Reduction of Tumor Growth with RNA-Targeting Treatment of the NAB2–STAT6 Fusion Transcript in Solitary Fibrous Tumor Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mammalian Cell Culture
2.2. Long-Range Genomic PCR and RT (Reverse Transcription)-PCR
2.3. Western Blot
2.4. Cell Proliferation Assay
2.5. Wound Healing Assay
2.6. RNA Sequencing (RNA-Seq)
2.7. ASO Treatment
2.8. Real-Time RT-PCR (Reverse Transcription-PCR)
2.9. Recombinant AAV2 Viral Vector Production
2.10. Fluorescence Microscopy
2.11. Flow Cytometry
2.12. Mouse Xenograft
2.13. H and E (Hematoxylin and Eosin) Staining
2.14. CT Scan
3. Results
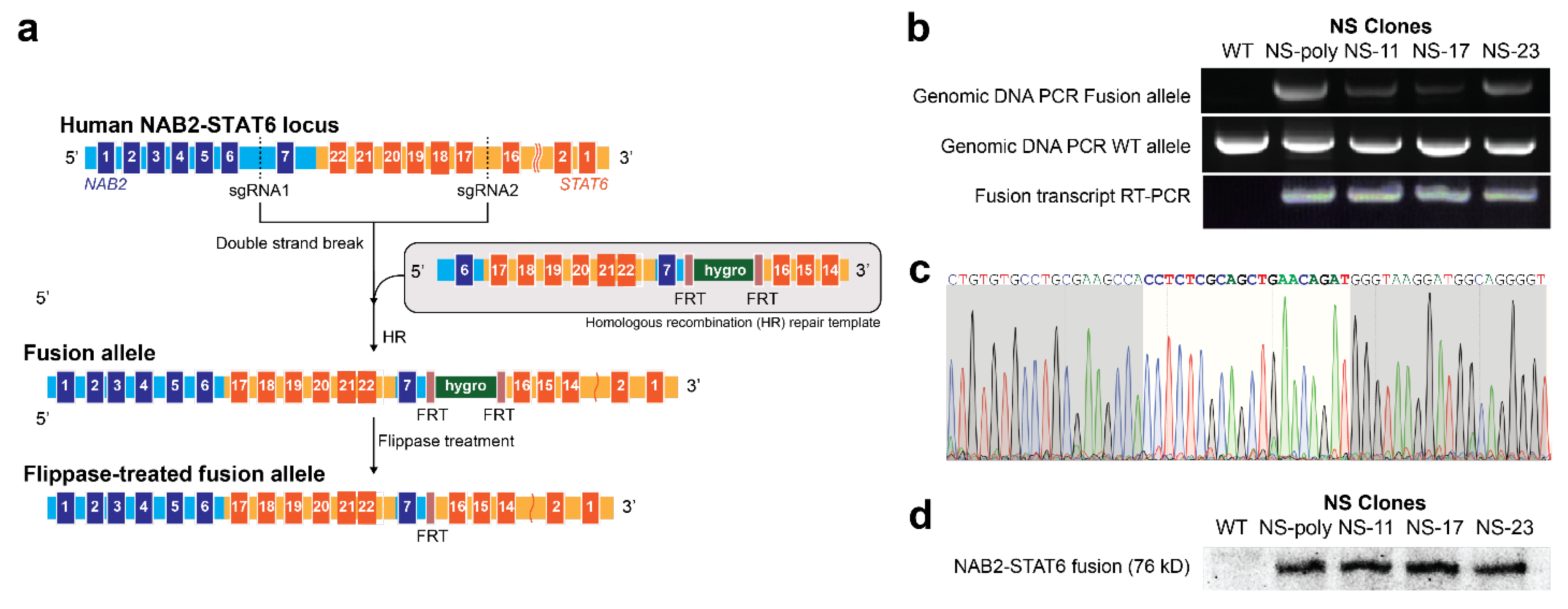
3.1. Generation of NAB2exon6–STAT6exon17 Gene Fusion Cell Models Using Genome Editing
3.2. RNA-Sequencing (RNA-Seq) Analysis of NAB2exon6–STAT6exon17 Gene Fusion Cell Models
3.3. In Vitro Targeting of NAB2–STAT6 Fusion Transcripts Using Fusion-Specific Antisense Oligonucleotides (ASOs)
3.4. In Vitro Targeting of NAB2–STAT6 Fusion Transcripts Using AAV2-Mediated Fusion-Specific CRISPR/CasRx
3.5. Ex Vivo Targeting of NAB2–STAT6 Fusion Transcripts Using AAV2-Mediated Fusion-Specific CRISPR/CasRx System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheehan, J.; Kondziolka, D.; Flickinger, J.; Lunsford, L.D.; Coffey, R.J.; Loeffler, J.S.; Sawaya, R.; Gutin, P.H. Radiosurgery for Treatment of Recurrent Intracranial Hemangiopericytomas. Neurosurgery 2002, 51, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; Endo, T.; Endo, H.; Murakami, K.; Tominaga, T. Intraspinal Dissemination of Intracranial Hemangiopericytoma: Case Report and Literature Review. Surg. Neurol. Int. 2016, 7 (Suppl. S40), S1016–S1020. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Scheithauer, B.W.; Kimmel, D.W.; Schomberg, P.J.; Piepgras, D.G. Management of Recurrent Meningeal Hemangiopericytoma. Cancer 1998, 82, 1915–1920. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.M.; Kalyana-Sundaram, S.; Cao, X.; Lonigro, R.J.; Sung, Y.S.; Chen, C.L.; Zhang, L.; Wang, R.; Su, F.; et al. Identification of Recurrent NAB2-STAT6 Gene Fusions in Solitary Fibrous Tumor by Integrative Sequencing. Nat. Genet. 2013, 45, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Crago, A.M.; Rosenberg, M.; O’Connor, R.; Walker, S.R.; Ambrogio, L.; Auclair, D.; McKenna, A.; Heinrich, M.C.; Frank, D.A.; et al. Whole-Exome Sequencing Identifies a Recurrent NAB2-STAT6 Fusion in Solitary Fibrous Tumors. Nat. Genet. 2013, 45, 131–132. [Google Scholar] [CrossRef]
