Role of Therapeutic Endoscopic Ultrasound in Management of Pancreatic Cancer: An Endoscopic Oncologist Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Discussion
2.1. EUS-Guided Fine Needle Injections of Anti-Tumor Agents
2.1.1. Cytoimplant
2.1.2. Dendritic Cell-Based Immunotherapy
2.1.3. Oncolytic Viruses
2.1.4. DNA Plasmids
2.1.5. Chemotherapeutic Agents
2.2. EUS-Assisted Radiotherapy
2.2.1. Brachytherapy
2.2.2. Stereotactic Body Radiotherapy
2.3. EUS-Guided Ablation
2.3.1. Radiofrequency Ablation
2.3.2. Hybrid Cryothermal Ablation
2.3.3. Microwave Ablation
2.3.4. Photodynamic Therapy
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.-J.; Wong, M.C. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Lowenfels, A.B. The Epidemiology of Pancreatitis and Pancreatic Cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B. Epidemiology of Pancreatic Cancer: An Update. Dig. Dis. 2010, 28, 645–656. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Al-Haddad, M.; Chandan, S.; Gangwani, M.K.; Aziz, M.; Mohan, B.P.; Ramai, D.; Canakis, A.; Bapaye, J.; Sharma, N. Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Cancer: Where Are We Now and What Does the Future Entail? J. Clin. Med. 2022, 11, 7476. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Donahue, T. Pancreatic Cancer. JAMA 2019, 322, 1426. [Google Scholar] [CrossRef]
- McAllister, F.; Montiel, M.F.; Uberoi, G.S.; Uberoi, A.S.; Maitra, A.; Bhutani, M.S. Current Status and Future Directions for Screening Patients at High Risk for Pancreatic Cancer. Gastroenterol. Hepatol. 2017, 13, 268–275. [Google Scholar]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Chaudhary, F.S.; Ehsan, A.; Suarez, A.L.; Muniraj, T.; Jamidar, P.; Aslanian, H.R.; Farrell, J.J. Endoscopic ul-trasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterol. 2020, 7, e000408. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Marín, J.; Vila, J.J.; Perez-Miranda, M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J. Gastrointest. Oncol. 2014, 6, 360–368. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Poley, J.-W.; Larghi, A.; Giovannini, M.; Petrone, M.C.; Abdulkader, I.; Monges, G.; Costamagna, G.; Arcidiacono, P.; Biermann, K.; et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest. Endosc. 2011, 73, 1189–1196. [Google Scholar] [CrossRef]
- Crinò, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. Endoscopic Ultrasound–guided Fine-needle Biopsy With or Without Rapid On-site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology 2021, 161, 899–909.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Bhutani, M.S.; Sun, S. Artificial intelligence: The new wave of innovation in EUS. Endosc Ultrasound. 2021, 10, 79–83. [Google Scholar] [PubMed]
- Hsieh, M.H.; Sun, L.-M.; Lin, C.-L.; Hsieh, M.J.; Hsu, C.Y.; Kao, C.H. Development of a prediction model for pancreatic cancer in patients with type 2 diabetes using logistic regression and artificial neural network models. Cancer Manag. Res. 2018, ume 10, 6317–6324. [Google Scholar] [CrossRef]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N.; et al. Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas 2021, 50, 251–279. [Google Scholar] [CrossRef]
- Prager, M.; Prager, E.; Sebesta, C.; Sebesta, C. Diagnostic and Therapeutic Indications for Endoscopic Ultrasound (EUS) in Patients with Pancreatic and Biliary Disease—Novel Interventional Procedures. Curr. Oncol. 2022, 29, 6211–6225. [Google Scholar] [CrossRef]
- Kanji, Z.S.; Gallinger, S. Diagnosis and management of pancreatic cancer. Can. Med Assoc. J. 2013, 185, 1219–1226. [Google Scholar] [CrossRef]
- Tzeng, C.W.; Tran Cao, H.S.; Lee, J.E.; Pisters, P.W.; Varadhachary, G.R.; Wolff, R.A.; Abbruzzese, J.L.; Crane, C.H.; Evans, D.B.; Wang, H.; et al. Treatment sequencing for resectable pancreatic cancer: Influence of early metastases and surgical complications on multimo-dality therapy completion and survival. J. Gastrointest. Surg. 2014, 18, 16–24; discussion-5. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef]
- De Dosso, S.; Siebenhüner, A.R.; Winder, T.; Meisel, A.; Fritsch, R.; Astaras, C.; Szturz, P.; Borner, M. Treatment landscape of metastatic pancreatic cancer. Cancer Treat. Rev. 2021, 96, 102180. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3217–3230. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Minami, T.; Shimizu, A.; Fukuhara, M.; Yano, S.; Sasaki, K.; Koda, M.; Sugiyama, K.; Yonehara, S.; Yanagisawa, A. Roles of ERCP in the Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 30. [Google Scholar] [CrossRef]
- Yan, B.M.; Van Dam, J. Endoscopic Ultrasound-Guided Intratumoural Therapy for Pancreatic Cancer. Can. J. Gastroenterol. 2008, 22, 405–410. [Google Scholar] [CrossRef]
- Gouma, D.; Busch, O.; van Gulik, T. Pancreatic carcinoma: Palliative surgical and endoscopic treatment⋆. HPB 2006, 8, 369–376. [Google Scholar] [CrossRef]
- Chang, K.J.; Nguyen, P.T.; Thompson, J.A.; Kurosaki, T.T.; Casey, L.R.; Leung, E.C.; Granger, G.A. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound?guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer 2000, 88, 1325–1335. [Google Scholar] [CrossRef]
- Irisawa, A.; Shibukawa, G.; Takagi, T.; Abe, Y.; Saito, A.; Imbe, K.; Hoshi, K.; Yamabe, A.; Igarashi, R. Endoscopic ultra-sound-guided immunotherapy. Gastrointest. Interv. 2014, 3, 24–26. [Google Scholar] [CrossRef]
- Liu, K. Dendritic Cells. Encyclopedia of Cell Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 741–749. [Google Scholar]
