External-Beam-Accelerated Partial-Breast Irradiation Reduces Organ-at-Risk Doses Compared to Whole-Breast Irradiation after Breast-Conserving Surgery
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
3.1. Contralateral Breast
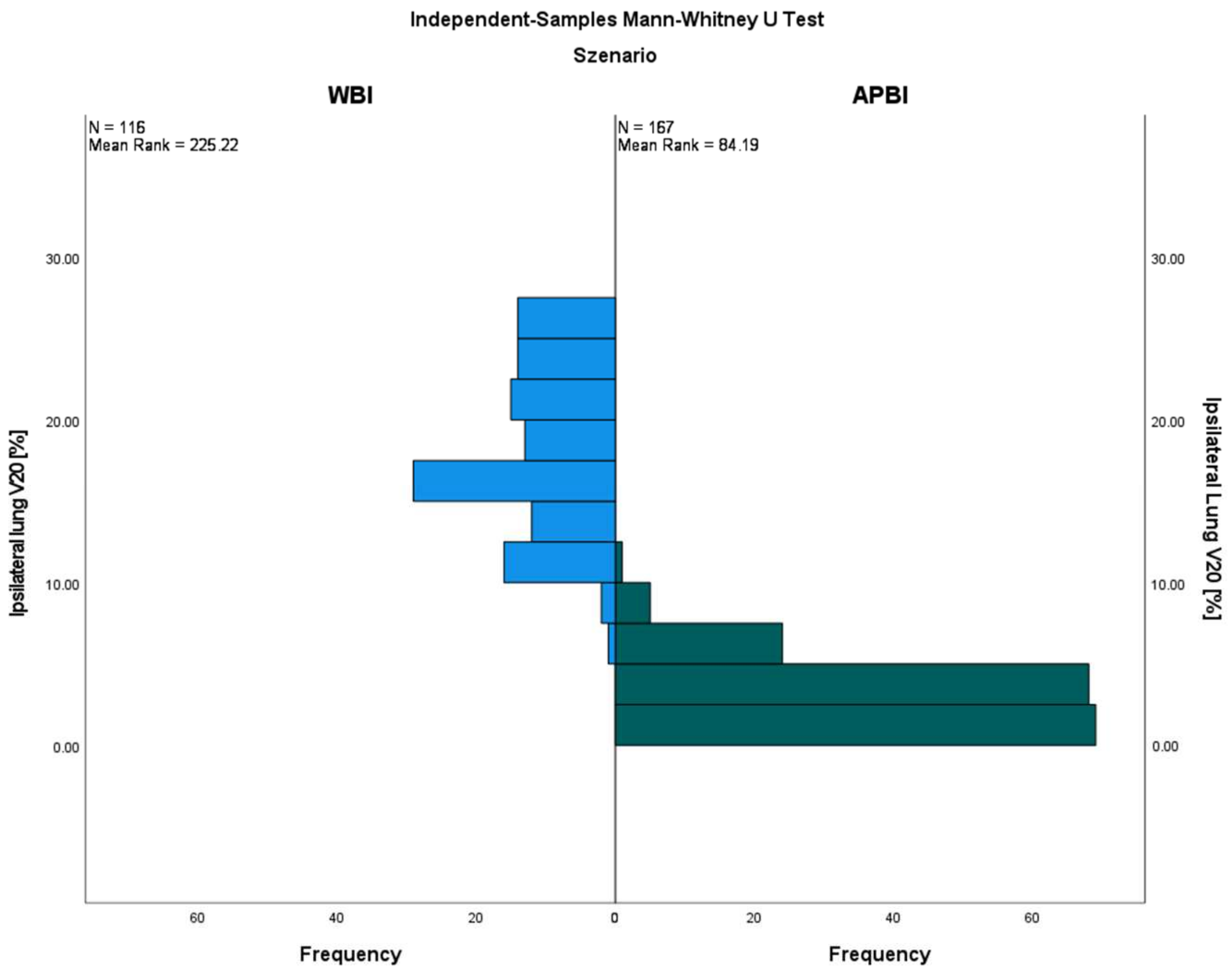
3.2. Lungs
3.3. Heart
3.4. Spinal Cord and Skin
4. Discussion
4.1. Target Volumes
4.2. Contralateral Breast
4.3. Lungs
4.4. Heart
4.5. Spinal Cord and Skin
4.6. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda, F.A.; Teixeira, L.A.B.; Heinzen, R.N.; de Andrade, F.E.M.; Hijal, T.; Buchholz, T.A.; Moraes, F.Y.; Poortmans, P.; Marta, G.N. Accelerated partial breast irradiation: Current status with a focus on clinical practice. Breast J. 2019, 25, 124–128. [Google Scholar] [CrossRef]
- Strnad, V.; Krug, D.; Sedlmayer, F.; Piroth, M.D.; Budach, W.; Baumann, R.; Feyer, P.; Duma, M.N.; Haase, W.; Harms, W.; et al. DEGRO practical guideline for partial-breast irradiation. Strahlenther. Onkol. 2020, 196, 749–763. [Google Scholar] [CrossRef]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef]
- Polat, B.; Arribas, L.; Fietkau, R.; Kauer-Dorner, D.; Guinot, J.L.; Kulik, A.; Strnad, V.; Hindemith, M.; Fischedick, A.-R.; Uter, W.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar] [CrossRef]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; Ganz, P.A.; Parda, D.S.; Scheier, M.F.; et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef]
- Ott, O.J.; Strnad, V.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Łyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. GEC-ESTRO multicenter phase 3-trial: Accelerated partial breast irradiation with interstitial multicatheter brachytherapy versus external beam whole breast irradiation: Early toxicity and patient compliance. Radiother. Oncol. 2016, 120, 119–123. [Google Scholar] [CrossRef]