- Guseva, N.V.; Tanas, M.R.; Stence, A.A.; Sompallae, R.; Schade, J.C.; Bossler, A.D.; Bellizzi, A.M.; Ma, D. The NAB2–STAT6 Gene Fusion in Solitary Fibrous Tumor Can Be Reliably Detected by Anchored Multiplexed PCR for Targeted next-Generation Sequencing. Cancer Genet. 2016, 209, 303–312. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, H.S.; Kim, J.H.; Choi, S.H.; Kim, D.S.; Ryoo, Z.Y.; Kim, J.Y.; Lee, S. NAB2-STAT6 Fusion Protein Mediates Cell Proliferation and Oncogenic Progression via EGR-1 Regulation. Biochem. Biophys. Res. Commun. 2020, 526, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bieg, M.; Moskalev, E.A.; Will, R.; Hebele, S.; Schwarzbach, M.; Schmeck, S.; Hohenberger, P.; Jakob, J.; Kasper, B.; Gaiser, T.; et al. Gene Expression in Solitary Fibrous Tumors (SFTs) Correlates with Anatomic Localization and NAB2-STAT6 Gene Fusion Variants. Am. J. Pathol. 2021, 191, 602–617. [Google Scholar] [CrossRef]
- Gao, Q.; Liang, W.W.; Foltz, S.M.; Mutharasu, G.; Jayasinghe, R.G.; Cao, S.; Liao, W.W.; Reynolds, S.M.; Wyczalkowski, M.A.; Yao, L.; et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018, 23, 227–238.e3. [Google Scholar] [CrossRef] [PubMed]
- de Bernardi, A.; Dufresne, A.; Mishellany, F.; Blay, J.-Y.; Ray-Coquard, I.; Brahmi, M. Novel Therapeutic Options for Solitary Fibrous Tumor: Antiangiogenic Therapy and Beyond. Cancers 2022, 14, 1064. [Google Scholar] [CrossRef] [PubMed]
- Zogg, H.; Singh, R.; Ro, S. Current Advances in RNA Therapeutics for Human Diseases. Int. J. Mol. Sci. 2022, 23, 2736. [Google Scholar] [CrossRef]
- Shadid, M.; Badawi, M.; Abulrob, A. Antisense Oligonucleotides: Absorption, Distribution, Metabolism, and Excretion. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1281–1292. [Google Scholar] [CrossRef]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic Acid Delivery for Therapeutic Applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef]
- Saifullah; Motohashi, N.; Tsukahara, T.; Aoki, Y. Development of Therapeutic RNA Manipulation for Muscular Dystrophy. Front. Genome Ed. 2022, 4, 863651. [Google Scholar] [CrossRef]
- Sartorius, K.; Antwi, S.O.; Chuturgoon, A.; Roberts, L.R.; Kramvis, A. RNA Therapeutic Options to Manage Aberrant Signaling Pathways in Hepatocellular Carcinoma: Dream or Reality? Front. Oncol. 2022, 12, 891812. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Rapezzi, C.; Franzini, M.; Panichella, G.; Vergaro, G.; Gillmore, J.; Fontana, M.; Passino, C.; Emdin, M. RNA-Targeting and Gene Editing Therapies for Transthyretin Amyloidosis. Nat. Rev. Cardiol. 2022, 19, 655–667. [Google Scholar] [CrossRef]
