The French Cohort of DNA Repair-Deficient Xeroderma Pigmentosum Patients: Risk of Hematological Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. French Cohort
2.2. Statistical Analysis
3. Results
3.1. The French Cohort of Xeroderma Pigmentosum Patients
3.2. Skin Cancers in the French XP Cohort
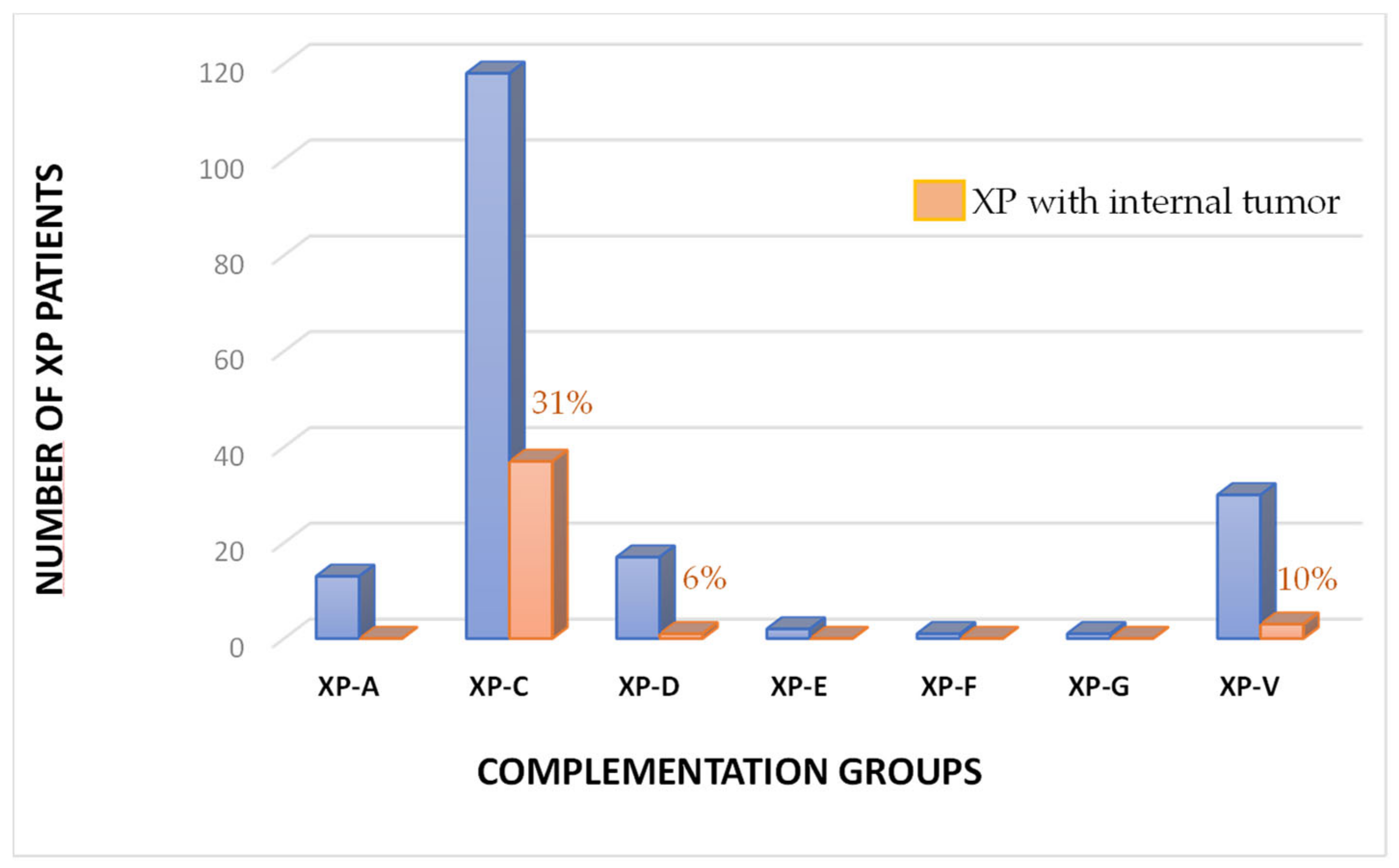
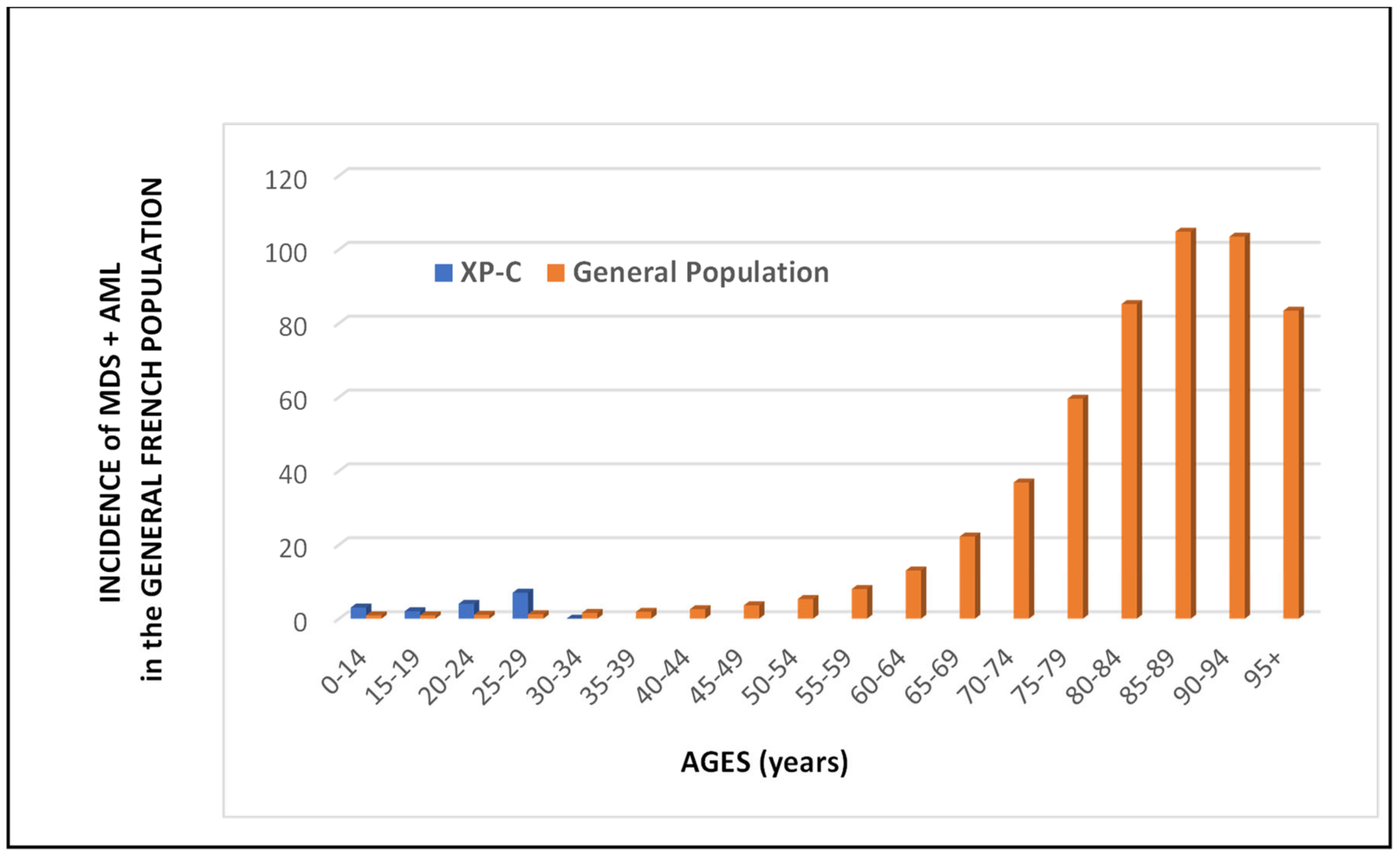
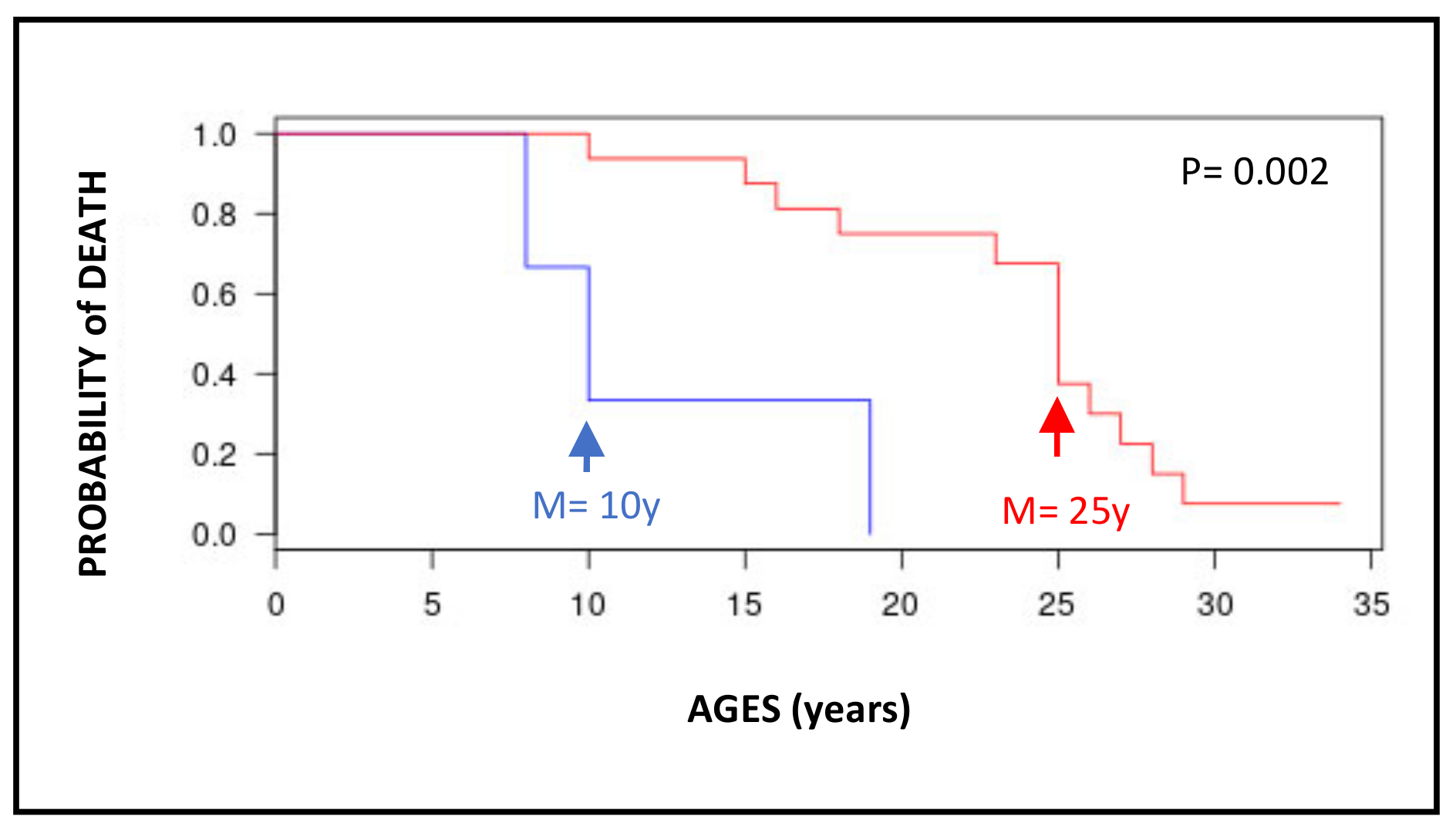
3.3. Internal Tumors in the French XP Cohort
3.4. Cautious Use of Chemotherapy for Treating Cancers in XP-C Patients
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanawalt, P.C.; Spivak, G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008, 9, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Sarasin, A.; Menck, C.F.M.; Cabral-Neto, J.B. Xeroderma Pigmentosum: When the Sun Is the Enemy. In Encyclopedia of Cancer, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 562–571. [Google Scholar] [CrossRef]
- Bradford, P.T.; Goldstein, A.M.; Tamura, D.; Khan, S.G.; Ueda, T.; Boyle, J.; Oh, K.S.; Imoto, K.; Inui, H.; Moriwaki, S.; et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J. Med. Genet. 2011, 48, 168–176. [Google Scholar] [CrossRef]
- Hadj-Rabia, S.; Oriot, D.; Soufir, N.; Dufresne, H.; Bourrat, E.; Mallet, S.; Poulhalon, N.; Ezzedine, K.; Grandchamp, B.; Taïeb, A.; et al. Unexpected extradermatological findings in 31 patients with xeroderma pigmentosum type C. Br. J. Dermatol. 2013, 168, 1109–1113. [Google Scholar] [CrossRef]
- Cartault, F.; Nava, C.; Malbrunot, A.C.; Munier, P.; Hebert, J.C.; N’guyen, P.; Djeridi, N.; Pariaud, P.; Pariaud, J.; Dupuy, A.; et al. A new XPC gene splicing mutation has led to the highest worldwide prevalence of xeroderma pigmentosum in black Mahori patients. DNA Repair 2011, 10, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.R.; McGibbon, D.; Stefanini, M. Xeroderma pigmentosum. Orphanet. J. Rare Dis. 2011, 6, 70. [Google Scholar] [CrossRef]