- Irisawa, A.; Takagi, T.; Kanazawa, M.; Ogata, T.; Sato, Y.; Takenoshita, S.; Ohto, H.; Ohira, H. Endoscopic Ultrasound-Guided Fine-Needle Injection of Immature Dendritic Cells Into Advanced Pancreatic Cancer Refractory to Gemcitabine: A Pilot Study. Pancreas 2007, 35, 189–190. [Google Scholar] [CrossRef]
- Hirooka, Y.; Itoh, A.; Kawashima, H.; Hara, K.; Nonogaki, K.; Kasugai, T.; Ohno, E.; Ishikawa, T.; Matsubara, H.; Ishigami, M.; et al. A Combination Therapy of Gemcitabine With Immunotherapy for Patients With Inoperable Locally Advanced Pancreatic Cancer. Pancreas 2009, 38, e69–e74. [Google Scholar] [CrossRef]
- Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Kamigaki, T.; Goto, S.; Takahara, M.; Goto, H. Comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma: A phase I/II trial. Oncotarget 2017, 9, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Saito, T.; Kenjo, A.; Hoshino, M.; Terashima, M.; Sato, T.; Anazawa, T.; Kimura, T.; Tsuchiya, T.; Irisawa, A.; et al. Phase I trial of preoperative intratumoral injection of immature dendritic cells and OK-432 for resectable pancreatic cancer patients. J. Hepato-Biliary-Pancreatic Sci. 2011, 19, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Apolonio, J.S.; Gonçalves, V.L.d.S.; Santos, M.L.C.; Luz, M.S.; Souza, J.V.S.; Pinheiro, S.L.R.; de Souza, W.R.; Loureiro, M.S.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef]
- Suzuki, R.; Irisawa, A.; Bhutani, M.S. Endoscopic Ultrasound-Guided Oncologic Therapy for Pancreatic Cancer. Diagn. Ther. Endosc. 2013, 2013, 157581. [Google Scholar] [CrossRef]
- Yoo, J.; Kistler, C.A.; Yan, L.; Dargan, A.; Siddiqui, A.A. Endoscopic ultrasound in pancreatic cancer: Innovative applications beyond the basics. J. Gastrointest. Oncol. 2016, 7, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Bedford, R.; Abbruzzese, J.L.; Lahoti, S.; Reid, T.R.; Soetikno, R.M.; Kirn, D.H.; Freeman, S.M. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carci-noma. Clin. Cancer Res. 2003, 9, 555–561. [Google Scholar] [PubMed]
- Hirooka, Y.; Kasuya, H.; Ishikawa, T.; Kawashima, H.; Ohno, E.; Villalobos, I.B.; Naoe, Y.; Ichinose, T.; Koyama, N.; Tanaka, M.; et al. A Phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Shin, D.W.; Park, H.; Kim, J.; Youn, Y.; Kim, J.H.; Kim, J.; Hwang, J.H. Tolerability and safety of EUS-injected ade-novirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: A phase 1 trial. Gastrointest Endosc. 2020, 92, 1044–1052.e1. [Google Scholar] [CrossRef]
- Zhao, X.; Fan, W.; Xu, Z.; Chen, H.; He, Y.; Yang, G.; Yang, G.; Hu, H.; Tang, S.; Wang, P.; et al. Inhibiting tumor necrosis factor-alpha diminishes desmoplasia and inflammation to overcome chemoresistance in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 81110–81122. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jaruvongvanich, V.; Chandrasekhara, V. Endoscopic ultrasound-guided injectable therapy for pancreatic cancer: A systematic review. World J. Gastroenterol. 2022, 28, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Kali, A. TNFerade, an innovative cancer immunotherapeutic. Indian J. Pharmacol. 2015, 47, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.E.; E Vokes, E.; Rubin, S.J.; O’Brien, S.; Samuels, B.; Vijaykumar, S.; Kufe, D.W.; Phillips, R.; Weichselbaum, R.R. Phase I dose-escalation study of tumor necrosis factor-alpha and concomitant radiation therapy. Cancer J. Sci. Am. 1995, 1, 204–209. [Google Scholar]
- Hecht, J.R.; Farrell, J.J.; Senzer, N.; Nemunaitis, J.; Rosemurgy, A.; Chung, T.; Hanna, N.; Chang, K.J.; Javle, M.; Posner, M.; et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: A phase I/II study. Gastrointest Endosc. 2012, 75, 332–338. [Google Scholar] [CrossRef]
- Herman, J.M.; Wild, A.T.; Wang, H.; Tran, P.T.; Chang, K.J.; Taylor, G.E.; Donehower, R.C.; Pawlik, T.M.; Ziegler, M.A.; Cai, H.; et al. Ran-domized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: Final results. J Clin Oncol. 2013, 31, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Scaiewicz, V.; Sorin, V.; Fellig, Y.; Birman, T.; Mizrahi, A.; Galula, J.; Abu-Lail, R.; Shneider, T.; Ohana, P.; Buscail, L.; et al. Use of H19 Gene Regulatory Sequences in DNA-Based Therapy for Pancreatic Cancer. J. Oncol. 2010, 2010, 178174. [Google Scholar] [CrossRef]
- Ma, C.; Nong, K.; Zhu, H.; Wang, W.; Huang, X.; Yuan, Z. Ai KH19 promotes pancreatic cancer metastasis by derepressing let-7’s suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014, 35, 9163–9169. [Google Scholar] [CrossRef]
- Luo, Y.; Han, S.; Yan, B.; Ji, H.; Zhao, L.; Gladkich, J.; Herr, I. UHMK1 Is a Novel Marker for Personalized Prediction of Pancreatic Cancer Prognosis. Front. Oncol. 2022, 12, 834647. [Google Scholar] [CrossRef]
- Hanna, N.; Ohana, P.; Konikoff, F.M.; Leichtmann, G.; Hubert, A.; Appelbaum, L.; Kopelman, Y.; Czerniak, A.; Hochberg, A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012, 19, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Matthes, K.; Mino-Kenudson, M.; Sahani, D.V.; Holalkere, N.; Fowers, K.D.; Rathi, R.; Brugge, W.R. EUS-guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video). Gastrointest. Endosc. 2007, 65, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.; Alberts, S.R.; Bamlet, W.R.; Burch, P.A.; Farnell, M.B.; Gleeson, F.C.; Haddock, M.G.; Kendrick, M.L.; Oberg, A.L.; Petersen, G.M.; et al. EUS-guided fine-needle injection of gemcitabine for locally advanced and metastatic pancreatic cancer. Gastrointest Endosc. 2017, 86, 161–169. [Google Scholar] [CrossRef]
- Verco, S. Trial of NanoPac® in Subjects with Locally Advanced Pancreatic Adenocarcinoma. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03077685 (accessed on 10 May 2023).
