Dose Optimization in Oncology Drug Development: The Emerging Role of Pharmacogenomics, Pharmacokinetics, and Pharmacodynamics
Abstract
Simple Summary
Abstract
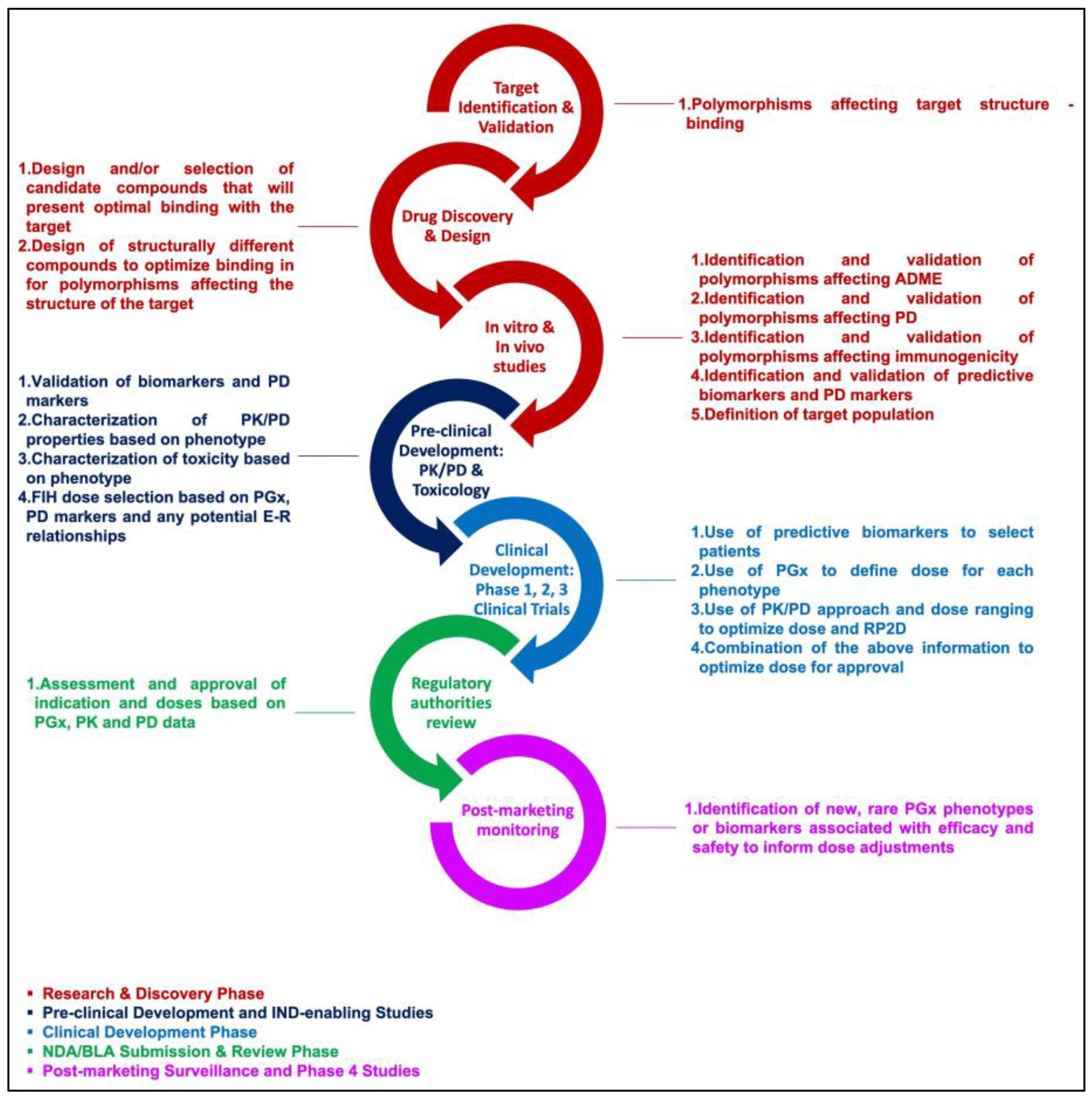
1. Introduction
2. Dose-Finding Trials Design—Project Optimus
3. Modeling Simulation (M&S) in Dose Selection
4. Tyrosine Kinase Inhibitors and Therapeutic Drug Monitoring to Optimize Dose/Exposure
5. Tyrosine Kinase Inhibitors and Therapeutic Drug Monitoring to Optimize Dose/Exposure
6. Tyrosine Kinase Inhibitors and Pharmacodynamics to Optimize Dose/Exposure
7. Monoclonal Antibodies (mAbs) and Pharmacological Methods to Optimize Dose/Exposure
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vogel, F. Moderne Probleme der Humangenetik; Springer: Berlin/Heidelberg, Germany, 1959; pp. 52–125. [Google Scholar]
- Eichelbaum, M.; Spannbrucker, N.; Steincke, B.; Dengler, H.J. Defective N-oxidation of sparteine in man: A new pharmacogenetic defect. Eur. J. Clin. Pharmacol. 1979, 16, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, A.; Dring, L.G.; Idle, J.R.; Lancaster, R.; Smith, R.L. Polymorphic hydroxylation of debrisoquine in man. Lancet 1977, 310, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Skodat, R.C.; Kimura, S.; Umeno, M.; Zanger, U.M.; Nebert, D.W.; Gelboin, H.V.; Hardwick, J.P.; Meyer, U.A. Characterization of the common genetic defect in humans deficient in debrisoquine metabolism. Nature 1988, 331, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.N. Application of genotype-guided cancer therapy in solid tumors. Pharmacogenomics 2014, 15, 79–93. [Google Scholar] [CrossRef]
- Watters, J.W.; McLeod, H.L. Cancer pharmacogenomics: Current and future applications. Biochim. Biophys. Acta (BBA) Rev. Cancer 2003, 1603, 99–111. [Google Scholar] [CrossRef]
- Wheeler, H.E.; Maitland, M.L.; Dolan, M.E.; Cox, N.J.; Ratain, M.J. Cancer pharmacogenomics: Strategies and challenges. Nat. Rev. Genet. 2013, 14, 23–34. [Google Scholar] [CrossRef]
- Schwab, M.; Zanger, U.M.; Marx, C.; Schaeffeler, E.; Klein, K.; Dippon, J.; Kerb, R.; Blievernicht, J.; Fischer, J.; Hofmann, U.; et al. Role of Genetic and Nongenetic Factors for Fluorouracil Treatment-Related Severe Toxicity: A Prospective Clinical Trial by the German 5-FU Toxicity Study Group. J. Clin. Oncol. 2008, 26, 2131–2138. [Google Scholar] [CrossRef]
- Patel, J.N.; Papachristos, A. Personalizing chemotherapy dosing using pharmacological methods. Cancer Chemother. Pharm. 2015, 76, 879–896. [Google Scholar] [CrossRef]