- Stary, A.; Kannouche, P.; Lehmann, A.R.; Sarasin, A. Role of DNA polymerase eta in the UV mutation spectrum in human cells. J. Biol. Chem. 2003, 278, 18767–18775. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, K.; Bourillon, A.; Yang, W.; Pouvelle, C.; Armier, J.; Despras, E.; Ludovic, M.; Mateus, C.; Robert, C.; Kannouche, P.; et al. Correlation of phenotype/genotype in a cohort of 23 xeroderma pigmentosum-variant patients reveals 12 new disease-causing POLH mutations. Hum. Mutat. 2014, 35, 117–128. [Google Scholar] [CrossRef]
- Kleijer, W.J.; Laugel, V.; Berneburg, M.; Nardo, T.; Fawcett, H.; Gratchev, A.; Jaspers, N.G.; Sarasin, A.; Stefanini, M.; Lehmann, A.R. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair 2008, 7, 744–750. [Google Scholar] [CrossRef]
- Robbins, J.H.; Kraemer, K.H.; Lutzner, M.A.; Festoff, B.W.; Coon, H.G. Xeroderma pigmentosum. An inherited disease with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann. Intern. Med. 1974, 80, 221–248. [Google Scholar] [CrossRef]
- Takebe, H.; Nishigori, C.; Satoh, Y. Genetics and skin cancer of xeroderma pigmentosum in Japan. Jpn. J. Cancer Res. 1987, 78, 1135–1143. [Google Scholar]
- Jerbi, M.; Ben Rekaya, M.; Naouali, C.; Jones, M.; Messaoud, O.; Tounsi, H.; Nagara, M.; Chargui, M.; Kefi, R.; Boussen, H.; et al. Clinical, genealogical and molecular investigation of the xeroderma pigmentosum type C complementation group in Tunisia. Br. J. Dermatol. 2016, 174, 439–443. [Google Scholar] [CrossRef]
- Marionnet, C.; Armier, J.; Sarasin, A.; Stary, A. Cyclobutane pyrimidine dimers are the main mutagenic DNA photoproducts in DNA repair-deficient trichothiodystrophy cells. Cancer Res. 1998, 58, 102–108. [Google Scholar]