- Verco, S.; Maulhardt, H.; Baltezor, M.; Williams, E.; Iacobucci, M.; Wendt, A.; Verco, J.; Marin, A.; Campbell, S.; Dorman, P.; et al. Local administration of submicron particle paclitaxel to solid carcinomas induces direct cytotoxicity and immune-mediated tumor-icidal effects without local or systemic toxicity: Preclinical and clinical studies. Drug Deliv. Transl. Res. 2021, 11, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.R.; Othman, M.O.; Ladd, A.H.; Verco, S.; Verco, J.; diZerega, G.; Lo, S.K. Sa2006 EUS-guided injection of intra-tumoral submicron particle paclitaxel (SPP) for the treatment of locally advanced pancreatic adenocarcinoma (LAPC): Phase 2 study. Gastrointest Endosc. 2020, 91, AB238. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pan-creatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Hall, W.A.; Erickson, B.; Crane, C.H. Evolving Concepts Regarding Radiation Therapy for Pancreatic Cancer. Surg. Oncol. Clin. North Am. 2021, 30, 719–730. [Google Scholar] [CrossRef]
- Chaput, G.; Regnier, L. Radiotherapy: Clinical pearls for primary care. Can. Fam. Physician. 2021, 67, 753–757. [Google Scholar] [CrossRef]
- Shirato, H.; Le, Q.-T.; Kobashi, K.; Prayongrat, A.; Takao, S.; Shimizu, S.; Giaccia, A.; Xing, L.; Umegaki, K. Selection of external beam radiotherapy approaches for precise and accurate cancer treatment. J. Radiat. Res. 2018, 59 (Suppl. 1), i2–i10. [Google Scholar] [CrossRef]
- Moutinho-Ribeiro, P.; Liberal, R.; Macedo, G. Endoscopic ultrasound in pancreatic cancer treatment: Facts and hopes. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 513–521. [Google Scholar] [CrossRef]
- Widmer, J.L.; Michel, K. Endoscopic Ultrasound-Guided Treatment beyond Drainage: Hemostasis, Anastomosis, and Others. Clin. Endosc. 2014, 47, 432–439. [Google Scholar] [CrossRef]
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guérin, F.; Dumas, I.; Bossi, A.; Morice, P.; Viswanathan, A.N.; et al. Brachytherapy: An overview for clinicians. CA Cancer J. Clin. 2019, 69, 386–401. [Google Scholar] [CrossRef]
- Du, Y.-Q.; Li, Z.-S.; Jin, Z.-D. Endoscope-assisted brachytherapy for pancreatic cancer: From tumor killing to pain relief and drainage. J. Interv. Gastroenterol. 2011, 1, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Hawes, R.H. EUS-Guided FNA of Solid Pancreas Tumors. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, H.; Xin, J.; Liu, J.; Guo, Q.; Li, S. Endoscopic Ultrasound-Guided Interstitial Brachytherapy of Unresectable Pancreatic Cancer: Results of a Pilot Trial. Endoscopy 2006, 38, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, Y.; Li, Z.; Jiang, Y.; Chen, J.; Liu, Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: A prospective pilot study. Endoscopy 2008, 40, 314–320. [Google Scholar] [CrossRef]