- Agency, E.M. EMA Recommendations on DPD Testing Prior to Treatment with Fluorouracil, Capecitabine, Tegafur and Flucytosine. Available online: https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine (accessed on 10 February 2023).
- FDA. Table of Pharmacogenomic Biomarkers in Drug Labeling. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 13 February 2023).
- Groenland, S.L.; Ratain, M.J.; Chen, L.S.; Gandhi, V. The Right Dose: From Phase I to Clinical Practice. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 92–106. [Google Scholar] [CrossRef]
- Kurzrock, R.; Lin, C.C.; Wu, T.C.; Hobbs, B.P.; Pestana, R.M.; Hong, D.S. Moving Beyond 3 + 3: The Future of Clinical Trial Design. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e133–e144. [Google Scholar] [CrossRef]
- Shah, M.; Rahman, A.; Theoret, M.R.; Pazdur, R. The Drug-Dosing Conundrum in Oncology—When Less Is More. N. Engl. J. Med. 2021, 385, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.J.; Peters, M.; Weber, B.L.; Davis, C.B. Optimizing the Therapeutic Window of Targeted Drugs in Oncology: Potency-Guided First-in-Human Studies. Clin. Transl. Sci. 2021, 14, 536–543. [Google Scholar] [CrossRef]
- Blumenthal, G.; Jain, L.; Loeser, A.L.; Pithaval, Y.K.; Rahman, A.; Ratain, M.J.; Shah, M.; Strawn, L.; Theoret, M.R. Optimizing Dosing in Oncology Drug Development. In Proceedings of the Friends of Cancer Research Annual Meeting 2021, Washington, DC, USA, 10 November 2021. [Google Scholar]
- Rogatko, A.; Babb, J.S.; Tighiouart, M.; Khuri, F.R.; Hudes, G. New paradigm in dose-finding trials: Patient-specific dosing and beyond phase I. Clin. Cancer Res. 2005, 11, 5342–5346. [Google Scholar] [CrossRef]
- Zirkelbach, J.F.; Shah, M.; Vallejo, J.; Cheng, J.; Ayyoub, A.; Liu, J.; Hudson, R.; Sridhara, R.; Ison, G.; Amiri-Kordestani, L.; et al. Improving Dose-Optimization Processes Used in Oncology Drug Development to Minimize Toxicity and Maximize Benefit to Patients. J. Clin. Oncol. 2022, 40, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Soltantabar, P.; Lon, H.-K.; Parivar, K.; Wang, D.D.; Elmeliegy, M. Optimizing benefit/risk in oncology: Review of post-marketing dose optimization and reflections on the road ahead. Crit. Rev. Oncol. Hematol. 2023, 182, 103913. [Google Scholar] [CrossRef]
- FDA. Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases Guidance for Industry. Available online: https://www.fda.gov/media/164555/download (accessed on 13 February 2023).