- Sarasin, A.R.; Smith, C.A.; Hanawalt, P.C. Repair of DNA in human cells after treatment with activated aflatoxin B1. Cancer Res. 1977, 37, 1786–1793. [Google Scholar]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Sarasin, A.; Blanchet-Bardon, C.; Renault, G.; Lehmann, A.; Arlett, C.; Dumez, Y. Prenatal diagnosis in a subset of trichothiodystrophy patients defective in DNA repair. Br. J. Dermatol. 1992, 127, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Daya-Grosjean, L.; James, M.R.; Drougard, C.; Sarasin, A. An immortalized xeroderma pigmentosum, group C, cell line which replicates SV40 shuttle vectors. Mutat. Res. 1987, 183, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Giglia, G.; Dumaz, N.; Drougard, C.; Avril, M.F.; Daya-Grosjean, L.; Sarasin, A. p53 mutations in skin and internal tumors of xeroderma pigmentosum patients belonging to the complementation group C. Cancer Res. 1998, 58, 4402–4409. [Google Scholar]
- Giglia, G.; Bouffet, E.; Jouvet, A.; Ohgaki, H.; Kleihus, P.; Sarasin, A. Molecular analysis of glioma and skin-tumour alterations in a xeroderma-pigmentosum child. Int. J. Cancer 1999, 81, 345–350. [Google Scholar] [CrossRef]
- Sarasin, A.; Quentin, S.; Droin, N.; Sahbatou, M.; Saada, V.; Auger, N.; Boursin, Y.; Dessen, P.; Raimbault, A.; Asnafi, V.; et al. Familial predisposition to TP53/complex karyotype MDS and leukemia in DNA repair-deficient xeroderma pigmentosum. Blood 2019, 133, 2718–2724. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Padioleau, I.; Matkarimov, B.T.; Soulier, J.; Sarasin, A.; Nikolaev, S. XPC deficiency increases risk of hematologic malignancies through mutator phenotype and characteristic mutational signature. Nat. Commun. 2020, 11, 5834. [Google Scholar] [CrossRef]