- Bhutani, M.S.; Klapman, J.B.; Tuli, R.; El-Haddad, G.; Hoffe, S.; Wong, F.C.L.; Chasen, B.; Fogelman, D.R.; Lo, S.K.; Nissen, N.N.; et al. An open-label, single-arm pilot study of EUS-guided brachytherapy with phosphorus-32 microparticles in combination with gemcitabine +/- nab-paclitaxel in unresectable locally advanced pancreatic cancer (OncoPaC-1): Technical details and study protocol. Endosc Ultrasound. 2020, 9, 24–30. [Google Scholar] [CrossRef]
- Ross, P.J.; Meenan, J.; O’Doherty, M.; Palmer, D.; Heatley, S.; Calara, J.; Chow, P. Novel delivery via endoscopic ultrasound of a 32P brachytherapy device in addition to gemcitabine (G) in advanced pancreatic cancer. ASCO 2008. [Google Scholar]
- Potters, L.; Kavanagh, B.; Galvin, J.M.; Hevezi, J.M.; Janjan, N.A.; Larson, D.A.; Mehta, M.P.; Ryu, S.; Steinberg, M.; Timmerman, R.; et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) Practice Guideline for the Performance of Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 326–332. [Google Scholar] [CrossRef]
- Haridass, A. Developments in Stereotactic Body Radiotherapy. Cancers 2018, 10, 497. [Google Scholar] [CrossRef]
- Burkoň, P.; Trna, J.; Slávik, M.; Němeček, R.; Kazda, T.; Pospíšil, P.; Dastych, M.; Eid, M.; Novotný, I.; Procházka, T.; et al. Stereotactic Body Radiotherapy (SBRT) of Pancreatic Cancer—A Critical Review and Practical Consideration. Biomedicines 2022, 10, 2480. [Google Scholar] [CrossRef]
- Koong, A.C.; Christofferson, E.; Le, Q.-T.; Goodman, K.A.; Ho, A.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Greco, R.; Norton, J.; et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. 2005, 63, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Didolkar, M.S.; Coleman, C.W.; Brenner, M.J.; Chu, K.U.; Olexa, N.; Stanwyck, E.; Yu, A.; Neerchal, N.; Rabinowitz, S. Image-Guided Stereotactic Radiosurgery for Locally Advanced Pancreatic Adenocarcinoma Results of First 85 Patients. J. Gastrointest. Surg. 2010, 14, 1547–1559. [Google Scholar] [CrossRef]
- Rwigema, J.-C.M.; Parikh, S.D.; Heron, D.E.; Howell, M.; Zeh, H.; Moser, A.J.; Bahary, N.; Quinn, A.; Burton, S.A. Stereotactic Body Radiotherapy in the Treatment of Advanced Adenocarcinoma of the Pancreas. Am. J. Clin. Oncol. 2011, 34, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.W.; Saif, M.W. Stereotactic body radiation therapy (SBRT) in pancreatic cancer: Is it ready for prime time? JOP 2008, 9, 676–682. [Google Scholar] [PubMed]
- Kothary, N.; Dieterich, S.; Louie, J.D.; Chang, D.T.; Hofmann, L.V.; Sze, D.Y. Percutaneous Implantation of Fiducial Markers for Imaging-Guided Radiation Therapy. Am. J. Roentgenol. 2009, 192, 1090–1096. [Google Scholar] [CrossRef]
- Pishvaian, A.C.; Collins, B.; Gagnon, G.; Ahlawat, S.; Haddad, N.G. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest. Endosc. 2006, 64, 412–417. [Google Scholar] [CrossRef]
- Ammar, T.; Coté, G.A.; Creach, K.M.; Kohlmeier, C.; Parikh, P.J.; Azar, R.R. Fiducial placement for stereotactic radiation by using EUS: Feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest. Endosc. 2010, 71, 630–633. [Google Scholar] [CrossRef]