- Tarn, W.-Y.; Cheng, Y.; Ko, S.-H.; Huang, L.-M. Antisense Oligonucleotide-Based Therapy of Viral Infections. Pharmaceutics 2021, 13, 2015. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Pyrzewicz, W.; Want, A.; Leszek, J.; Wojda, U. Antisense Oligonucleotides for Alzheimer’s Disease Therapy: From the MRNA to MiRNA Paradigm. EBioMedicine 2021, 74, 103691. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nguyen, L.H.; Odisho, A.S.; Maxey, B.S.; Pruitt, J.W.; Girma, B.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. The Antisense Oligonucleotide Nusinersen for Treatment of Spinal Muscular Atrophy. Orthop. Rev. 2021, 13, 24934. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, R.; Feigin, A. Emerging Therapeutics in Huntington’s Disease. Expert Opin. Emerg. Drugs 2021, 26, 295–302. [Google Scholar] [CrossRef]
- Amado, D.A.; Davidson, B.L. Gene Therapy for ALS: A Review. Mol. Ther. 2021, 29, 3345–3358. [Google Scholar] [CrossRef] [PubMed]
- Robson, F.; Khan, K.S.; Le, T.K.; Paris, C.; Demirbag, S.; Barfuss, P.; Rocchi, P.; Ng, W.-L. Coronavirus RNA Proofreading: Molecular Basis and Therapeutic Targeting. Mol. Cell 2020, 79, 710–727. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Aach, J.; Stranges, P.B.; Esvelt, K.M.; Moosburner, M.; Kosuri, S.; Yang, L.; Church, G.M. CAS9 Transcriptional Activators for Target Specificity Screening and Paired Nickases for Cooperative Genome Engineering. Nat. Biotechnol. 2013, 31, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, S.; Kim, J.M.; Kim, J.S. Targeted Genome Engineering in Human Cells with the Cas9 RNA-Guided Endonuclease. Nat. Biotechnol. 2013, 31, 230–232. [Google Scholar] [CrossRef]
- Kuscu, C.; Arslan, S.; Singh, R.; Thorpe, J.; Adli, M. Genome-Wide Analysis Reveals Characteristics of off-Target Sites Bound by the Cas9 Endonuclease. Nat. Biotechnol. 2014, 32, 677–683. [Google Scholar] [CrossRef]
- Zarei, A.; Razban, V.; Hosseini, S.E.; Tabei, S.M.B. Creating Cell and Animal Models of Human Disease by Genome Editing Using CRISPR/Cas9. J. Gene Med. 2019, 21, e3082. [Google Scholar] [CrossRef]
- Karimian, A.; Azizian, K.; Parsian, H.; Rafieian, S.; Shafiei-Irannejad, V.; Kheyrollah, M.; Yousefi, M.; Majidinia, M.; Yousefi, B. CRISPR/Cas9 Technology as a Potent Molecular Tool for Gene Therapy. J. Cell. Physiol. 2019, 234, 12267–12277. [Google Scholar] [CrossRef]
- Chen, M.; Mao, A.; Xu, M.; Weng, Q.; Mao, J.; Ji, J. CRISPR-Cas9 for Cancer Therapy: Opportunities and Challenges. Cancer Lett. 2019, 447, 48–55. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef]
- Tong, H.; Huang, J.; Xiao, Q.; He, B.; Dong, X.; Liu, Y.; Yang, X.; Han, D.; Wang, Z.; Wang, X.; et al. High-Fidelity Cas13 Variants for Targeted RNA Degradation with Minimal Collateral Effects. Nat. Biotechnol. 2023, 41, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.H.; Méndez-Mancilla, A.; Guo, X.; Legut, M.; Daniloski, Z.; Sanjana, N.E. Massively Parallel Cas13 Screens Reveal Principles for Guide RNA Design. Nat. Biotechnol. 