- Nikolaev, S.; Yurchenko, A.A.; Sarasin, A. Increased risk of internal tumors in DNA repair-deficient xeroderma pigmentosum patients: Analysis of four international cohorts. Orphanet J. Rare Dis. 2022, 17, 104. [Google Scholar] [CrossRef]
- Boutros, C.; Rouleau, E.; Majer, M.; Nikolaev, S.; Robert, C. Combination of targeted therapy and immune checkpoint blocker in a patient with xeroderma pigmentosum presenting an aggressive angiosarcoma and a recurrent non-resectable basal cell carcinoma. Eur. J. Cancer 2021, 150, 130–132. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Fresneau, B.; Borghese, B.; Rajabi, F.; Tata, Z.; Genestie, C.; Sarasin, A.; Nikolaev, S.I. Early-onset gynecological tumors in xeroderma pigmentosum group C patients. Commun. Med. 2023, in press.
- Fréchet, M.; Bergoglio, V.; Chevallier-Lagente, O.; Sarasin, A.; Magnaldo, T. Complementation assays adapted for DNA repair-deficient keratinocytes. Methods Mol. Biol. 2006, 314, 9–23. [Google Scholar]
- Zeng, L.; Quilliet, X.; Chevallier-Lagente, O.; Eveno, E.; Sarasin, A.; Mezzina, M. Retrovirus mediated gene transfer corrects DNA repair defect of xeroderma pigmentosum cells of complementation groups A, B and C. Gene Ther. 1997, 4, 1077–1084. [Google Scholar] [CrossRef]
- Soufir, N.; Ged, C.; Bourillon, A.; Austerlitz, F.; Chemin, C.; Stary, A.; Armier, J.; Pham, D.; Khadir, K.; Roume, J.; et al. A prevalent mutation with founder effect in xeroderma pigmentosum group C from North Africa. J. Investig. Dermatol. 2010, 130, 1537–1542. [Google Scholar] [CrossRef]
- National Estimates of Cancer Incidence and Mortality in Metropolitan France between 1990 and 2018. In Hematological Malignancies; Santé Publique France: Saint-Maurice, France, 2019; Volume 2, Available online: https://www.e-cancer.fr/ (accessed on 18 January 2023).
