Fat-Containing Soft Tissue Tumors in Children, Adolescents, and Young Adults: Which Require Biopsy?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Population
2.3. Clinical, Histopathologic, and Imaging Data
2.4. Statistical Analysis
3. Results
3.1. Lipoblastoma and Lipoma
3.2. Fibrous Hamartoma of Infancy
3.3. Lipofibromatosis
3.4. Other Benign Tumors
3.5. Liposarcoma
3.6. Liposarcoma vs. Benign Tumor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brisse, H.J.; Orbach, D.; Klijanienko, J. Soft tissue tumours: Imaging strategy. Pediatr. Radiol. 2010, 40, 1019–1028. [Google Scholar] [CrossRef]
- Brisse, H.; Orbach, D.; Klijanienko, J.; Freneaux, P.; Neuenschwander, S. Imaging and diagnostic strategy of soft tissue tumors in children. Eur. Radiol. 2006, 16, 1147–1164. [Google Scholar] [CrossRef]
- Moulin, B.; Messiou, C.; Crombe, A.; Kind, M.; Hohenberger, P.; Rutkowski, P.; van Houdt, W.J.; Strauss, D.; Gronchi, A.; Bonvalot, S. Diagnosis strategy of adipocytic soft-tissue tumors in adults: A consensus from European experts. Eur. J. Surg. Oncol. 2022, 48, 518–525. [Google Scholar] [CrossRef]
- Navarro, O.M.; Laffan, E.E.; Ngan, B.Y. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: Part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics 2009, 29, 887–906. [Google Scholar] [CrossRef]
- Miller, G.G.; Yanchar, N.L.; Magee, J.F.; Blair, G.K. Lipoblastoma and liposarcoma in children: An analysis of 9 cases and a review of the literature. Can. J. Surg. 1998, 41, 455–458. [Google Scholar]
- Coffin, C.M.; Alaggio, R. Adipose and myxoid tumors of childhood and adolescence. Pediatr. Dev. Pathol. 2012, 15 (Suppl. S1), 239–254. [Google Scholar] [CrossRef]
- Sheybani, E.F.; Eutsler, E.P.; Navarro, O.M. Fat-containing soft-tissue masses in children. Pediatr. Radiol. 2016, 46, 1760–1773. [Google Scholar] [CrossRef]
- Putra, J.; Al-Ibraheemi, A. Adipocytic tumors in Children: A contemporary review. Semin. Diagn. Pathol. 2019, 36, 95–104. [Google Scholar] [CrossRef]
- Gupta, P.; Potti, T.A.; Wuertzer, S.D.; Lenchik, L.; Pacholke, D.A. Spectrum of Fat-containing Soft-Tissue Masses at MR Imaging: The Common, the Uncommon, the Characteristic, and the Sometimes Confusing. Radiographics 2016, 36, 753–766. [Google Scholar] [CrossRef]
- Huh, W.W.; Yuen, C.; Munsell, M.; Hayes-Jordan, A.; Lazar, A.J.; Patel, S.; Wang, W.L.; Barahmani, N.; Okcu, M.F.; Hicks, J.; et al. Liposarcoma in children and young adults: A multi-institutional experience. Pediatr. Blood Cancer 2011, 57, 1142–1146. [Google Scholar] [CrossRef]
- Alaggio, R.; Coffin, C.M.; Weiss, S.W.; Bridge, J.A.; Issakov, J.; Oliveira, A.M.; Folpe, A.L. Liposarcomas in young patients: A study of 82 cases occurring in patients younger than 22 years of age. Am. J. Surg. Pathol. 2009, 33, 645–658. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Tumours of Soft Tissue and Bone, 5th ed.; World Health Organization: Geneva, Switzerland, 2020.