2020, 38, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau Staining as a Loading Control Alternative to Actin in Western Blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Goasdoue, K.; Awabdy, D.; Bjorkman, S.T.; Miller, S. Standard Loading Controls Are Not Reliable for Western Blot Quantification across Brain Development or in Pathological Conditions. Electrophoresis 2016, 37, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mendiratta, S.; Ehrhardt, K.; Kashyap, N.; White, M.A.; Bleris, L. Exploiting the CRISPR/Cas9 PAM Constraint for Single-Nucleotide Resolution Interventions. PLoS ONE 2016, 11, e0144970. [Google Scholar] [CrossRef]
- Li, Y.; Nowak, C.M.; Withers, D.; Pertsemlidis, A.; Bleris, L. CRISPR-Based Editing Reveals Edge-Specific Effects in Biological Networks. CRISPR J. 2018, 1, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.M.C.M.; Lawson, S.; Zerez, M.; Bleris, L. Guide RNA Engineering for Versatile Cas9 Functionality. Nucleic Acids Res. 2016, 44, 9555–9564. [Google Scholar] [CrossRef]
- Moore, R.; Spinhirne, A.; Lai, M.J.; Preisser, S.; Li, Y.; Kang, T.; Bleris, L. CRISPR-Based Self-Cleaving Mechanism for Controllable Gene Delivery in Human Cells. Nucleic Acids Res. 2015, 43, 1297–1303. [Google Scholar] [CrossRef]
- Quarton, T.; Kang, T.; Papakis, V.; Nguyen, K.; Nowak, C.; Li, Y.; Bleris, L. Uncoupling Gene Expression Noise along the Central Dogma Using Genome Engineered Human Cell Lines. Nucleic Acids Res. 2020, 48, 9406–9413. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Choe, J.H.; Gadhvi, J.; Kim, Y.J.; Arguez, M.A.; Palmer, M.; Gerold, H.; Nowak, C.; Do, H.; Mazambani, S.; et al. P63 and SOX2 Dictate Glucose Reliance and Metabolic Vulnerabilities in Squamous Cell Carcinomas. Cell Rep. 2019, 28, 1860–1878.e9. [Google Scholar] [CrossRef]
- Davanzo, B.; Emerson, R.E.; Lisy, M.; Koniaris, L.G.; Kays, J.K. Solitary Fibrous Tumor. Transl. Gastroenterol. Hepatol. 2018, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.-P.; Mushayamaha, T.; Thomas, P.D. PANTHER Version 16: A Revised Family Classification, Tree-Based Classification Tool, Enhancer Regions and Extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef] [PubMed]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 Pathway in Tumor-Associated Macrophages Reduces Tumor Growth and Metastatic Niche Formation in Breast Cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef]
- Prakash, T.P.; Yu, J.; Shen, W.; De Hoyos, C.L.; Berdeja, A.; Gaus, H.; Liang, X.-H.; Crooke, S.T.; Seth, P.P. Site-Specific Incorporation of 2’,5’-Linked Nucleic Acids Enhances Therapeutic Profile of Antisense Oligonucleotides. ACS Med. Chem. Lett. 2021, 12, 922–927. [Google Scholar] [CrossRef]