- Walburn, J.; Canfield, M.; Norton, S.; Sainsbury, K.; Araújo-Soares, V.; Foster, L.; Berneburg, M.; Sarasin, A.; Morrison-Bowen, N.; Sniehotta, F.F.; et al. Psychological correlates of adherence to photoprotection in a rare disease: International survey of people with Xeroderma Pigmentosum. Br. J. Health Psychol. 2019, 24, 668–686. [Google Scholar] [CrossRef]
- Saffi, J.; Agnoletto, M.H.; Guecheva, T.N.; Batista, L.F.; Carvalho, H.; Henriques, J.A.; Stary, A.; Menck, C.F.; Sarasin, A. Effect of the anti-neoplastic drug doxorubicin on XPD-mutated DNA repair-deficient human cells. DNA Repair 2010, 9, 40–47. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Soda, H.; Sadanaga, N.; Taniguchi, H.; Ikeda, T.; Maruta, H.; Dotsu, Y.; Ogawara, D.; Fukuda, Y.; Mukae, H. Alert regarding Cisplatin-induced severe adverse events in cancer patients with xeroderma pigmentosum. Intern. Med. 2017, 56, 979–982. [Google Scholar] [CrossRef]
- Gilbar, P.J.; Pokharel, K. Severe cisplatin-induced renal toxicity in a patient with xeroderma pigmentosum. J. Oncol. Pharm. Pract. 2022, 28, 466–470. [Google Scholar] [CrossRef]
- Wu, X.; Fan, W.; Xu, S.; Zhou, Y. Sensitization to the cytotoxicity of cisplatin by transfection with nucleotide excision repair gene xeroderma pigmentosun group A antisense RNA in human lung adenocarcinoma cells. Clin. Cancer Res. 2003, 9, 5874–5879. [Google Scholar] [PubMed]
- Janjetovic, S.; Bacher, U.; Haalck, T.; Janning, M.; Bokemeyer, C.; Fiedler, W. Acute Megakaryoblastic Leukemia in a Patient with Xeroderma Pigmentosum: Discussion of Pathophysiological, Prognostic, and Toxicological Aspects. Acta Haematol. 2013, 129, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.; Rajabi, F.; Braz-Petta, T.; Fassihi, H.; Lehmann, A.R.; Nishigori, C.; Wang, J.; Padioleau, I.; Gunbin, K.; Panunzi, L.; et al. Genomic mutation landscape of skin cancers from DNA repair-deficient xeroderma pigmentosum patients. Nat. Commun. 2023, in press. [CrossRef] [PubMed]
- Corradi, C.; Vilar, J.B.; Buzzatto, V.C.; De Souza, T.A.; Castro, L.P.; Munford, V.; De Vecchi, R.; Galante, P.; Orpinelli, F.; Miller, T.; et al. Mutational signatures and increased retrotransposon insertions in xeroderma pigmentosum variant skin tumors. Carcinogenesis 2023, in press.
- D’Errico, M.; Parlanti, E.; Teson, M.; Bernades de Jesus, B.M.; Degan, P.; Calcagnile, A.; Jaruga, P.; Bjoras, M.; Crescenzi, M.; Pedrini, A.M.; et al. New functions of XPC in the protection of human skin cells from oxidative damage. EMBO J. 2006, 25, 4305–4315. [Google Scholar] [CrossRef]
- Cleaver, J.E.; Revet, I. Clinical implications of the basic defects in Cockayne syndrome and xeroderma pigmentosum and the DNA lesions responsible for cancer, neurodegeneration and aging. Mech. Ageing Dev. 2008, 129, 492–497. [Google Scholar] [CrossRef]
- Sarasin, A. UVSSA and USP7: New players regulating transcription-coupled nucleotide excision repair in human cells. Genome Med. 2012, 4, 44–45. [Google Scholar] [CrossRef]