- Ferrari, A.; Casanova, M.; Spreafico, F.; Luksch, R.; Terenziani, M.; Cefalo, G.; Massimino, M.; Gandola, L.; Navarria, P.; Fossati-Bellani, F. Childhood liposarcoma: A single-institutional twenty-year experience. Pediatr. Hematol. Oncol. 1999, 16, 415–421. [Google Scholar] [CrossRef]
- La Quaglia, M.P.; Spiro, S.A.; Ghavimi, F.; Hajdu, S.I.; Meyers, P.; Exelby, P.R. Liposarcoma in patients younger than or equal to 22 years of age. Cancer 1993, 72, 3114–3119. [Google Scholar] [CrossRef]
- Shmookler, B.M.; Enzinger, F.M. Liposarcoma occurring in children. An analysis of 17 cases and review of the literature. Cancer 1983, 52, 567–574. [Google Scholar] [CrossRef]
- Stanelle, E.J.; Christison-Lagay, E.R.; Sidebotham, E.L.; Singer, S.; Antonescu, C.R.; Meyers, P.A.; La Quaglia, M.P. Prognostic factors and survival in pediatric and adolescent liposarcoma. Sarcoma 2012, 2012, 870910. [Google Scholar] [CrossRef]
- Murphey, M.D.; Carroll, J.F.; Flemming, D.J.; Pope, T.L.; Gannon, F.H.; Kransdorf, M.J. From the archives of the AFIP: Benign musculoskeletal lipomatous lesions. Radiographics 2004, 24, 1433–1466. [Google Scholar] [CrossRef]
- Degnan, A.J.; Jelinek, J.S.; Murphey, M.D. Lipoblastoma: Computed tomographic and magnetic resonance imaging features correlate with tumor behavior and pathology. Pediatr. Radiol. 2021, 51, 614–621. [Google Scholar] [CrossRef]
- Bancroft, L.W.; Kransdorf, M.J.; Peterson, J.J.; O’Connor, M.I. Benign fatty tumors: Classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006, 35, 719–733. [Google Scholar] [CrossRef]
- Warren, M.; Turpin, B.K.; Mark, M.; Smolarek, T.A.; Li, X. Undifferentiated myxoid lipoblastoma with PLAG1-HAS2 fusion in an infant; morphologically mimicking primitive myxoid mesenchymal tumor of infancy (PMMTI)--diagnostic importance of cytogenetic and molecular testing and literature review. Cancer Genet. 2016, 209, 21–29. [Google Scholar] [CrossRef]