- DiMaio, C.J.; Nagula, S.; Goodman, K.A.; Ho, A.Y.; Markowitz, A.J.; Schattner, M.A.; Gerdes, H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc. 2010, 71, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Park, W.G.; Yan, B.M.; Schellenberg, D.; Kim, J.; Chang, D.T.; Koong, A.; Patalano, C.; Van Dam, J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest. Endosc. 2010, 71, 513–518. [Google Scholar] [CrossRef]
- Sanders, M.K.; Moser, A.J.; Khalid, A.; Fasanella, K.E.; Zeh, H.J.; Burton, S.; McGrath, K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest. Endosc. 2010, 71, 1178–1184. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Trevino, J.M.; Shen, S.; Jacob, R. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: A case series. Endoscopy 2010, 42, 423–425. [Google Scholar] [CrossRef]
- Choi, J.-H.; Seo, D.-W.; Park, D.H.; Lee, S.K.; Kim, M.-H. Fiducial Placement for Stereotactic Body Radiation Therapy under Only Endoscopic Ultrasonography Guidance in Pancreatic and Hepatic Malignancy: Practical Feasibility and Safety. Gut Liver 2014, 8, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Dávila Fajardo, R.; Lekkerkerker, S.J.; van der Horst, A.; Lens, E.; Bergman, J.J.; Fockens, P.; Bel, A.; van Hooft, J.E. EUS-guided fiducial markers placement with a 22-gauge needle for image-guided radiation therapy in pancreatic cancer. Gastrointest Endosc. 2014, 79, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Dhadham, G.C.; Hoffe, S.; Harris, C.L.; Klapman, J.B. Endoscopic ultrasound-guided fiducial marker placement for im-age-guided radiation therapy without fluoroscopy: Safety and technical feasibility. Endosc. Int. Open 2016, 4, E378–E382. [Google Scholar] [PubMed]
- Tabernero, S.; Prados, S.; Rubio, M.D.C.; de la Morena, F.; López, M.; Sánchez, E. Endoscopic ultrasound-guided fiducial placement in pancreatic tumors: Safety and technical feasibility. Rev. Esp. Enferm. Dig. 2019, 111, 425–430. [Google Scholar] [CrossRef]
- Khashab, M.A.; Kim, K.J.; Tryggestad, E.J.; Wild, A.T.; Roland, T.; Singh, V.K.; Lennon, A.M.; Shin, E.J.; Ziegler, M.A.; Sharaiha, R.Z.; et al. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stere-otactic body radiation therapy. Gastrointest. Endosc. 2012, 76, 962–971. [Google Scholar] [CrossRef]
- Gollapudi, L.A.; Tyberg, A. EUS-RFA of the pancreas: Where are we and future directions. Transl. Gastroenterol. Hepatol. 2022, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- McGahan, J.P.; Brock, J.M.; Tesluk, H.; Gu, W.-Z.; Schneider, P.; Browning, P.D. Hepatic Ablation with Use of Radio-Frequency Electrocautery in the Animal Model. J. Vasc. Interv. Radiol. 1992, 3, 291–297. [Google Scholar] [CrossRef]
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofre-quency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 2009, 251, 58–66. [Google Scholar] [CrossRef]