- Giglia-Mari, G.; Sarasin, A. TP53 mutations in human skin cancers. Hum. Mutat. 2003, 21, 217–228. [Google Scholar] [CrossRef]
- Dumaz, N.; Duthu, A.; Ehrhart, J.C.; Drougard, C.; Appella, E.; Anderson, C.W.; May, P.; Sarasin, A.; Daya-Grosjean, L. prolonged p53 protein accumulation in trichothiodystrophy fibroblasts dependent on unrepaired pyrimidine dimers on the transcribed strands of cellular genes. Mol. Carcinog. 1997, 20, 340–347. [Google Scholar] [CrossRef]
- Ljungman, M.; Zhang, F. Blockage of RNA polymerase as a possible trigger for u.v. light-induced apoptosis. Oncogene 1996, 13, 823–831. [Google Scholar]
- Da Costa, R.M.A.; Quayle, C.; De Fatima Jacysyn, J.; Amarante-Mendes, G.P.; Sarasin, A.; Menck, C.F.M. Resistance to ultraviolet-induced apoptosis in DNA repair deficient growth arrested human fibroblasts is not related to recovery from RNA transcription blockage. Mutat. Res. 2008, 640, 1–7. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Kool, H.J.M.; Mullenders, L.H.F.; Van Zeeland, A.A.; Friedberg, E.C.; Van der Horst, G.T.J.; Van Steeg, H.; Vrieling, H. Age-dependent spontaneous mutagenesis in Xpc mice defective in nucleotide excision repair. Oncogene 2000, 19, 5034–5037. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Stratton, M.R. Mutational Signatures: The patterns of somatic mutations hidden in cancer genomes. Curr. Opin. Genet. Dev. 2014, 24, 52–60. [Google Scholar] [CrossRef]
- Kuraoka, I.; Bender, C.; Romieu, A.; Cadet, J.; Wood, R.D.; Lindahl, T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3832–3837. [Google Scholar] [CrossRef]
- Pastoriza Gallego, M.; Sarasin, A. Transcription-coupled repair of 8-oxoguanine in human cells and its deficiency in some DNA repair diseases. Biochimie 2003, 85, 1073–1082. [Google Scholar] [CrossRef]
- Kraemer, K.H.; Lee, M.M.; Scotto, J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch. Dermatol. 1987, 123, 241–250. [Google Scholar] [CrossRef]
- Fassihi, H.; Sethi, M.; Fawcett, H.; Wing, J.; Chandler, N.; Mohammed, S.; Craythorne, E.; Morley, A.M.S.; Lim, R.; Turner, S.; et al. Deep phenotyping of 89 xeroderma pigmentosum patients reveals unexpected heterogeneity dependent on the precise molecular defect. Proc. Natl. Acad. Sci. USA 2016, 113, E1236–E1245. [Google Scholar] [CrossRef]
- Kouatcheu, S.D.; Marko, J.; Tamura, D.; Khan, S.G.; Lee, C.R.; DiGiovanna, J.J.; Kraemer, K.H. Thyroid Nodules in Xeroderma Pigmentosum Patients: A Feature of Premature Aging. J. Endocrinol. Investig. 2021, 44, 1475–1482. [Google Scholar] [CrossRef]
- Oetjen, K.A.; Levoska, M.A.; Tamura, D.; Ito, S.; Douglas, D.; Khan, S.G.; Calvo, K.R.; Kraemer, K.H.; Digiovanna, J.J. Predisposition to hematologic malignancies in patients with xeroderma pigmentosum. Haematologica 2020, 105, e144–e146. [Google Scholar] [CrossRef]
- Leite, R.A.; Marchetto, M.C.; Muotri, A.R.; Vasconcelos, D.; De Oliveira, Z.N.; Machado, M.C.; Menck, C.F. Identification of XP complementation groups by recombinant adenovirus carrying DNA repair genes. J. Investig. Dermatol. 2009, 129, 502–506. [Google Scholar] [CrossRef]