- Mathijssen, R.H.; Sparreboom, A.; Verweij, J. Determining the optimal dose in the development of anticancer agents. Nat. Rev. Clin. Oncol. 2014, 11, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Murakami, H.; Nishina, T.; Hirashima, T.; Sugio, K.; Muro, K.; Takahashi, T.; Naito, T.; Yasui, H.; Akinaga, S.; et al. The effect of CYP2C19 polymorphism on the safety, tolerability, and pharmacokinetics of tivantinib (ARQ 197): Results from a phase I trial in advanced solid tumors. Ann. Oncol. 2013, 24, 1653–1659. [Google Scholar] [CrossRef]
- FDA. Clinical Pharmacology Considerations for Antibody-Drug Conjugates. Available online: https://www.fda.gov/media/155997/download (accessed on 13 February 2023).
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: A randomised, open-label, phase 3 trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef]
- Derendorf, H.; Meibohm, B. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: Concepts and perspectives. Pharm. Res. 1999, 16, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.R.; Cook, J.; Sinha, V.; Zhao, P.; Rostami-Hodjegan, A.; Sahasrabudhe, V.; Stockbridge, N.; Powell, J.R. A proposal for scientific framework enabling specific population drug dosing recommendations. J. Clin. Pharmacol. 2015, 55, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Buckman-Garner, S.; McCune, S.; ONeill, R.; Geanacopoulos, M.; Amur, S.; Clingman, C.; Barratt, R.; Rocca, M.; Hills, I.; et al. Catalyzing the Critical Path Initiative: FDA’s progress in drug development activities. Clin. Pharmacol. Ther. 2015, 97, 221–233. [Google Scholar] [CrossRef]
- Milligan, P.A.; Brown, M.J.; Marchant, B.; Martin, S.W.; van der Graaf, P.H.; Benson, N.; Nucci, G.; Nichols, D.J.; Boyd, R.A.; Mandema, J.W.; et al. Model-Based Drug Development: A Rational Approach to Efficiently Accelerate Drug Development. Clin. Pharmacol. Ther. 2013, 93, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Madabushi, R.; Seo, P.; Zhao, L.; Tegenge, M.; Zhu, H. Review: Role of Model-Informed Drug Development Approaches in the Lifecycle of Drug Development and Regulatory Decision-Making. Pharm. Res. 2022, 39, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.F.; Hemmings, R.; Josephson, F.; Karlsson, M.O.; Posch, M.; Steimer, J.L. Modeling and simulation to optimize the design and analysis of confirmatory trials, characterize risk-benefit, and support label claims. CPT Pharmacomet. Syst Pharm. 2013, 2, e27. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Karlsson, M.O.; Jönsson, S. Pharmacometrics-Based Considerations for the Design of a Pharmacogenomic Clinical Trial Assessing Irinotecan Safety. Pharm. Res. 2021, 38, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Steeghs, N.; Nijenhuis, C.M.; Schellens, J.H.; Beijnen, J.H.; Huitema, A.D. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: Focus on the pharmacokinetic targets. Clin. Pharm. 2014, 53, 305–325. [Google Scholar] [CrossRef]
- Josephs, D.H.; Fisher, D.S.; Spicer, J.; Flanagan, R.J. Clinical pharmacokinetics of tyrosine kinase inhibitors: Implications for therapeutic drug monitoring. Ther. Drug Monit. 2013, 35, 562–587. [Google Scholar] [CrossRef]
- Verheijen, R.B.; Yu, H.; Schellens, J.H.M.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin. Pharmacol. Ther. 2017, 102, 765–776. [Google Scholar] [CrossRef]