- Shah, C.; Jia, X.; Hobbs, B.P.; Tendulkar, R.D.; Sittenfeld, S.M.C.; Al-Hilli, Z.; Arthur, D.W.; Keisch, M.E.; Khan, A.J.; Shaitelman, S.F.; et al. Outcomes with Partial Breast Irradiation vs. Whole Breast Irradiation: A Meta-Analysis. Ann. Surg. Oncol. 2021, 28, 4985–4994. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Ott, O.J.; Stillkrieg, W.; Lambrecht, U.; Sauer, T.-O.; Schweizer, C.; Lamrani, A.; Strnad, V.; Hack, C.C.; Beckmann, M.W.; Uder, M.; et al. External Beam Accelerated Partial Breast Irradiation in Early Breast Cancer and the Risk for Radiogenic Pneumonitis. Cancers 2022, 14, 3520. [Google Scholar] [CrossRef]
- Ott, O.J.; Strnad, V.; Stillkrieg, W.; Uter, W.; Beckmann, M.W.; Fietkau, R. Accelerated partial breast irradiation with external beam radiotherapy: First results of the German phase 2 trial. Strahlenther Onkol 2017, 193, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Major, T.; Lössl, K.; Van Limbergen, E.; Resch, A.; Kauer-Dorner, D.; Guinot, J.-L.; Kovács, G.; Strnad, V.; Hannoun-Levi, J.-M. Recommendations from GEC ESTRO Breast Cancer Working Group (I): Target definition and target delineation for accelerated or boost Partial Breast Irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother. Oncol. 2015, 115, 342–348. [Google Scholar] [CrossRef]
- Duane, F.; Aznar, M.C.; Bartlett, F.; Cutter, D.J.; Darby, S.C.; Jagsi, R.; Lorenzen, E.L.; McArdle, O.; McGale, P.; Myerson, S.; et al. A cardiac contouring atlas for radiotherapy. Radiother. Oncol. 2017, 122, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.4, 2021, AWMF Registernummer: 032-045OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (accessed on 28 October 2021).