- Asmandar, S.; Ranganathan, S.; Ramirez, R.; Chamond, O.; Coulomb, A.; Boudjemaa, S. Myxoid Lipoblastoma and Mimickers on Fine-Needle Biopsy in a Child. Pediatr. Dev. Pathol. 2019, 22, 157–160. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Cai, Y.; Wang, L.; Yang, S.D. Undifferentiated myxoid lipoblastoma with PLAG1 gene rearrangement in infant. Pathol. Res. Pract. 2020, 216, 152765. [Google Scholar] [CrossRef]
- Nagano, A.; Ohno, T.; Nishimoto, Y.; Hirose, Y.; Miyake, S.; Shimizu, K. Lipoblastoma mimicking myxoid liposarcoma: A clinical report and literature review. Tohoku J. Exp. Med. 2011, 223, 75–78. [Google Scholar] [CrossRef]
- Al-Ibraheemi, A.; Martinez, A.; Weiss, S.W.; Kozakewich, H.P.; Perez-Atayde, A.R.; Tran, H.; Parham, D.M.; Sukov, W.R.; Fritchie, K.J.; Folpe, A.L. Fibrous hamartoma of infancy: A clinicopathologic study of 145 cases, including 2 with sarcomatous features. Mod. Pathol. 2017, 30, 474–485. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.H.; Cheon, J.E.; Kim, M.J.; Lee, M.J.; Koh, M.J. Ultrasonographic features of fibrous hamartoma of infancy. Skeletal Radiol. 2014, 43, 649–653. [Google Scholar] [CrossRef]
- Stensby, J.D.; Conces, M.R.; Nacey, N.C. Benign fibrous hamartoma of infancy: A case of MR imaging paralleling histologic findings. Skeletal Radiol. 2014, 43, 1639–1643. [Google Scholar] [CrossRef]
- Song, Y.S.; Lee, I.S.; Kim, H.T.; Choi, K.U.; Song, J.W. Fibrous hamartoma of infancy in the hand: Unusual location and MR imaging findings. Skeletal Radiol. 2010, 39, 1035–1038. [Google Scholar] [CrossRef]
- Bertino, F.; Braithwaite, K.A.; Hawkins, C.M.; Gill, A.E.; Briones, M.A.; Swerdlin, R.; Milla, S.S. Congenital Limb Overgrowth Syndromes Associated with Vascular Anomalies. Radiographics 2019, 39, 491–515. [Google Scholar] [CrossRef]
- Fetsch, J.F.; Miettinen, M.; Laskin, W.B.; Michal, M.; Enzinger, F.M. A clinicopathologic study of 45 pediatric soft tissue tumors with an admixture of adipose tissue and fibroblastic elements, and a proposal for classification as lipofibromatosis. Am. J. Surg. Pathol. 2000, 24, 1491–1500. [Google Scholar] [CrossRef]