- Zerbini, A.; Pilli, M.; Laccabue, D.; Pelosi, G.; Molinari, A.; Negri, E.; Cerioni, S.; Fagnoni, F.; Soliani, P.; Ferrari, C.; et al. Radiofrequency Thermal Ablation for Hepatocellular Carcinoma Stimulates Autologous NK-Cell Response. Gastroenterology 2010, 138, 1931–1942.e2. [Google Scholar] [CrossRef]
- Karaisz, F.G.; Elkelany, O.O.; Davies, B.; Lozanski, G.; Krishna, S.G. A Review on Endoscopic Ultrasound-Guided Radiofre-quency Ablation (EUS-RFA) of Pancreatic Lesions. Diagnostics 2023, 13, 536. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Napoleon, B.; Facciorusso, A.; Lakhtakia, S.; Borbath, I.; Caillol, F.; Pham, K.D.-C.; Rizzatti, G.; Forti, E.; Palazzo, L.; et al. Endoscopic Ultrasound-guided Radiofrequency Ablation Versus Surgical Resection for Treatment of Pancreatic Insulinoma. Clin. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Mallery, S.; Gazelle, G.; Brugge, W.R. EUS-guided radiofrequency ablation in the pancreas: Results in a porcine model. Gastrointest. Endosc. 1999, 50, 392–401. [Google Scholar] [CrossRef]
- Gaidhane, M.; Smith, I.; Ellen, K.; Gatesman, J.; Habib, N.; Foley, P.; Moskaluk, C.; Kahaleh, M. Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of the Pancreas in a Porcine Model. Gastroenterol. Res. Pr. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Seo, D.-W.; Hassanuddin, A.; Kim, S.-H.; Chae, H.J.; Jang, J.W.; Park, D.H.; Lee, S.S.; Lee, S.-K.; Kim, M.-H. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest. Endosc. 2012, 76, 1039–1043. [Google Scholar] [CrossRef]
- Song, T.J.; Seo, D.W.; Lakhtakia, S.; Reddy, N.; Oh, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest. Endosc. 2015, 83, 440–443. [Google Scholar] [CrossRef]
- Crinò, S.F.; D’Onofrio, M.; Bernardoni, L.; Frulloni, L.; Iannelli, M.; Malleo, G.; Paiella, S.; Larghi, A.; Gabbrielli, A. EUS-guided Radiof-requency Ablation (EUS-RFA) of Solid Pancreatic Neoplasm Using an 18-gauge Needle Electrode: Feasibility, Safety, and Technical Success. J. Gastrointestin. Liver Dis. 2018, 27, 67–72. [Google Scholar] [CrossRef]
- Thosani, N.; Sharma, N.R.; Raijman, I.; Thosani, A.J.; Kannadath, B.S.; Guider, J.C.; Raza, A.; Guha, S. 483 Safety and efficacy of endo-scopic ultrasound guided radiofrequency ablation (EUS-RFA) in the treatment of pancreatic lesions: A multi-center experience. Gastrointest. Endosc. 2018, 87, AB84. [Google Scholar] [CrossRef]
- Arcidiacono, P.G.; Carrara, S.; Reni, M.; Petrone, M.C.; Cappio, S.; Balzano, G.; Boemo, C.; Cereda, S.; Nicoletti, R.; Enderle, M.D.; et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest. Endosc. 2012, 76, 1142–1151. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Mangini, M.; Fontana, F.; Dionigi, G.; Boni, L.; Rovera, F.; Cuffari, S.; Fugazzola, C. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int. J. Surg. 2008, 6, S65–S69. [Google Scholar] [CrossRef]