- Sauer, T.-O.; Ott, O.J.; Lahmer, G.; Fietkau, R.; Bert, C. Prerequisites for the clinical implementation of a markerless SGRT-only workflow for the treatment of breast cancer patients. Strahlenther. Onkol. 2022, 199, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Sola, A.B.; Kirova, Y.M.; Pignol, J.-P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1. Radiother. Oncol. 2016, 118, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N.; Kulms, T.; Knippen, S.; Teichmann, T.; Wittig, A. Breast clinical target volume: HU-based glandular CTVs and ESTRO CTVs in modern and historical radiotherapy treatment planning. Strahlenther. Onkol. 2022, 198, 229–235. [Google Scholar] [CrossRef]
- Offersen, B.V.; Alsner, J.; Nielsen, H.M.; Jakobsen, E.H.; Nielsen, M.H.; Stenbygaard, L.; Pedersen, A.N.; Thomsen, M.S.; Yates, E.; Berg, M.; et al. Partial Breast Irradiation Versus Whole Breast Irradiation for Early Breast Cancer Patients in a Randomized Phase III Trial: The Danish Breast Cancer Group Partial Breast Irradiation Trial. J. Clin. Oncol. 2022, 40, 4189–4197. [Google Scholar] [CrossRef]
- Peterson, D.; Truong, P.T.; Parpia, S.; Olivotto, I.A.; Berrang, T.; Kim, D.-H.; Kong, I.; Germain, I.; Nichol, A.; Akra, M.; et al. Predictors of adverse cosmetic outcome in the RAPID trial: An exploratory analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 968–976. [Google Scholar] [CrossRef]
- Rodríguez, N.; Sanz, X.; Dengra, J.; Foro, P.; Membrive, I.; Reig, A.; Quera, J.; Fernández-Velilla, E.; Pera, Ó.; Lio, J.; et al. Five-Year Outcomes, Cosmesis, and Toxicity With 3-Dimensional Conformal External Beam Radiation Therapy to Deliver Accelerated Partial Breast Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 1051–1057. [Google Scholar] [CrossRef]
- Mészáros, N.; Major, T.; Stelczer, G.; Zaka, Z.; Mózsa, E.; Pukancsik, D.; Takácsi-Nagy, Z.; Fodor, J.; Polgár, C. Implementation of image-guided intensity-modulated accelerated partial breast irradiation: Three-year results of a phase II clinical study. Strahlenther. Onkol. 2017, 193, 70–79. [Google Scholar] [CrossRef]
- Roychoudhuri, R.; Evans, H.; Robinson, D.; Moller, H. Radiation-induced malignancies following radiotherapy for breast cancer. Br. J. Cancer 2004, 91, 868–872. [Google Scholar] [CrossRef]
- Gao, X.; Fisher, S.G.; Emami, B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D.; Shore, R.E.; Andersson, M.; Buchholz, T.A.; Capanu, M.; Bernstein, L.; Lynch, C.F.; et al. Faculty Opinions recommendation of Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef]
- Borghero, Y.O.; Salehpour, M.; McNeese, M.D.; Stovall, M.; Smith, S.A.; Johnson, J.; Perkins, G.H.; Strom, E.A.; Oh, J.L.; Kirsner, S.M.; et al. Multileaf field-in-field forward-planned intensity-modulated dose compensation for whole-breast irradiation is associated with reduced contralateral breast dose: A phantom model comparison. Radiother. Oncol. 2007, 82, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Ogino, I.; Seto, H.; Shigenaga, D.; Hata, M. Dose to contralateral breast from whole breast irradiation by automated tangential IMRT planning: Comparison of flattening-filter and flattening-filter-free modes. Rep. Pr. Oncol. Radiother. 2022, 27, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Chang, J.S.; Moon, J.Y.; Lee, W.H.; Shah, C.; Shim, J.S.; Han, M.C.; Baek, J.G.; Park, R.H.; Kim, Y.B.; et al. Dosimetric Comparison of Radiation Techniques for Comprehensive Regional Nodal Radiation Therapy for Left-Sided Breast Cancer: A Treatment Planning Study. Front. Oncol. 2021, 11, 645328. [Google Scholar] [CrossRef]