- Walton, J.R.; Green, B.A.; Donaldson, M.M.; Mazuru, D.G. Imaging characteristics of lipofibromatosis presenting as a shoulder mass in a 16-month-old girl. Pediatr. Radiol. 2010, 40 (Suppl. 1), S43–S46. [Google Scholar] [CrossRef]
- Vogel, D.; Righi, A.; Kreshak, J.; Dei Tos, A.P.; Merlino, B.; Brunocilla, E.; Vanel, D. Lipofibromatosis: Magnetic resonance imaging features and pathological correlation in three cases. Skeletal Radiol. 2014, 43, 633–639. [Google Scholar] [CrossRef]
- Beals, C.; Rogers, A.; Wakely, P.; Mayerson, J.L.; Scharschmidt, T.J. Hibernomas: A single-institution experience and review of literature. Med. Oncol. 2014, 31, 769. [Google Scholar] [CrossRef]
- Yilmaz, S.; Kozakewich, H.P.; Alomari, A.I.; Fishman, S.J.; Mulliken, J.B.; Chaudry, G. Intramuscular capillary-type hemangioma: Radiologic-pathologic correlation. Pediatr. Radiol. 2014, 44, 558–565. [Google Scholar] [CrossRef]
- Amarneh, M.; Shaikh, R. Clinical and imaging features in fibro-adipose vascular anomaly (FAVA). Pediatr. Radiol. 2020, 50, 380–387. [Google Scholar] [CrossRef]


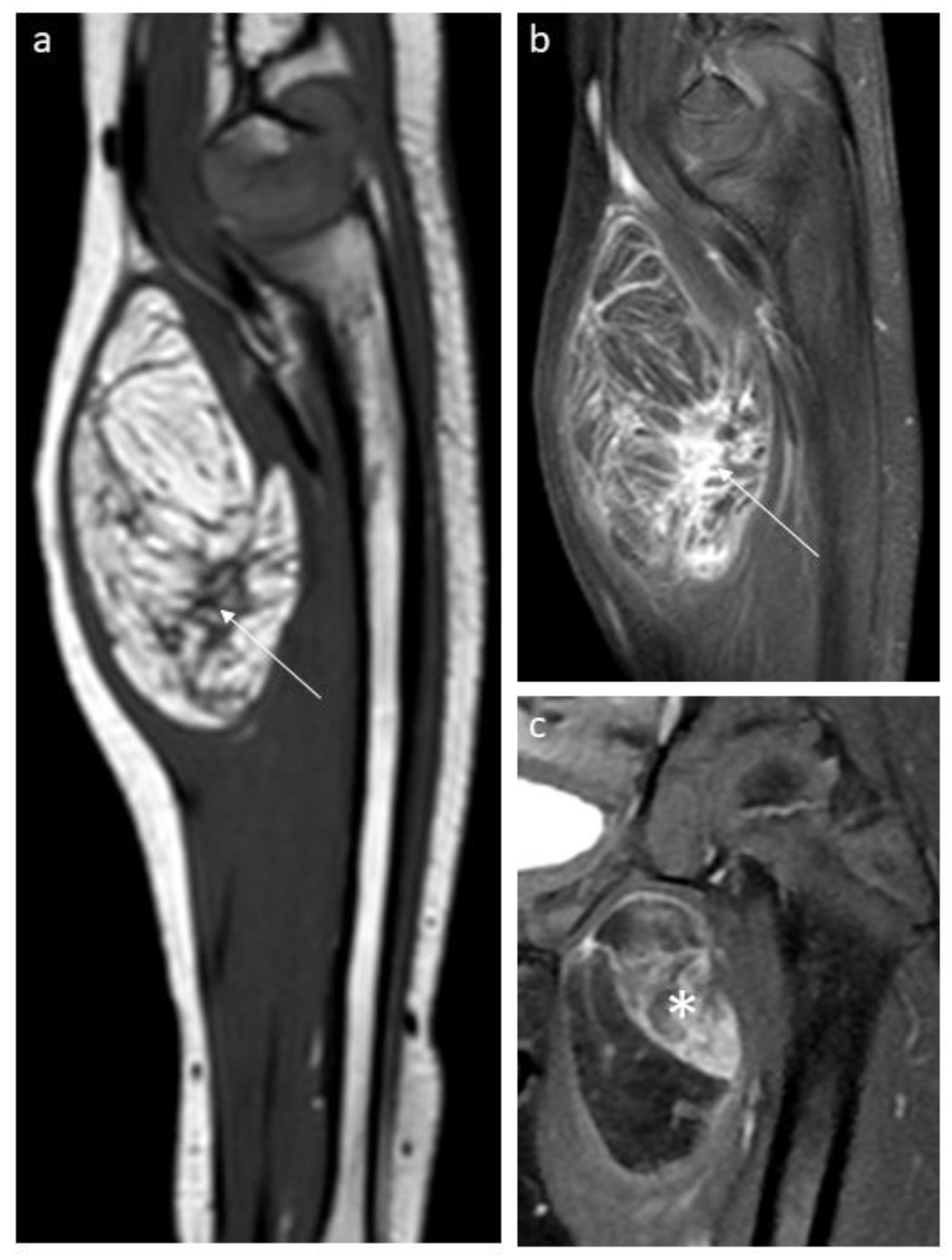
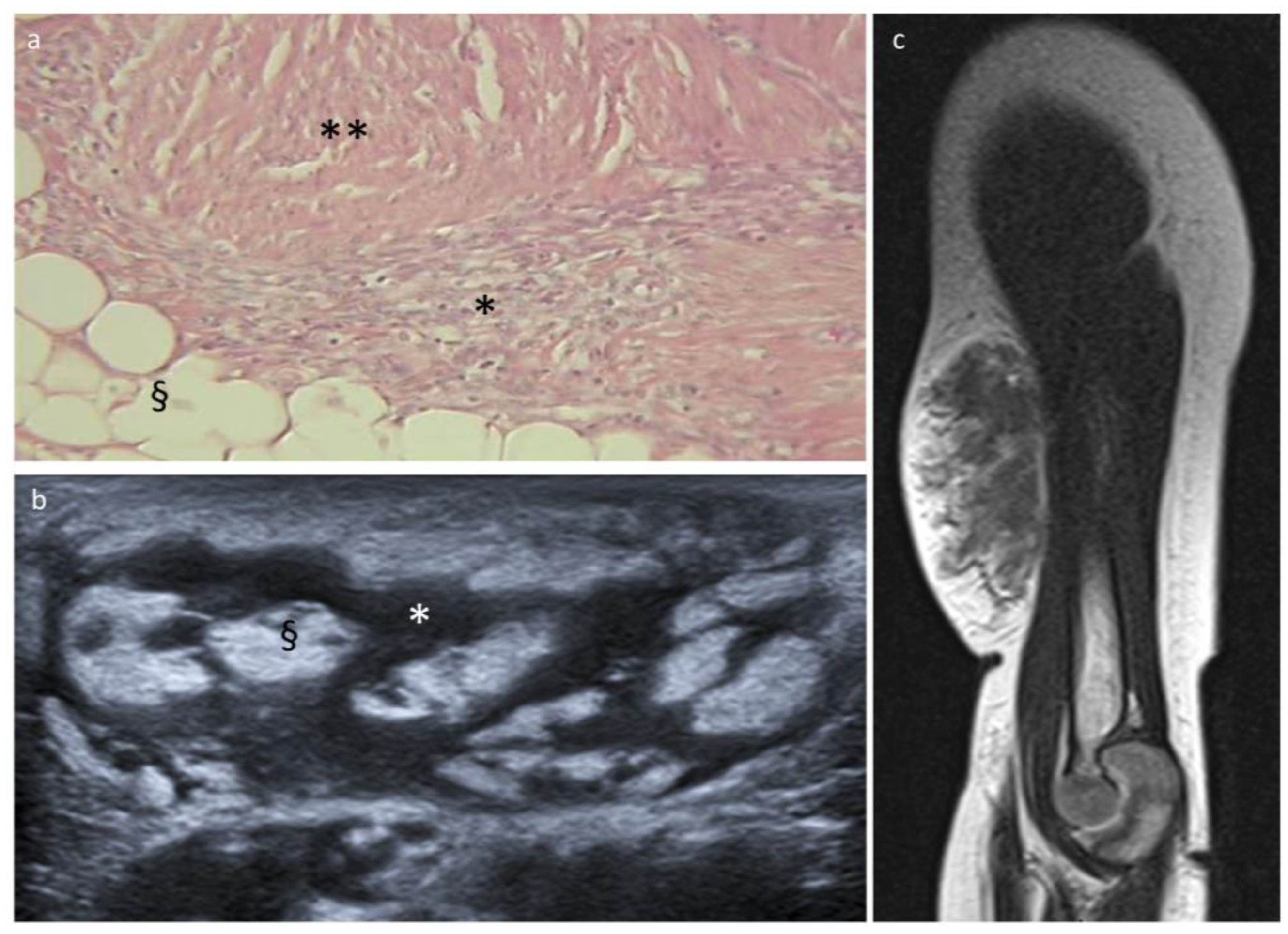
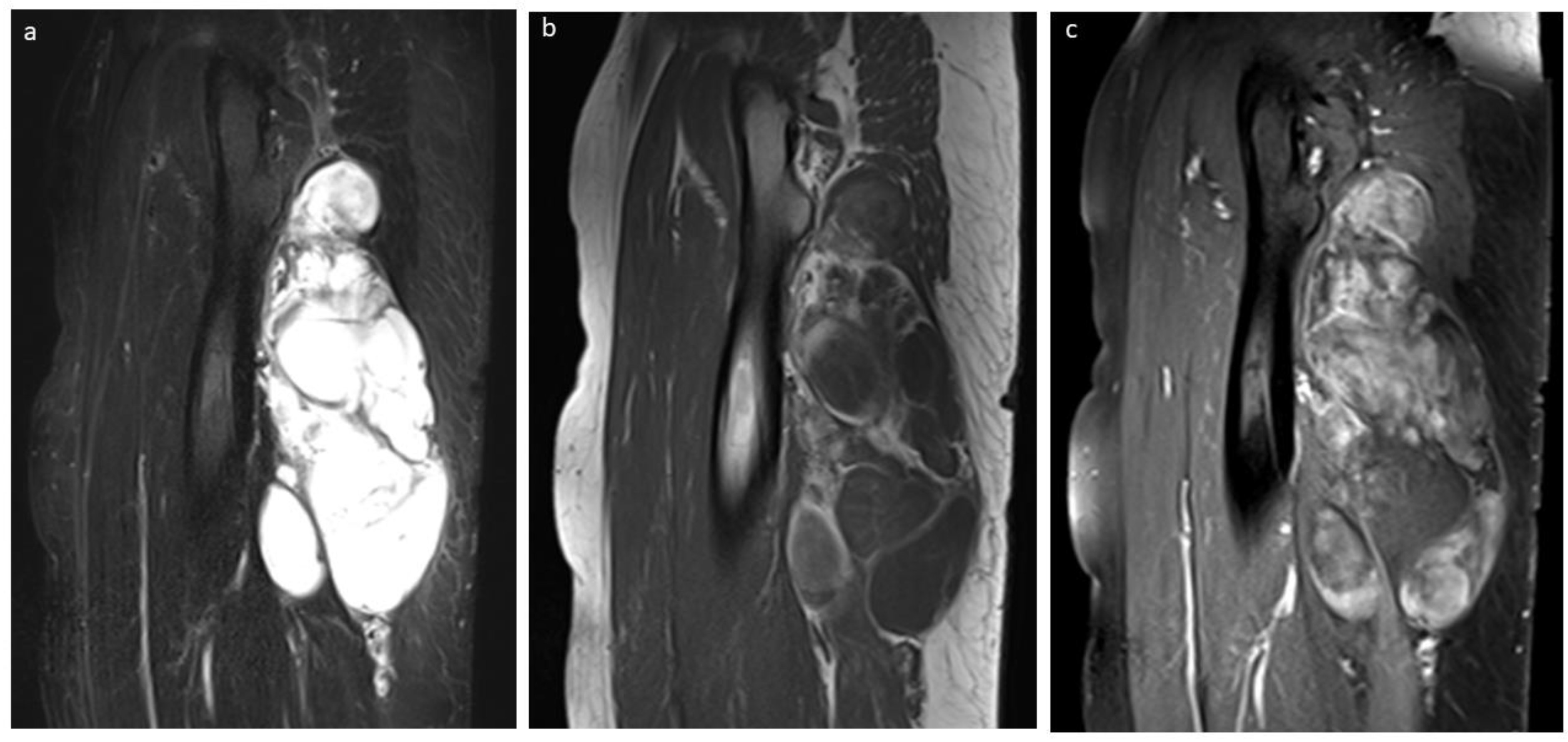
| Lipoblastoma/Lipoma | FHI | LF | Lipoma Arborescens | Lipomatosis | Lipomatosis of Nerve | Spindle Cell Lipoma | Liposarcoma | |
|---|---|---|---|---|---|---|---|---|
| Number of cases | 36 | 12 | 5 | 2 | 1 | 1 | 1 | 5 |
| Gender female/male | 11/25 | 3/9 | 1/4 | 1/1 | 0/1 | 0/1 | 1/0 | 2/3 |
| Age: median [range] | 3 yo [0–17 yo] | 3 mo [0–10 mo] | 2 mo [0–8 yo] | 7 yo [5–9 yo] | 7 yo | 8 yo | 23 yo | 14 yo [12–20 yo] |
| Predisposition | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Pain | 5 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Skin changes | 2 | 4 | 1 | 1 | 0 | 0 | 0 | 1 |
| Location | ||||||||
| Head and neck | 2 | 1 | - | - | - | - | - | 1 |
| Trunk | 17 | 5 | - | - | - | - | 1 | 1 |
| Upper extremity | 7 | 5 | 1 | - | - | - | - | - |
| Lower extremity | 8 | 1 | 4 | 1 | 1 | 1 | - | 3 |
| Scrotum | 2 | - | - | - | - | - | - | - |