- Gala, K.B.; Shetty, N.S.; Patel, P.; Kulkarni, S.S. Microwave ablation: How we do it? Indian J Radiol Imaging. 2020, 30, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.J.; Dupuy, D.; Mayo-Smith, W.W. Microwave Ablation: Principles and Applications. RadioGraphics 2005, 25, S69–S83. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.S.; Lee, F.T.; Mahvi, D.M., Jr. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003, 10, 275–283. [Google Scholar] [CrossRef]
- Robles-Medranda, C.; Arevalo-Mora, M.; Oleas, R.; Alcivar-Vasquez, J.; Del Valle, R. Novel EUS-guided microwave ablation of an unresectable pancreatic neuroendocrine tumor. Videogie 2022, 7, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, S.; Liu, F.; Wang, C.; Qin, R.; He, Y.; Cai, R.; Liu, L.; Wang, L. An experimental study on endoscopic ultrasound-guided microwave ablation for porcine liver and pancreas. Chin. J. Dig. Endosc. 2019, 12, 119–123. [Google Scholar]
- Lygidakis, N.J.; Sharma, S.K.; Papastratis, P.; Zivanovic, V.; Kefalourous, H.; Koshariya, M.; Lintzeris, I.; Porfiris, T.; Koutsiouroumba, D. Microwave ablation in locally advanced pancreatic carcinoma--a new look. Hepato-Gastroenterology 2007, 54, 1305–1310. [Google Scholar]
- Carrafiello, G.; Ierardi, A.M.; Fontana, F.; Petrillo, M.; Floridi, C.; Lucchina, N.; Cuffari, S.; Dionigi, G.; Rotondo, A.; Fugazzola, C. Microwave Ablation of Pancreatic Head Cancer: Safety and Efficacy. J. Vasc. Interv. Radiol. 2013, 24, 1513–1520. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Testoni, S.G.G.; Healey, A.J.; Arcidiacono, P.G. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc. Ultrasound 2020, 9, 83–100. [Google Scholar] [CrossRef]
- Barr, H.; Tralau, C.J.; Boulos, P.B.; MacRobert, A.J.; Tilly, R.; Bown, S.G. The Contrasting Mechanisms of Colonic Collagen Damage between Photodynamic Therapy and Thermal Injury. Photochem. Photobiol. 1987, 46, 795–800. [Google Scholar] [CrossRef]
- Bown, S.G.; Rogowska, A.Z.; Whitelaw, D.E.; Lees, W.R.; Lovat, L.B.; Ripley, P.; Jones, L.; Wyld, P.; Gillams, A.; Hatfield, A.W.R. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, T.E.; Matthes, K.; Brugge, W.R. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: A pilot study in a porcine model (with video). Gastrointest. Endosc. 2008, 67, 957–961. [Google Scholar] [CrossRef]
- Chan, H.-H.; Nishioka, N.S.; Mino, M.; Lauwers, G.Y.; Puricelli, W.P.; Collier, K.N.; Brugge, W.R. EUS-guided photodynamic therapy of the pancreas: A pilot study. Gastrointest. Endosc. 2004, 59, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Oh, D.; Lee, J.H.; Park, J.-H.; Kim, K.-P.; Lee, S.S.; Lee, Y.-J.; Lim, Y.-S.; Song, T.J.; Lee, S.S.; et al. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy 2015, 47, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2018, 89, 390–398. [Google Scholar] [CrossRef]
- Hanada, Y.; Pereira, S.P.; Pogue, B.; Maytin, E.V.; Hasan, T.; Linn, B.; Mangels-Dick, T.; Wang, K.K. EUS-guided verteporfin photody-namic therapy for pancreatic cancer. Gastrointest Endosc. 2021, 94, 179–186. [Google Scholar] [CrossRef]