- Cheng, F.; Zeng, F.; Li, Q.; Cui, Z.; Chen, Y.; Li, W.; Zhang, Y. Imatinib dose optimization based on therapeutic drug monitoring in Chinese patients with chronic-phase chronic myeloid leukemia. Cancer 2022, 128, 3951–3958. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Garrett, M.; Poland, B.; Dutcher, J.P.; Rixe, O.; Wilding, G.; Stadler, W.M.; Pithavala, Y.K.; Kim, S.; Tarazi, J.; et al. Axitinib in metastatic renal cell carcinoma: Results of a pharmacokinetic and pharmacodynamic analysis. J. Clin. Pharmacol. 2013, 53, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rugo, H.S.; Wilding, G.; McShane, T.M.; Evelhoch, J.L.; Ng, C.; Jackson, E.; Kelcz, F.; Yeh, B.M.; Lee, F.T., Jr.; et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: Results from a phase I study. J. Clin. Oncol. 2005, 23, 5464–5473. [Google Scholar] [CrossRef]
- Rini, B.I.; Melichar, B.; Fishman, M.N.; Oya, M.; Pithavala, Y.K.; Chen, Y.; Bair, A.H.; Grünwald, V. Axitinib dose titration: Analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann. Oncol. 2015, 26, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; van Eerden, R.A.G.; Westerdijk, K.; Meertens, M.; Koolen, S.L.W.; Moes, D.; de Vries, N.; Rosing, H.; Otten, H.; Vulink, A.J.E.; et al. Therapeutic drug monitoring-based precision dosing of oral targeted therapies in oncology: A prospective multicenter study. Ann. Oncol. 2022, 33, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; Mathijssen, R.H.J.; Beijnen, J.H.; Huitema, A.D.R.; Steeghs, N. Individualized dosing of oral targeted therapies in oncology is crucial in the era of precision medicine. Eur. J. Clin. Pharmacol. 2019, 75, 1309–1318. [Google Scholar] [CrossRef]
- Diekstra, M.H.; Swen, J.J.; Gelderblom, H.; Guchelaar, H.J. A decade of pharmacogenomics research on tyrosine kinase inhibitors in metastatic renal cell cancer: A systematic review. Expert Rev. Mol. Diagn. 2016, 16, 605–618. [Google Scholar] [CrossRef]
- Kaehler, M.; Cascorbi, I. Pharmacogenomics of Impaired Tyrosine Kinase Inhibitor Response: Lessons Learned From Chronic Myelogenous Leukemia. Front. Pharmacol. 2021, 12, 696960. [Google Scholar] [CrossRef]
- Kolesar, J.; Peh, S.; Thomas, L.; Baburaj, G.; Mukherjee, N.; Kantamneni, R.; Lewis, S.; Pai, A.; Udupa, K.S.; Kumar An, N.; et al. Integration of liquid biopsy and pharmacogenomics for precision therapy of EGFR mutant and resistant lung cancers. Mol. Cancer 2022, 21, 61. [Google Scholar] [CrossRef]
- Evelina, C.; Guidi, M.; Khoudour, N.; Pascaline, B.-R.; Fabre, E.; Tlemsani, C.; Arrondeau, J.; François, G.; Vidal, M.; Schneider, M.P.; et al. Population Pharmacokinetics of Erlotinib in Patients With Non-small Cell Lung Cancer: Its Application for Individualized Dosing Regimens in Older Patients. Clin. Ther. 2020, 42, 1302–1316. [Google Scholar] [CrossRef]
- Advani, R.H.; Buggy, J.J.; Sharman, J.P.; Smith, S.M.; Boyd, T.E.; Grant, B.; Kolibaba, K.S.; Furman, R.R.; Rodriguez, S.; Chang, B.Y.; et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013, 31, 88–94. [Google Scholar] [CrossRef]
- Chen, L.S.; Bose, P.; Cruz, N.D.; Jiang, Y.; Wu, Q.; Thompson, P.A.; Feng, S.; Kroll, M.H.; Qiao, W.; Huang, X.; et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood 2018, 132, 2249–2259. [Google Scholar] [CrossRef]