| Lipoblastoma/Lipoma | FHI | LF | Lipoma Arborescens | Lipomatosis | Lipomatosis of Nerve | Spindle Cell Lipoma | Liposarcoma | |
|---|---|---|---|---|---|---|---|---|
| Depth | ||||||||
| Superficial | 13 | 9 | 2 | - | 1 | 1 | 1 | 1 |
| Deep | 23 | 3 | 3 | 2 | - | - | - | 4 |
| Size (mm): mean [range] | 56 [15–170] | 39 [25–65] | 66 [15–82] | 92.5 [65–120] | 45 | 75 | 75 | 71 [15–215] |
| Margins | ||||||||
| Well-defined | 33 | 5 | 1 | 2 | - | 1 | 1 | 3 |
| Ill-defined | 3 | 7 | 4 | - | 1 | - | - | 2 |
| Shape | ||||||||
| Ovoid | 22 | 10 | 2 | - | - | 1 | - | 2 |
| Polylobate | 14 | 2 | - | 2 | - | - | 1 | 3 |
| Diffuse | - | - | 3 | - | 1 | - | - | - |
| Echostructure | - | - | ||||||
| Homogeneous | 9 (/23) | - | - | - | 1 | - | ||
| Heterogeneous | 14 | 10 (/10) | 2 (/2) | 1 | - | 3 (/3) | ||
| Echogenicity | - | - | ||||||
| Isoechoic | 1 (/23) | - | - | - | - | - | ||
| Hyperechoic | 16 | - | 2 (/2) | 1 | 1 | 3 (/3) | ||
| Hypoechoic | 2 | - | - | - | - | - | ||
| Mixed | 4 | 10 (/10) | - | - | - | - | ||
| % of fat | ||||||||
| 0% | - | - | 0 | - | - | - | - | 2 (/4) |
| <25% | - | 9 | 1 | - | - | - | - | 2 |
| 25–75% | 6 (/35) | 1 | 0 | 1 | - | - | - | - |
| >75% | 29 | 2 | 4 | 1 | 1 | 1 | 1 | - |
| Non-fatty components (NFC) | ||||||||
| None | 2 (/35) | - | 2 | - | 1 | - | - | - |
| Thin septa | 16 | - | - | - | - | 1 | - | - |
| Thick septa | 7 | - | 2 | 1 | - | - | - | - |
| Nodules | 5 | 2 | - | - | - | - | - | 1 (/4) |
| Patches | 5 | 10 | 1 | 1 | - | - | 1 | 3 |
| NFC: T1-weighted signal | - | |||||||
| Isosignal | 8 (/29) | 9 | 3 (/3) | 1 | - | 1 | 3 (/4) | |
| Hyposignal | 20 | 1 | - | 1 | 1 | - | 1 | |
| Hypersignal | 1 | 2 | - | - | - | - | - | |
| NFC: T2-weighted signal | - | |||||||
| Isosignal | 1 (/28) | - | - | - | - | 1 | - | |
| Hyposignal | 19 | 1 | 1 (/3) | 1 | 1 | - | - | |
| Hypersignal | 4 | - | - | 1 | - | - | 4 (/4) | |
| Intermediate | 4 | 11 | 2 | - | - | - | - | |
| Enhancement | 21 (/31) | 10 (/11) | 3 | 2 | - | - | 1 | 4 (/4) |
