | 71 (54, 82) | 0.003 |
| Male | 276 (57%) | 129 (54%) | 147 (61%) | 0.10 |
| Body Mass Index (BMI) | 23 (20, 28) | 23 (21, 27) | 24 (20, 28) | 0.9 |
| Charlson comorbidity index | 6 (3, 9) | 6 (4, 9) | 6 (3, 9) | 0.12 |
| Double-lumen PICC | 154 (32%) | 104 (43%) | 50 (21%) | <0.001 |
| PICC duration (days) | 24 (11, 54) | 32 (15, 76) | 16 (8, 34) | <0.001 |
| Number of catheter days | 22,432 | 15,108 | 7324 | |
| Accidental removal (rate) | 27 (5.6%) | 10 (4.2%) | 17 (7.1%) | 0.2 |
| Accidental removal per 1000 catheter days | 1.2 | 0.7 | 2.3 | |
| Vein thrombosis (rate) | 11 (2.3%) | 8 (3.3%) | 3 (1.3%) | 0.13 |
| Vein thrombosis per 1000 catheter days | 0.5 | 0.5 | 0.4 | |
| Catheter dysfunction (rate) | 6 (1.3%) | 2 (0.8%) | 4 (1.7%) | 0.7 |
| Catheter dysfunction per 1000 catheter days | 0.3 | 0.1 | 0.5 | |
| PICC colonization 3 (rate) | 23 (4.8%) | 15 (6.2%) | 8 (3.3%) | 0.13 |
| PICC colonization per 1000 catheter days | 1.0 | 0.9 | 1.1 | |
| NB-PICCRI 4 (rate) | 10 (2.1%) | 5 (2.1%) | 5 (2.1%) | >0.9 |
| NB-PICCRI per 1000 catheter days | 0.4 | 0.3 | 0.7 | |
| PICCR-BSI 5 (rate) | 47 (9.8%) | 40 (17%) | 7 (2.9%) | <0.001 |
| NB-PICCRI per 1000 catheter days | 2.1 | 2.6 | 1.0 |
| Variables | aHR 1 | 95%CI 2 | p-Value |
|---|---|---|---|
| Cancer | 1.83 | 0.86–3.86 | 0.11 |
| Age (year) | 1.003 | 0.98–1.02 | 0.75 |
| Double-lumen PICC | 1.81 | 1.01–3.22 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larcher, R.; Barrigah-Benissan, K.; Ory, J.; Simon, C.; Beregi, J.-P.; Lavigne, J.-P.; Sotto, A. Peripherally Inserted Central Venous Catheter (PICC) Related Bloodstream Infection in Cancer Patients Treated with Chemotherapy Compared with Noncancer Patients: A Propensity-Score-Matched Analysis. Cancers 2023, 15, 3253. https://doi.org/10.3390/cancers15123253
Larcher R, Barrigah-Benissan K, Ory J, Simon C, Beregi J-P, Lavigne J-P, Sotto A. Peripherally Inserted Central Venous Catheter (PICC) Related Bloodstream Infection in Cancer Patients Treated with Chemotherapy Compared with Noncancer Patients: A Propensity-Score-Matched Analysis. Cancers. 2023; 15(12):3253. https://doi.org/10.3390/cancers15123253
Chicago/Turabian StyleLarcher, Romaric, Koko Barrigah-Benissan, Jerome Ory, Claire Simon, Jean-Paul Beregi, Jean-Philippe Lavigne, and Albert Sotto. 2023. "Peripherally Inserted Central Venous Catheter (PICC) Related Bloodstream Infection in Cancer Patients Treated with Chemotherapy Compared with Noncancer Patients: A Propensity-Score-Matched Analysis" Cancers 15, no. 12: 3253. https://doi.org/10.3390/cancers15123253
APA StyleLarcher, R., Barrigah-Benissan, K., Ory, J., Simon, C., Beregi, J.-P., Lavigne, J.-P., & Sotto, A. (2023). Peripherally Inserted Central Venous Catheter (PICC) Related Bloodstream Infection in Cancer Patients Treated with Chemotherapy Compared with Noncancer Patients: A Propensity-Score-Matched Analysis. Cancers, 15(12), 3253. https://doi.org/10.3390/cancers15123253