- Mato, A.R.; Nabhan, C.; Thompson, M.C.; Lamanna, N.; Brander, D.M.; Hill, B.; Howlett, C.; Skarbnik, A.; Cheson, B.D.; Zent, C.; et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: A real-world analysis. Haematologica 2018, 103, 874–879. [Google Scholar] [CrossRef]
- Shigehiro, Y.; Akinobu, H. Monoclonal antibody pharmacogenomics in cancer treatment. Monoclon. Antib. Pharm. Cancer Treat. 2019, 5, 75. [Google Scholar] [CrossRef]
- Chatelut, E.; Hendrikx, J.; Martin, J.; Ciccolini, J.; Moes, D. Unraveling the complexity of therapeutic drug monitoring for monoclonal antibody therapies to individualize dose in oncology. Pharmacol. Res. Perspect. 2021, 9, e00757. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Read, S.A.; Ahlenstiel, G.; Piatkov, I. Pharmacogenetics of anticancer monoclonal antibodies. Cancer Drug Resist 2019, 2, 69–81. [Google Scholar] [CrossRef]
- Passot, C.; Azzopardi, N.; Renault, S.; Baroukh, N.; Arnoult, C.; Ohresser, M.; Boisdron-Celle, M.; Gamelin, E.; Watier, H.; Paintaud, G.; et al. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs 2013, 5, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Nomizo, T.; Ozasa, H.; Tsuji, T.; Funazo, T.; Yasuda, Y.; Yoshida, H.; Yagi, Y.; Sakamori, Y.; Nagai, H.; Hirai, T.; et al. Clinical Impact of Single Nucleotide Polymorphism in PD-L1 on Response to Nivolumab for Advanced Non-Small-Cell Lung Cancer Patients. Sci. Rep. 2017, 7, 45124. [Google Scholar] [CrossRef]
- Queirolo, P.; Dozin, B.; Morabito, A.; Banelli, B.; Piccioli, P.; Fava, C.; Leo, C.; Carosio, R.; Laurent, S.; Fontana, V.; et al. Association of CTLA-4 Gene Variants with Response to Therapy and Long-term Survival in Metastatic Melanoma Patients Treated with Ipilimumab: An Italian Melanoma Intergroup Study. Front. Immunol. 2017, 8, 386. [Google Scholar] [CrossRef]
- Queirolo, P.; Dozin, B.; Morabito, A.; Banelli, B.; Carosio, R.; Fontana, V.; Ferrucci, P.F.; Martinoli, C.; Cocorocchio, E.; Ascierto, P.A.; et al. CTLA-4 gene variant -1661A>G may predict the onset of endocrine adverse events in metastatic melanoma patients treated with ipilimumab. Eur. J. Cancer 2018, 97, 59–61. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papachristos, A.; Patel, J.; Vasileiou, M.; Patrinos, G.P. Dose Optimization in Oncology Drug Development: The Emerging Role of Pharmacogenomics, Pharmacokinetics, and Pharmacodynamics. Cancers 2023, 15, 3233. https://doi.org/10.3390/cancers15123233
Papachristos A, Patel J, Vasileiou M, Patrinos GP. Dose Optimization in Oncology Drug Development: The Emerging Role of Pharmacogenomics, Pharmacokinetics, and Pharmacodynamics. Cancers. 2023; 15(12):3233. https://doi.org/10.3390/cancers15123233
Chicago/Turabian StylePapachristos, Apostolos, Jai Patel, Maria Vasileiou, and George P. Patrinos. 2023. "Dose Optimization in Oncology Drug Development: The Emerging Role of Pharmacogenomics, Pharmacokinetics, and Pharmacodynamics" Cancers 15, no. 12: 3233. https://doi.org/10.3390/cancers15123233
APA StylePapachristos, A., Patel, J., Vasileiou, M., & Patrinos, G. P. (2023). Dose Optimization in Oncology Drug Development: The Emerging Role of Pharmacogenomics, Pharmacokinetics, and Pharmacodynamics. Cancers, 15(12), 3233. https://doi.org/10.3390/cancers15123233