| Myxoid zone | 3 (/28) | - | - | - | - | - | - | 4 (/4) |
| Neurovascular encasement | 4 | - | 3 | - | 1 | 1 | - | 1 |
| Bone involvement | - | - | - | - | - | - | - | - |
| Calcifications | - | - | - | - | - | - | - | - |
| Cystic zone | 1 | - | - | 1 | - | - | - | - |
| Factor | Benign | Malignant | Total | Fisher Test | |
|---|---|---|---|---|---|
| Sex | Female | 17 (29.3%) | 2 (40%) | 19 (30.2%) | p = 0.63 |
| Male | 41 (70.7%) | 3 (60%) | 44 (69.8%) | ||
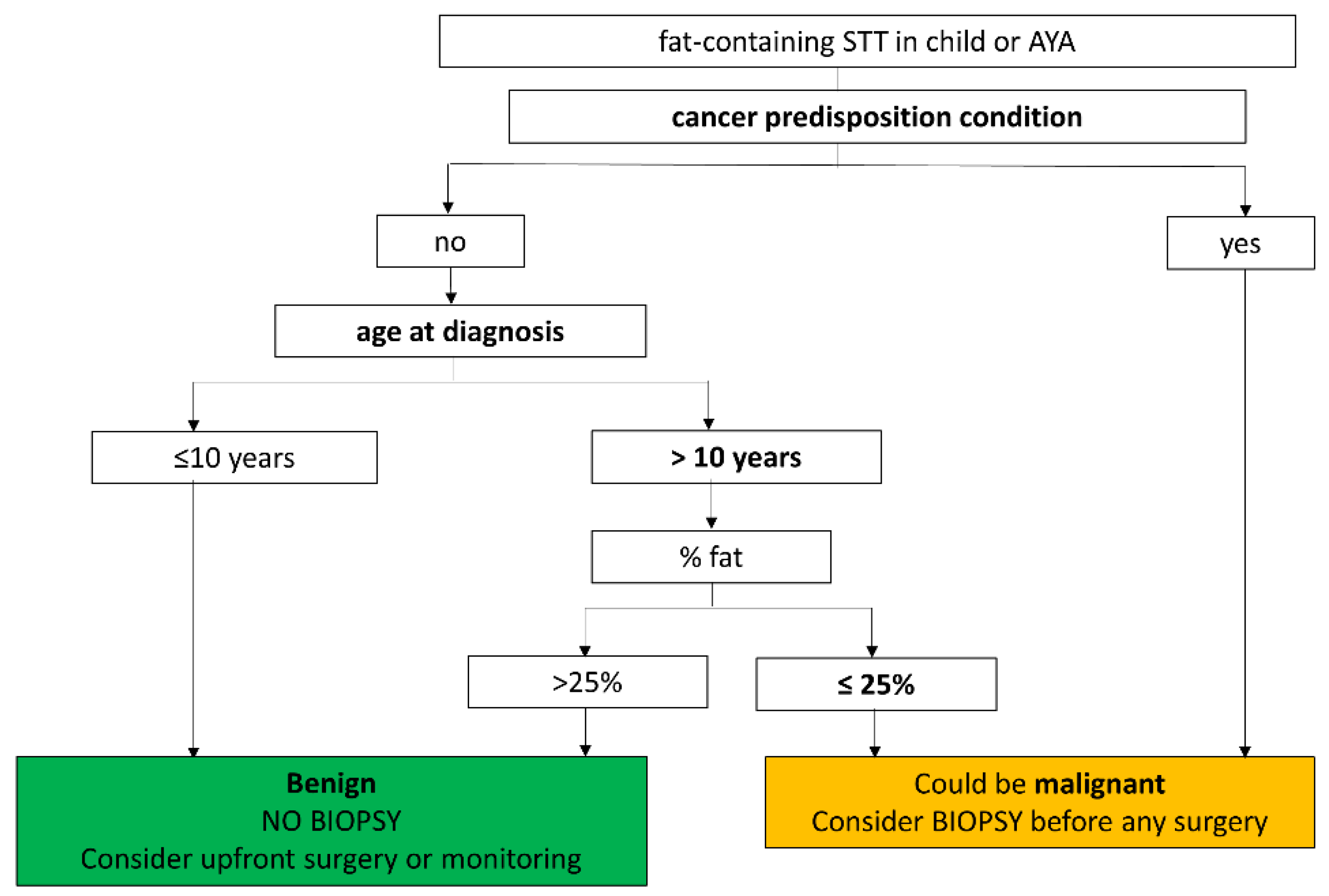
| Predisposition | Yes No | 0 (0%) 58 (100%) | 3 (60%) 2 (40%) | 3 (4.8%) 60 (95.2%) | p < 0.001 |
| Age at diagnosis | ≤10 y.o. >10 y.o. | 52 (89.7%) 6 (10.3%) | 0 (0%) 5 (100%) | 52 (82.5%) 11 (17.5%) | p < 0.001 |
| Location | Upper extremity | 13 (22.4%) | 0 (0%) | 13 (20.6%) | p = 0.26 |
| Lower extremity | 17 (29.3%) | 3 (60%) | 20 (31.7%) | ||
| Trunk | 23 (39.7%) | 1 (20%) | 24 (38.1%) | ||
| Head and neck | 3 (5.2%) | 1 (20%) | 4 (6.3%) | ||
| Other | 2 (3.4%) | 0 (0%) | 2 (3.2%) | ||
| Pain | Yes | 7 (12.7%) | 0 (0%) | 7 (11.7%) | p = 1 |
| No | 48 (87.3%) | 5 (100%) | 53 (88.3%) | ||
| NA | 3 | 0 | 3 | ||
| Skin changes | No | 46 (83.6%) | 4 (80%) | 50 (83.3%) | p = 1 |
| Yes | 9 (16.4%) | 1 (20%) | 10 (16.7%) | ||
| NA | 3 | 0 | 3 | ||
| Depth | Supra-aponeurotic | 27 (46.6%) | 1 (20%) | 28 (44.4%) | p = 0.5 |
| Sub-aponeurotic | 31 (53.4%) | 4 (80%) | 35 (55.6%) | ||
| Shape | Ovoid or round | 35 (60.3%) | 2 (40%) | 37 (58.7%) | p = 0.53 |
| Polylobed | 19 (32.8%) | 3 (60%) | 22 (34.9%) | ||
| Diffuse | 4 (6.9%) | 0 (0%) | 4 (6.3%) | ||
| Margins | Well-defined | 43 (74.1%) | 3 (60%) | 46 (73%) | p = 0.65 |
| Ill-defined | 13 (22.4%) | 2 (40%) | 15 (23.8%) | ||
| ND | 2 (3.4%) | 0 (0%) | 2 (3.2%) | ||
| Echostructure | Homogeneous | 10 (27%) | 0 (0%) | 10 (25%) | p = 0.56 |
| Heterogeneous | 27 (73%) | 3 (100%) | 30 (75%) | ||
| NA | 21 | 2 | 23 | ||
| Echogenicity | Iso-echoic | 1 (2.7%) | 0 (0%) | 1 (2.5%) | p = 0.41 |
| Hyper-echoic | 20 (54.1%) | 3 (100%) | 23 (57.5%) | ||
| Hypo-echoic | 2 (5.4%) | 0 (0%) | 2 (5%) | ||