- Jones, J.; Muttath, G.; Yahiya, N.; Nawaz, S.; Narendran, A.P.; Reja, A. Organ at risk doses in hypo fractionated three dimensional conformal radiotherapy in carcinoma breast following breast conservation surgery. Oncol. Radiother. 2020, 52, 1–3. [Google Scholar]
- Williams, T.M.; Moran, J.M.; Hsu, S.-H.; Marsh, R.; Yanke, B.; Fraass, B.A.; Pierce, L.J. Contralateral Breast Dose After Whole-Breast Irradiation: An Analysis by Treatment Technique. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 2079–2085. [Google Scholar] [CrossRef]
- Rather, S.A.; Haq, M.M.-U.; Khan, N.A.; Khan, A.A.; Sofi, A. Determining the contralateral breast dose during radiotherapy of breast cancer using rainbow dosimeter. J. Radiat. Res. Appl. Sci. 2014, 7, 384–389. [Google Scholar] [CrossRef]
- Boice, J.D.; Harvey, E.B.; Blettner, M.; Stovall, M.; Flannery, J.T. Cancer in the Contralateral Breast after Radiotherapy for Breast Cancer. New Engl. J. Med. 1992, 326, 781–785. [Google Scholar] [CrossRef]
- Bhatnagar, A.K.; Heron, D.E.; Deutsch, M.; Brandner, E.; Wu, A.; Kalnicki, S. Does breast size affect the scatter dose to the ipsilateral lung, heart, or contralateral breast in primary breast irradiation using intensity-modulated radiation therapy (IMRT)? Am. J. Clin. Oncol. 2006, 29, 80–84. [Google Scholar] [CrossRef]
- Tolia, M.; Platoni, K.; Foteineas, A.; Kalogeridi, M.-A.; Zygogianni, A.; Tsoukalas, N.; Caimi, M.; Margari, N.; Dilvoi, M.; Pantelakos, P.; et al. Assessment of contralateral mammary gland dose in the treatment of breast cancer using accelerated hypofractionated radiotherapy. World J. Radiol. 2011, 3, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zurl, B.; Stranzl, H.; Winkler, P.; Kapp, K.S. Quantification of contralateral breast dose and risk estimate of radiation-induced contralateral breast cancer among young women using tangential fields and different modes of breathing. Int. J. Radiat. Oncol. 2013, 85, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Johansen, S.; Cozzi, L.; Olsen, D.R. A planning comparison of dose patterns in organs at risk and predicted risk for radiation induced malignancy in the contralateral breast following radiation therapy of primary breast using conventional, IMRT and Volumetric modulated arc treatment techniques. Acta Oncol. 2009, 48, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.C.; Nelson, C.L.; Bloom, E.S.; Kisling, K.; Mason, B.E.; Fisher, G.D.; Kirsner, S.M. Contralateral breast dose from partial breast brachytherapy. J. Appl. Clin. Med. Phys. 2015, 16, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pignol, J.-P.; Keller, B.M.; Ravi, A. Doses to internal organs for various breast radiation techniques-implications on the risk of secondary cancers and cardiomyopathy. Radiat. Oncol. 2011, 6, 5. [Google Scholar] [CrossRef]
- Grantzau, T.; Overgaard, J. Risk of second non-breast cancer after radiotherapy for breast cancer: A systematic review and meta-analysis of 762,468 patients. Radiother. Oncol. 2015, 114, 56–65. [Google Scholar] [CrossRef]
- Aznar, M.C.; Duane, F.K.; Darby, S.C.; Wang, Z.; Taylor, C.W. Exposure of the lungs in breast cancer radiotherapy: A systematic review of lung doses published 2010–2015. Radiother. Oncol. 2018, 126, 148–154. [Google Scholar] [CrossRef]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef]
- Beaton, L.; Bergman, A.; Nichol, A.; Aparicio, M.; Wong, G.; Gondara, L.; Speers, C.; Weir, L.; Davis, M.; Tyldesley, S. Cardiac death after breast radiotherapy and the QUANTEC cardiac guidelines. Clin. Transl. Radiat. Oncol. 2019, 19, 39–45. [Google Scholar] [CrossRef]