- Shen, W.; De Hoyos, C.L.; Migawa, M.T.; Vickers, T.A.; Sun, H.; Low, A.; Bell, T.A., 3rd; Rahdar, M.; Mukhopadhyay, S.; Hart, C.E.; et al. Chemical Modification of PS-ASO Therapeutics Reduces Cellular Protein-Binding and Improves the Therapeutic Index. Nat. Biotechnol. 2019, 37, 640–650. [Google Scholar] [CrossRef]
- Kamola, P.J.; Maratou, K.; Wilson, P.A.; Rush, K.; Mullaney, T.; McKevitt, T.; Evans, P.; Ridings, J.; Chowdhury, P.; Roulois, A.; et al. Strategies for In Vivo Screening and Mitigation of Hepatotoxicity Associated with Antisense Drugs. Mol. Ther. Nucleic Acids 2017, 8, 383–394. [Google Scholar] [CrossRef]
- Howard, D.B.; Powers, K.; Wang, Y.; Harvey, B.K. Tropism and Toxicity of Adeno-Associated Viral Vector Serotypes 1, 2, 5, 6, 7, 8, and 9 in Rat Neurons and Glia in Vitro. Virology 2008, 372, 24–34. [Google Scholar] [CrossRef]
- Kamerkar, S.; Leng, C.; Burenkova, O.; Jang, S.C.; McCoy, C.; Zhang, K.; Dooley, K.; Kasera, S.; Zi, T.; Sisó, S.; et al. Exosome-Mediated Genetic Reprogramming of Tumor-Associated Macrophages by ExoASO-STAT6 Leads to Potent Monotherapy Antitumor Activity. Sci. Adv. 2022, 8, eabj7002. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; Punt, C.J.A.; Hato, S.V.; Eleveld-Trancikova, D.; Jansen, B.J.H.; Nierkens, S.; Schreibelt, G.; de Boer, A.; Van Herpen, C.M.L.; Kaanders, J.H.; et al. Platinum-Based Drugs Disrupt STAT6-Mediated Suppression of Immune Responses against Cancer in Humans and Mice. J. Clin. Investig. 2011, 121, 3100–3108. [Google Scholar] [CrossRef]
- Haselager, M.V.; Thijssen, R.; Bax, D.; Both, D.; De Boer, F.; Mackay, S.; Dubois, J.; Mellink, C.; Kater, A.P.; Eldering, E. JAK-STAT Signaling Shapes the NF-ΚB Response in CLL towards Venetoclax Sensitivity or Resistance via Bcl-XL. Mol. Oncol. 2022. [Google Scholar] [CrossRef]
- Shi, P.; Murphy, M.R.; Aparicio, A.O.; Kesner, J.S.; Fang, Z.; Chen, Z.; Trehan, A.; Guo, Y.; Wu, X. Collateral Activity of the CRISPR/RfxCas13d System in Human Cells. Commun. Biol. 2023, 6, 334. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Guo, X.; Li, Z.; Cao, L.; Liu, S.; Guo, Y.; Wang, G.; Luo, Y.; Zhang, Z.; et al. The Collateral Activity of RfxCas13d Can Induce Lethality in a RfxCas13d Knock-in Mouse Model. Genome Biol. 2023, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Z.E.; Tan, S.X.; Hoon, S.; Yeo, G.W. Pre-Existing Adaptive Immunity to the RNA-Editing Enzyme Cas13d in Humans. Nat. Med. 2022, 28, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Farhang Ghahremani, M.; Goossens, S.; Nittner, D.; Bisteau, X.; Bartunkova, S.; Zwolinska, A.; Hulpiau, P.; Haigh, K.; Haenebalcke, L.; Drogat, B.; et al. P53 Promotes VEGF Expression and Angiogenesis in the Absence of an Intact P21-Rb Pathway. Cell Death Differ. 2013, 20, 888–897. [Google Scholar] [CrossRef]