| Mixt hyper and hypo-echoic | 14 (37.8%) | 0 (0%) | 14 (35%) | ||
| NA | 21 | 2 | 23 | ||
| Percentage of fat | 0% | 0 (0%) | 2 (50%) | 2 (3.2%) | p < 0.001 |
| <25% | 10 (17.2%) | 2 (50%) | 12 (19.4%) | ||
| 25–75% | 8 (13.8%) | 0 (0%) | 8 (12.9%) | ||
| >75% | 40 (69%) | 0 (0%) | 40 (64.5%) | ||
| NA | 0 | 1 | 1 | ||
| Non-fatty components | None | 5 (8.8%) | 0 (0%) | 5 (8.2%) | p = 0.38 |
| Thin septa < 1 mm | 17 (29.8%) | 0 (0%) | 17 (27.9%) | ||
| Thick septa 1–5 mm | 10 (17.5%) | 0 (0%) | 10 (16.4%) | ||
| Nodules | 7 (12.3%) | 1 (25%) | 8 (13.1%) | ||
| Patches | 18 (31.6%) | 3 (75%) | 21 (34.4%) | ||
| NA | 1 | 1 | 2 | ||
| NFC: T1-weighted signal | Isosignal | 22 (45.8%) | 3 (75%) | 25 (48.1%) | p = 0.68 |
| Hyposignal | 23 (47.9%) | 1 (25%) | 24 (46.2%) | ||
| Hypersignal | 3 (6.2%) | 0 (0%) | 3 (5.8%) | ||
| NA | 10 | 1 | 11 | ||
| NFC: T2-weighted signal | Iso T2 | 2 (4.3%) | 0 (0%) | 2 (3.9%) | p = 0.001 |
| Hyposignal | 23 (48.9%) | 0 (0%) | 23 (45.1%) | ||
| Hypersignal | 5 (10.6%) | 4 (100%) | 9 (17.6%) | ||
| Intermediate signal | 17 (36.2%) | 0 (0%) | 17 (33.3%) | ||
| NA | 11 | 1 | 12 | ||
| Enhancement | Yes | 37 (72.5%) | 4 (100%) | 41 (74.5%) | p = 0.56 |
| No | 14 (27.5%) | 0 (0%) | 14 (25.5%) | ||
| NA | 7 | 1 | 8 | ||
| Cystic zones | Yes | 2 (3.6%) | 0 (0%) | 2 (3.4%) | p = 1 |
| No | 54 (96.4%) | 3 (100%) | 57 (96.6%) | ||
| NA | 2 | 2 | 4 | ||
| Myxoid zones | No | 50 (94.3%) | 0 (0%) | 50 (87.7%) | p < 0.001 |
| Yes | 3 (5.7%) | 4 (100%) | 7 (12.3%) | ||
| NA | 5 | 1 | 6 | ||
| Neurovascular encasement | Yes | 9 (16.1%) | 1 (25%) | 10 (16.7%) | p = 0.53 |
| No | 47 (83.9%) | 3 (75%) | 50 (83.3%) | ||
| NA | 2 | 1 | 3 |
| Factors | Age at Diagnosis (>10 Years Old) | Cancer Predisposition | % Fat | Myxoid Component |
|---|---|---|---|---|
| N patients | 63 | 63 | 62 | 57 |
| % of population | 100.0% | 100.0% | 98.4% | 90.5% |
| true positive | 5 | 3 | 4 | 4 |
| false negative | 0 | 2 | 0 | 0 |
| true negative | 52 | 58 | 48 | 50 |
| false positive | 6 | 0 | 10 | 3 |