- Wang, K.K. Ultrasound-Guided Verteporfin Photodynamic Therapy for the Treatment of Unresectable Solid Pancreatic Tumors or Advanced Pancreatic Cancer, VERTPAC-02 Study. 2017. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03033225 (accessed on 10 May 2023).
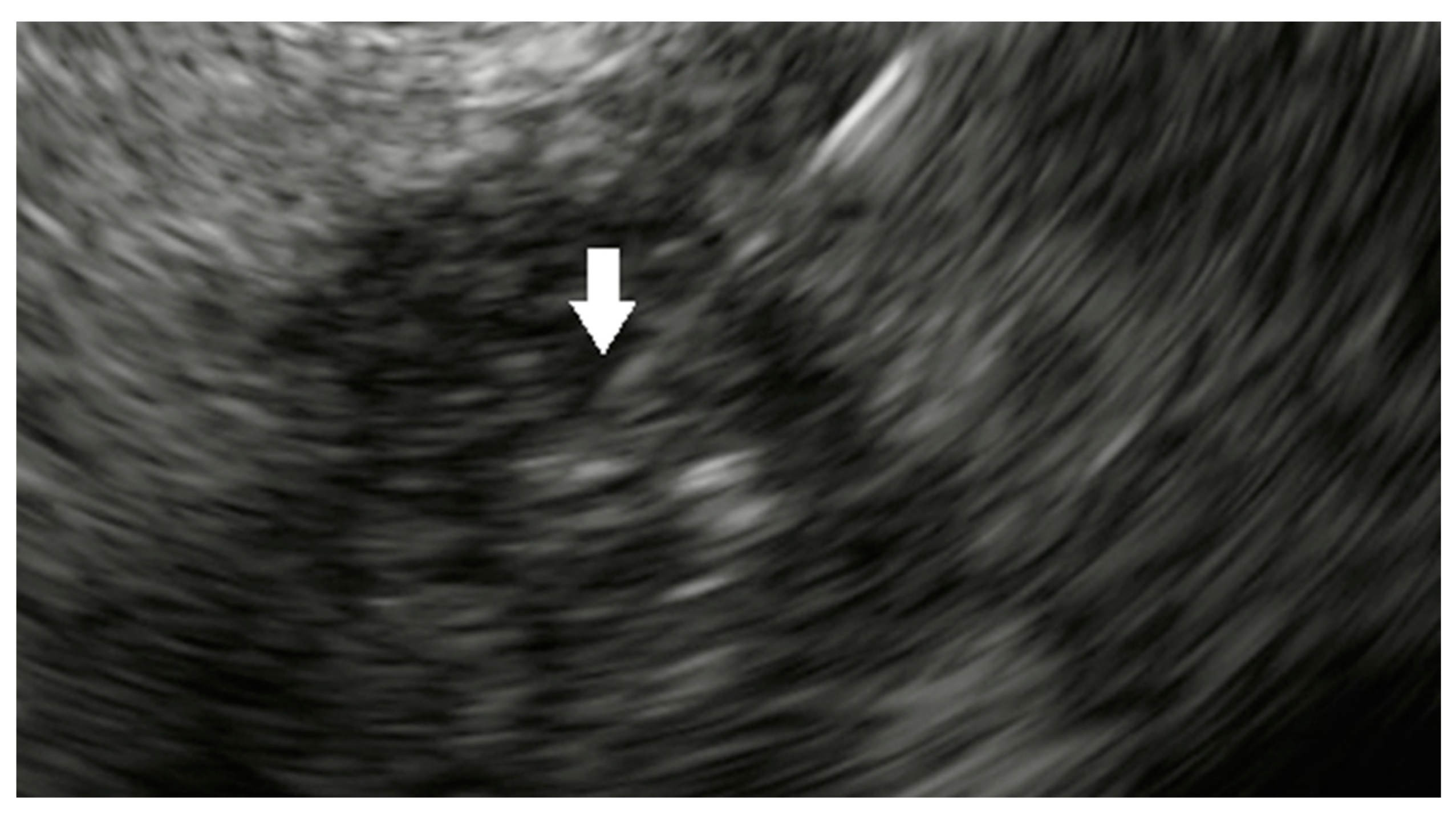
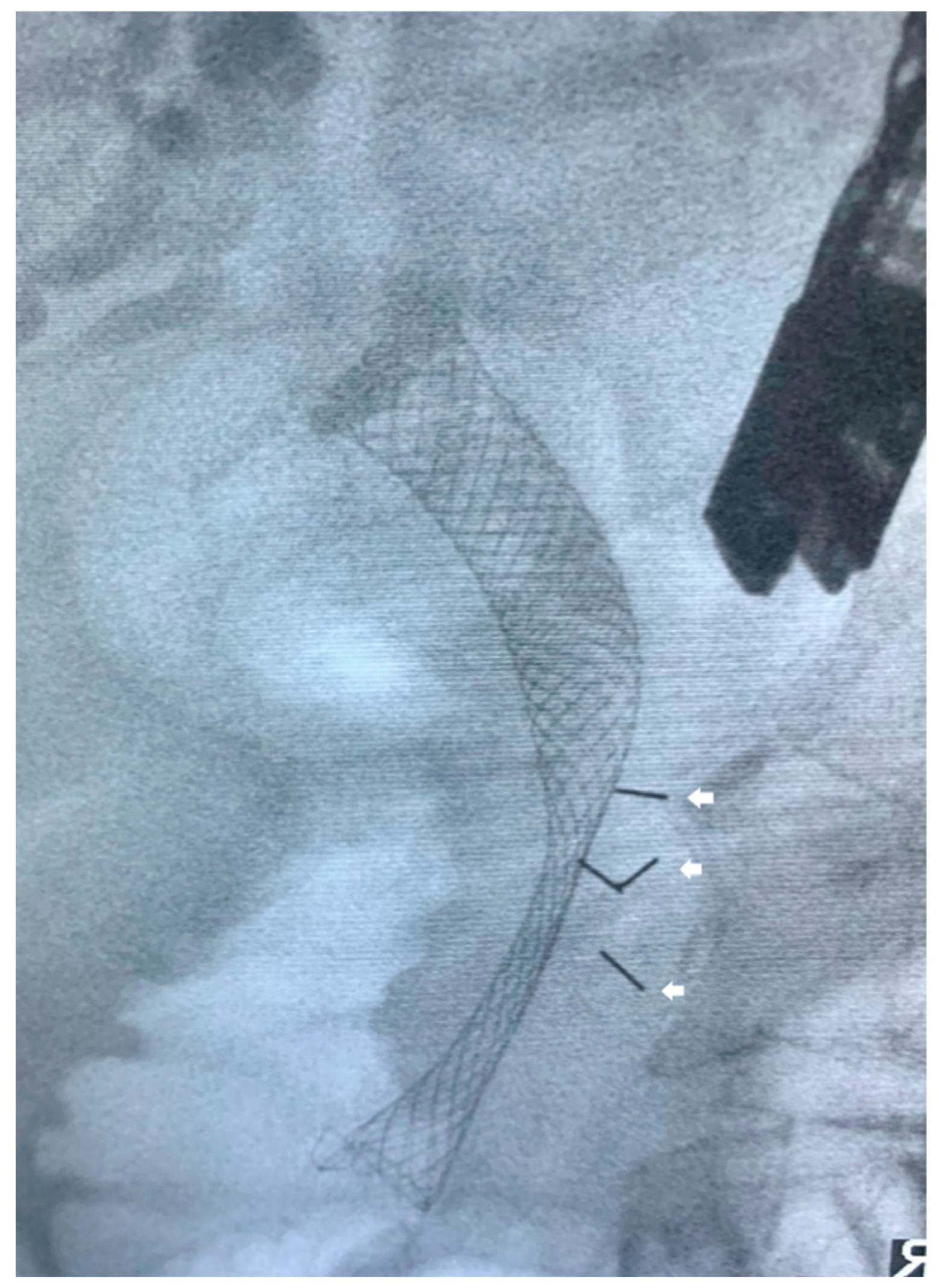
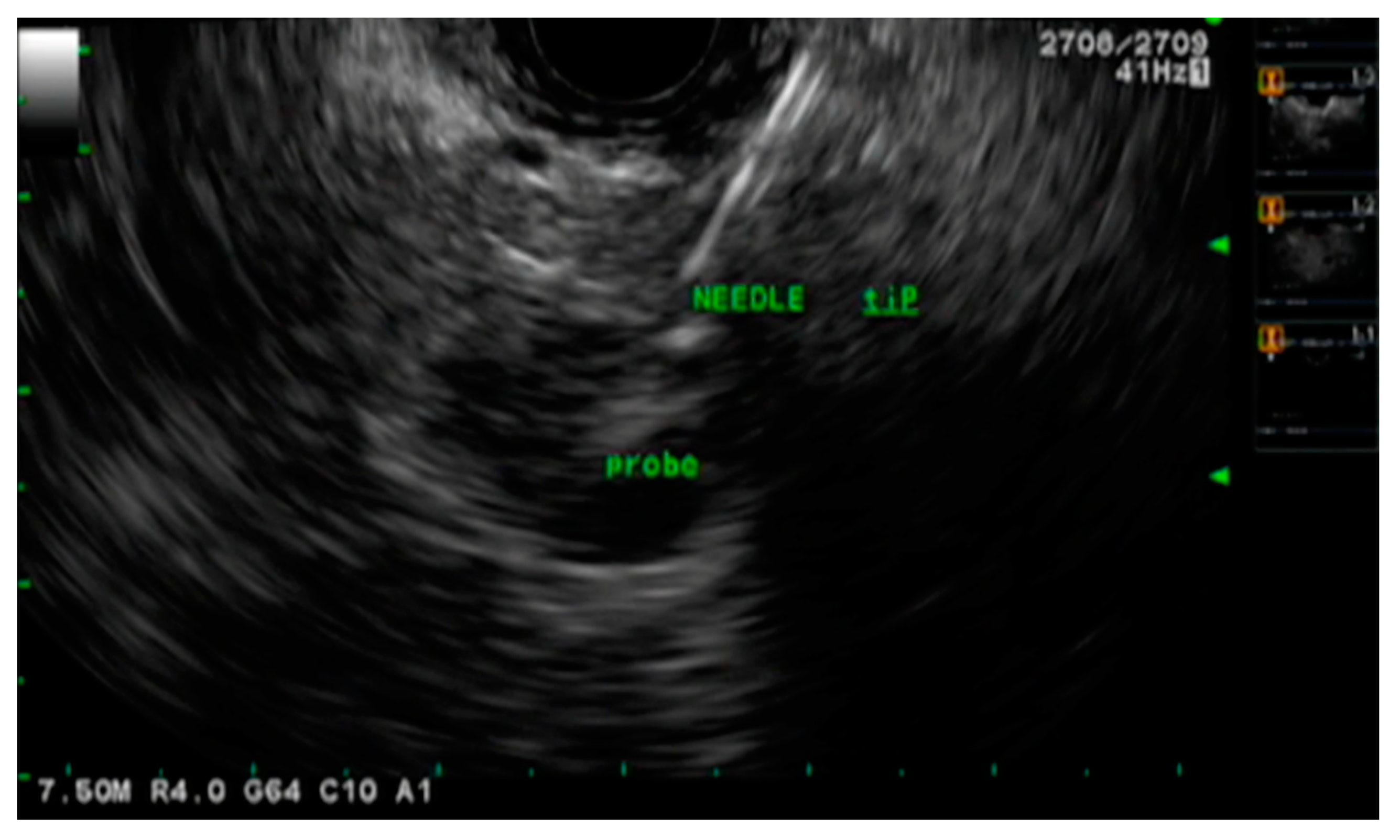
| Study | Year | Study Characteristics | Total Number of Patients | Technical Success (%) | Intraprocedural Complications | Postprocedural Complications |
|---|---|---|---|---|---|---|
| Radiofrequency Ablation | ||||||
| Song et al. [98] | 2016 | Single Center Prospective | 6 (unresectable PDAC) | 100% | None | 2 (mild abdominal pain) |
| Crinò et al. [99] | 2018 | Single Center Retrospective | 9 (8 locally advanced PDAC, 1 pancreatic head metastasis) | 100% | None | 3 (mild abdominal pain) |
| Thosani et al. [100] | 2018 | Multi-center Retrospective | 21 (10 PDAC) | 100% | None | 1 (abdominal pain) |
| Hybrid Cryothermal Ablation | ||||||
| Arcidiacono et al. [101] | 2012 | Multi-center Prospective | 22 (locally advanced PDAC) | 72.8% | None | 4 (3 abdominal pain with hyperamylasemia, 1 minor GI bleeding) |
| Photodynamic Therapy | ||||||
| Choi et al. [116] | 2015 | Single Center Prospective | 4 (1 pancreatic cancer) | 100% | None | None |
| DeWitt et al. [117] | 2018 | Open-label Phase I Single Center Prospective | 12 (locally advanced PDAC) | 100% | None | None |
| Hanada et al. [118] | 2021 | Pilot Study | 8 (locally advanced PDAC) | 100% | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahiya, D.S.; Chandan, S.; Ali, H.; Pinnam, B.S.M.; Gangwani, M.K.; Al Bunni, H.; Canakis, A.; Gopakumar, H.; Vohra, I.; Bapaye, J.; et al. Role of Therapeutic Endoscopic Ultrasound in Management of Pancreatic Cancer: An Endoscopic Oncologist Perspective. Cancers 2023, 15, 3235. https://doi.org/10.3390/cancers15123235
Dahiya DS, Chandan S, Ali H, Pinnam BSM, Gangwani MK, Al Bunni H, Canakis A, Gopakumar H, Vohra I, Bapaye J, et al. Role of Therapeutic Endoscopic Ultrasound in Management of Pancreatic Cancer: An Endoscopic Oncologist Perspective. Cancers. 2023; 15(12):3235. https://doi.org/10.3390/cancers15123235
Chicago/Turabian StyleDahiya, Dushyant Singh, Saurabh Chandan, Hassam Ali, Bhanu Siva Mohan Pinnam, Manesh Kumar Gangwani, Hashem Al Bunni, Andrew Canakis, Harishankar Gopakumar, Ishaan Vohra, Jay Bapaye, and et al. 2023. "Role of Therapeutic Endoscopic Ultrasound in Management of Pancreatic Cancer: An Endoscopic Oncologist Perspective" Cancers 15, no. 12: 3235. https://doi.org/10.3390/cancers15123235
APA StyleDahiya, D. S., Chandan, S., Ali, H., Pinnam, B. S. M., Gangwani, M. K., Al Bunni, H., Canakis, A., Gopakumar, H., Vohra, I., Bapaye, J., Al-Haddad, M., & Sharma, N. R. (2023). Role of Therapeutic Endoscopic Ultrasound in Management of Pancreatic Cancer: An Endoscopic Oncologist Perspective. Cancers, 15(12), 3235. https://doi.org/10.3390/cancers15123235