- Okawa, T.; Michaylira, C.Z.; Kalabis, J.; Stairs, D.B.; Nakagawa, H.; Andl, C.D.; Johnstone, C.N.; Klein-Szanto, A.J.; El-Deiry, W.S.; Cukierman, E.; et al. The Functional Interplay between EGFR Overexpression, HTERT Activation, and P53 Mutation in Esophageal Epithelial Cells with Activation of Stromal Fibroblasts Induces Tumor Development, Invasion, and Differentiation. Genes Dev. 2007, 21, 2788–2803. [Google Scholar] [CrossRef]
- Katerji, R.; Agostini-Vulaj, D. Solitary Fibrous Tumor Presenting as a Colonic Polyp: Report of a Case and Literature Review. Hum. Pathol. Case Rep. 2021, 25, 200547. [Google Scholar] [CrossRef]
- Ghanim, B.; Baier, D.; Pirker, C.; Müllauer, L.; Sinn, K.; Lang, G.; Hoetzenecker, K.; Berger, W. Trabectedin Is Active against Two Novel, Patient-Derived Solitary Fibrous Pleural Tumor Cell Lines and Synergizes with Ponatinib. Cancers 2022, 14, 5602. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/MTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Mondaza-Hernandez, J.L.; Moura, D.S.; Lopez-Alvarez, M.; Sanchez-Bustos, P.; Blanco-Alcaina, E.; Castilla-Ramirez, C.; Collini, P.; Merino-Garcia, J.; Zamora, J.; Carrillo-Garcia, J.; et al. ISG15 as a Prognostic Biomarker in Solitary Fibrous Tumour. Cell. Mol. Life Sci. 2022, 79, 434. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Nguyen, J.T.; Ammanamanchi, M.; Zhou, Z.; Harbut, E.F.; Mondaza-Hernandez, J.L.; Meyer, C.A.; Moura, D.S.; Martin-Broto, J.; Hayenga, H.N.; et al. Reduction of Tumor Growth with RNA-Targeting Treatment of the NAB2–STAT6 Fusion Transcript in Solitary Fibrous Tumor Models. Cancers 2023, 15, 3127. https://doi.org/10.3390/cancers15123127
Li Y, Nguyen JT, Ammanamanchi M, Zhou Z, Harbut EF, Mondaza-Hernandez JL, Meyer CA, Moura DS, Martin-Broto J, Hayenga HN, et al. Reduction of Tumor Growth with RNA-Targeting Treatment of the NAB2–STAT6 Fusion Transcript in Solitary Fibrous Tumor Models. Cancers. 2023; 15(12):3127. https://doi.org/10.3390/cancers15123127
Chicago/Turabian StyleLi, Yi, John T. Nguyen, Manasvini Ammanamanchi, Zikun Zhou, Elijah F. Harbut, Jose L. Mondaza-Hernandez, Clark A. Meyer, David S. Moura, Javier Martin-Broto, Heather N. Hayenga, and et al. 2023. "Reduction of Tumor Growth with RNA-Targeting Treatment of the NAB2–STAT6 Fusion Transcript in Solitary Fibrous Tumor Models" Cancers 15, no. 12: 3127. https://doi.org/10.3390/cancers15123127
APA StyleLi, Y., Nguyen, J. T., Ammanamanchi, M., Zhou, Z., Harbut, E. F., Mondaza-Hernandez, J. L., Meyer, C. A., Moura, D. S., Martin-Broto, J., Hayenga, H. N., & Bleris, L. (2023). Reduction of Tumor Growth with RNA-Targeting Treatment of the NAB2–STAT6 Fusion Transcript in Solitary Fibrous Tumor Models. Cancers, 15(12), 3127. https://doi.org/10.3390/cancers15123127