| apparent prevalence | 17.5% (9.1–29.1%) | 4.8% (1.0–13.3%) | 22.6% (12.9–35.0%) | 12.3% (5.1–23.7%) |
| true prevalence | 7.9% (2.6–17.6%) | 7.9% (2.6–17.6%) | 6.5% (1.8–15.7%) | 7.0% (1.9–17.0%) |
| sensitivity | 100.0% (47.8–100.0%) | 60.0% (14.7–94.7%) | 100.0% (39.8–100.0%) | 100.0% (39.8–100.0%) |
| specificity | 89.7% (78.8–96.1%) | 100.0% (93.8–100.0%) | 82.8% (70.6–91.4%) | 94.3% (84.3–98.8%) |
| positive predictive value | 45.5% (16.7–76.6%) | 100.0% (29.2–100.0%) | 28.6% (8.4–58.1%) | 57.1% (18.4–90.1%) |
| negative predictive value | 100.0% (93.2–100.0%) | 96.7% (88.5–99.6%) | 100.0% (92.6–100.0%) | 100.0% (92.9–100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoen, L.; Nicolas, N.; Le Gaudu, V.; Gauthier, A.; Carton, M.; Berrebi, D.; Cyrta, J.; Collignon, C.; Cordero, C.; Pierron, G.; et al. Fat-Containing Soft Tissue Tumors in Children, Adolescents, and Young Adults: Which Require Biopsy? Cancers 2023, 15, 3228. https://doi.org/10.3390/cancers15123228
Cardoen L, Nicolas N, Le Gaudu V, Gauthier A, Carton M, Berrebi D, Cyrta J, Collignon C, Cordero C, Pierron G, et al. Fat-Containing Soft Tissue Tumors in Children, Adolescents, and Young Adults: Which Require Biopsy? Cancers. 2023; 15(12):3228. https://doi.org/10.3390/cancers15123228
Chicago/Turabian StyleCardoen, Liesbeth, Nayla Nicolas, Violette Le Gaudu, Arnaud Gauthier, Matthieu Carton, Dominique Berrebi, Joanna Cyrta, Charlotte Collignon, Camille Cordero, Gaëlle Pierron, and et al. 2023. "Fat-Containing Soft Tissue Tumors in Children, Adolescents, and Young Adults: Which Require Biopsy?" Cancers 15, no. 12: 3228. https://doi.org/10.3390/cancers15123228
APA StyleCardoen, L., Nicolas, N., Le Gaudu, V., Gauthier, A., Carton, M., Berrebi, D., Cyrta, J., Collignon, C., Cordero, C., Pierron, G., Pannier, S., Philippe-Chomette, P., Orbach, D., & Brisse, H. J. (2023). Fat-Containing Soft Tissue Tumors in Children, Adolescents, and Young Adults: Which Require Biopsy? Cancers, 15(12), 3228. https://doi.org/10.3390/cancers15123228