- Khan, S.G.; Oh, K.S.; Shahlavi, T.; Ueda, T.; Busch, D.B.; Inui, H.; Emmert, S.; Imoto, K.; Muniz-Medina, V.; Baker, C.C.; et al. Reduced XPC DNA repair gene mRNA levels in clinically normal parents of xeroderma pigmentosum patients. Carcinogenesis 2006, 27, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of Acrolein Toxicity: Relevance to Human Disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Kuloglu, S.S.; Yalcin, E.; Cavusoglu, K.; Acar, A. Paraquat toxicity in different cell types of Swiss albino mice. Sci. Rep. 2022, 12, 4818. [Google Scholar]
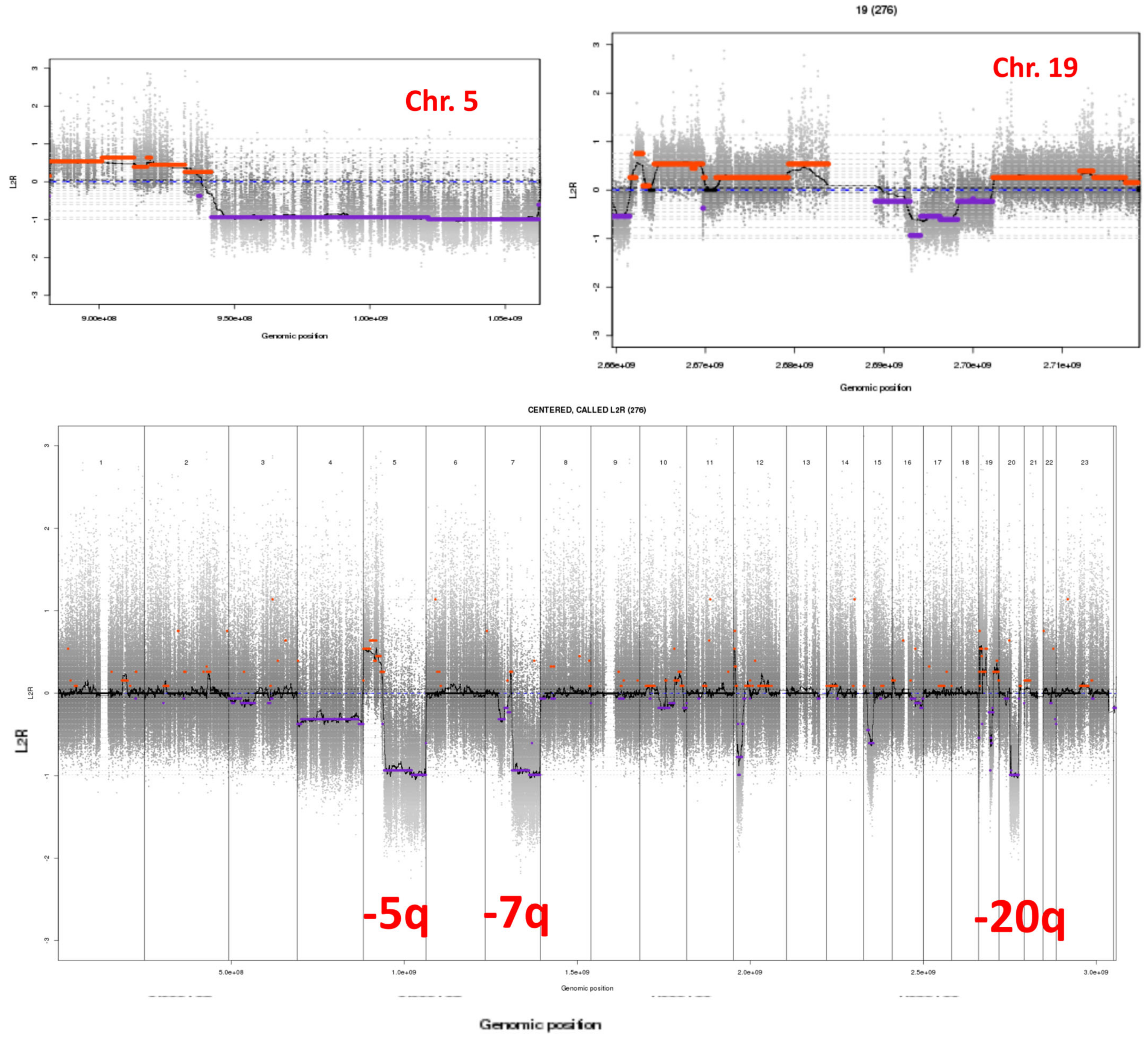
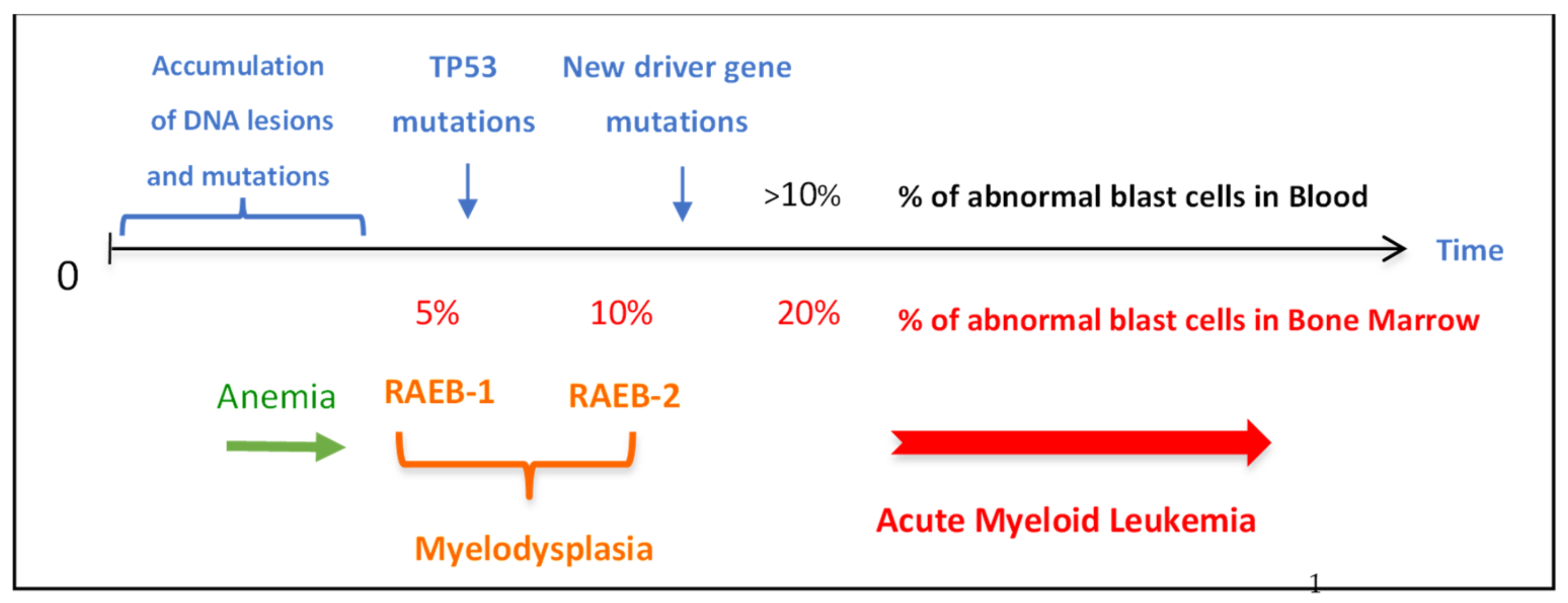
| Patient’s | Countries of | Sex | Ages | Ages at | XP | XP Gene | Clinical Information | References |
|---|---|---|---|---|---|---|---|---|
| Code | Family’s Origin | In 2023 (Years) | Death (Years) | Group | Mutations | |||
| XP10VI * | Morocco | M | 28 | XP-C | delTG | AML-4 (27y); death after chemotherapy at 28. | [20] | |
| XP82VI * | Tunisia | M | 18 | XP-C | delTG | AML-6 (16y); HSCT (17y); death of toxicity. | [20] | |
| XPAlHaVI | Tunisia | M | 25 | XP-C | delTG | Death of AML. | [20] | |
| XP128VI | North Africa | F | 16 | XP-C | delTG | Leukemia (15y); death following chemotherapy. | This paper | |
| XP148VI | Algeria | F | 19 | XP-C | del TG | Poorly differentiated follicular thyroid carcinoma (13y). | [18] | |
| XP155VI | USA | M | 42 | XP-V | ? | Death of T-lymphoma following chemotherapy. | This paper | |
| XP165VI | Morocco | F | 25 | XP-C | delTG | Kidney adenocarcinoma (23y). | [4] | |
| XP167VI | Algeria | M | 26 | XP-C | delTG | Trisomy 21; MDS (25y); death of AML. | [20] | |
| XP185VI | Spain | F | 25 | XP-C | delTG | MDS (24y); death of AML. | [20] | |
| XP208VI | Algeria | M | 17 | XP-C | delTG | Mediastinal lymphoma (8y). | [22] | |
| XP233VI | Tunisia | M | 8 | XP-C | delTG | Death of anaplastic astrocytoma. | [19] | |
| XP235VI | Tunisia | F | 29 | XP-C | delTG | MDS (24); death of AML-6. | [20] | |
| XP269VI | Morocco | F | 23 | XP-C | delTG | Cervical tumor (18y). | [4] | |
| XP309VI | Morocco | F | 10 | XP-C | delTG | B-ALL (7y); death of MDS following chemotherapy. | [20] | |
| XP420VI | Morocco | F | 25 | XP-C | delTG | Death of cutaneous lymphoma NK. | [22] | |
| XP538VI | Algeria | M | 29 | XP-C | delTG | Death of AML-2. | [20] | |
| XP664VI | Tunisia | M | 10 | XP-C | delTG | Death of astrocytoma. | This paper | |
| XPAdSaVI | Morocco | M | 19 | XP-C | delTG | Cerebellar astrocytoma (14y). | [22] | |
| XP673VI | Morocco | F | 22 | XP-C | delTG | Death of T-ALL. | [20] | |
| XP694VI | North Africa | F | 21 | XP-C | delTG | Left ovarian juvenile granulosa-cell tumor at 19y | [24] | |
| and right ovarian Sertoli–Leydig cell tumor at 19y. | ||||||||
| XP757VI | French | M | 20 | XP-D | ? | Hodgkin’s disease (20y); death following chemotherapy. | This paper | |
| AS802VI | Algeria | M | 33 | XP-C | delTG | Poorly differentiated follicular thyroid carcinoma (20y). | [4] | |
| XP819VI | French | M | 73 | XP-V | c.1727_1728delCT; c.883G>A | Prostate tumor at 60y. | [22] | |
| XP820VI | North Africa | F | 19 | XP-C | delTG | MDS/AML in January 2023; waiting for HSCT. | This paper | |
| XP924VI | Morocco | M | 15 | XP-C | delTG | T-ALL (12y); MDS (13y); death of AML-6. | [4] | |
| XP2003VI ** | Algeria | F | 28 | XP-C | delTG | Vaginal embryonal rhabdomyosarcoma at 16y. | [22] | |
| XP2004VI ** | Algeria | F | 23 | XP-C | delTG | Vaginal rhabdomyosarcoma at 16y and death of AML. | [22] | |
| XP2006VI | Morocco | F | 34 | XP-C | delTG | AML-6 (29y); HSCT (29y). | [20] | |
| XPGAVI | French | F | 54 | XP-V | ? | Gastric tumor (48y). | [17] | |
| XPAHVI | Tunisia | M | 25 | XP-C | delTG | AML (24y); curietherapy (24y); death at 25. | [20] | |
| XPChFa2VI | North Africa | M | 24 | XP-C | ? | Death of leukemia. | This paper | |
| XP2020VI | Tunisia | F | 14 | XP-C | delTG; c.G850T | Ovarian Sertoli–Leydig cell tumor (11y). | [24] | |
| XPAAVI | Algeria | F | 29 | XP-C | delTG | Papillary thyroid carcinomas (17y) and | [22] | |
| pancreatic tumor (29y) under chemotherapy. | This paper | |||||||
| XPElHaVI $ | North Africa | F | 22 | XP-C | delTG | Ovarian sarcoma (18y). | [22] | |
| XPElKaVI $ | North Africa | F | 28 | XP-C | delTG | Uterine adenomyosarcoma (15y); death at 28. | [22] | |
| XPMaAbVI | Morocco | M | 22 | XP-C | delTG | AML-3 (14y). | [22] | |
| XPGaViVI * | Algeria | F | 25 | XP-C | delTG | Death of RAEB-2. | [22] | |
| XPGaMVI * | Algeria | M | 27 | XP-C | delTG | Death of RAEB-t. | [22] | |
| XPWaVI | Pakistan | M | 33 | XP-C | c.1243C>G; c.1934delC | Angiosarcoma of the inner canthus of the left eye (22). | [23] | |
| XPMYVI | Comoros | F | 30 | XP-C | IVS 12-1G>C | Death of breast tumor at 30y. | [21] |
| Tumor Types * | Nb of Tumors ** | Ages at Diagnosis | Ages at Death |
|---|---|---|---|
| (Median Age in Years) | (Median Age in Years) | ||
| Hematological malignancies | 23 (1 XP-D; 1 XP-V) | 7–42 (25y) | 10–42 (25y) |
| Gynecology | 8 | 11–19 (17y) | 13–28 (22y) |
| Brain | 3 | 8–19 (10y) | 8–19 (10y) |
| Thyroid | 3 | 13–17 (17y) | 19 |
| Gastric | 1 (XP-V) | 48 | 54 |
| Prostate | 1 (XP-V) | 60 | na *** |
| Pancreas | 1 | 29 | na |
| Breast | 1 | 30 | 30 |
| Kidney | 1 | 23 | 25 |
| Angiosarcoma | 1 | 22 | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasin, A. The French Cohort of DNA Repair-Deficient Xeroderma Pigmentosum Patients: Risk of Hematological Malignancies. Cancers 2023, 15, 2706. https://doi.org/10.3390/cancers15102706
Sarasin A. The French Cohort of DNA Repair-Deficient Xeroderma Pigmentosum Patients: Risk of Hematological Malignancies. Cancers. 2023; 15(10):2706. https://doi.org/10.3390/cancers15102706
Chicago/Turabian StyleSarasin, Alain. 2023. "The French Cohort of DNA Repair-Deficient Xeroderma Pigmentosum Patients: Risk of Hematological Malignancies" Cancers 15, no. 10: 2706. https://doi.org/10.3390/cancers15102706
APA StyleSarasin, A. (2023). The French Cohort of DNA Repair-Deficient Xeroderma Pigmentosum Patients: Risk of Hematological Malignancies. Cancers, 15(10), 2706. https://doi.org/10.3390/cancers15102706