- Correa, C.R.; Litt, H.I.; Hwang, W.T.; Ferrari, V.A.; Solin, L.J.; Harris, E.E. Faculty Opinions recommendation of Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J. Clin. Oncol. 2007, 25, 3031–3037. [Google Scholar] [CrossRef]
- Taylor, C.W.; Wang, Z.; Macaulay, E.; Jagsi, R.; Duane, F.; Darby, S.C. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-W.; Hsueh, H.-P.; Liu, W.-S. Cardiac dosage comparison among whole breast irradiation and partial breast irradiation techniques. Ther. Radiol. Oncol. 2021, 6, 3. [Google Scholar] [CrossRef]
- Piroth, M.D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther. Onkol. 2019, 195, 1–12. [Google Scholar] [CrossRef]
- Waheed, A.; Butt, S.; Ishtiaq, A.; Mansha, M.A.; Mehreen, S.; Raza, M.; Yousaf, M. Dosimetric Comparison of Whole Breast Radiotherapy Using Field-in-Field and Volumetric Modulated Arc Therapy Techniques in Left-Sided Breast Cancer Patients. Cureus 2021, 13, e15732. [Google Scholar] [CrossRef]
- Dumane, V.A.; Bakst, R.; Green, S. Dose to organs in the supraclavicular region when covering the Internal Mammary Nodes (IMNs) in breast cancer patients: A comparison of Volumetric Modulated Arc Therapy (VMAT) versus 3D and VMAT. PLoS ONE 2018, 13, e0205770. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Badiyan, S.; Berry, S.; Khan, A.; Goyal, S.; Schulte, K.; Nanavati, A.; Lynch, M.; Vicini, F.A. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother. Oncol. 2014, 112, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, N.; Fleury, E.; Lara, T.R.M.; van der Baan, P.; Bahnerth, A.; Struik, G.; Hoogeman, M.; Pignol, J.-P. Long-term risks of secondary cancer for various whole and partial breast irradiation techniques. Radiother. Oncol. 2018, 128, 428–433. [Google Scholar] [CrossRef]
| APBI (n = 170) | WBI (n = 116) | p-Value | |
|---|---|---|---|
| Median age (range) | 62 (48–86) | 56 (30–81) | <0.001 |
| Lateralization [n/N (%)] | |||
| 84/170 (49) | 61/116 (53) | n.s. |
| 86/170 (51) | 55/116 (47) | |
| Tumor site [n/N (%)] | |||
| 100/170 (59) | 66/116 (57) | n.s. |
| 70/170 (41) | 49/116 (42) | |
| - | 1/116 (1) | |
| Radiotherapy technique [n/N (%)] | |||
| 170/170 (100) | - | - |
| - | 62/116 (53) | |
| - | 54/116 (47) | |
| Breathing technique [n/N (%)] | |||
| 169/170 (99) | - | - |
| 1/170 (1) | 86/116 (74) | |
| - | 30/116 (26) | |
| Radiotherapy dose regimen [n/N (%)] | |||
| 170/170 (100) | - | - |
| - | 60/116 (52) | |
| - | 55/116 (47) | |
| - | 1/116 (1) | |
| Mean PTVeval/PTV size [ml ± SD] | 178 ± 86 | 836 ± 451 | <0.001 |
| Left-Sided Breast Cancer | Right-Sided Breast Cancer | All Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| APBI (n = 84) | WBI (n = 61) | p-Value | APBI (n = 86) | WBI (n = 55) | p-Value | APBI (n = 170) | WBI (n = 116) | p-Value | |
| Contralateral breast | |||||||||
| 3.2 ± 5.7 | 8.2 ± 10.3 | <0.001 | 3.3 ± 7.0 | 13.6 ± 13.5 | <0.001 | 3.2 ± 6.4 | 10.8 ± 12.2 | 0.000 |
| 0.4 ± 0.6 | 0.7 ± 0.5 | <0.001 | 0.4 ± 0.7 | 0.9 ± 1.2 | <0.001 | 0.4 ± 0.6 | 0.8 ± 0.9 | 0.000 |
| Ipsilateral lung | |||||||||
| 4.9 ± 1.8 | 7.6 ± 4.0 | <0.001 | 5.0 ± 1.9 | 7.7 ± 3.5 | <0.001 | 5.0 ± 1.9 | 7.7 ± 3.7 | <0.001 |
| 29.8 ± 12.6 | 35.1 ± 10.4 | 0.010 | 30.3 ± 13.5 | 36.1 ± 9.3 | 0.005 | 30.1 ± 13.1 | 35.6 ± 9.9 | <0.001 |
| 10.8 ± 5.9 | 25.0 ± 7.1 | 0.000 | 10.1 ± 5.3 | 25.2 ± 6.5 | 0.000 | 10.5 ± 5.6 | 25.1 ± 6.8 | 0.000 |
| 3.1 ± 2.2 | 17.9 ± 5.2 | 0.000 | 2.9 ± 1.9 | 17.9 ± 4.5 | 0.000 | 3.0 ± 2.0 | 17.9 ± 4.9 | 0.000 |
| 1.1 ± 1.0 | 13.3 ± 4.5 | 0.000 | 1.0 ± 0.9 | 13.3 ± 3.9 | 0.000 | 1.0 ± 1.0 | 13.3 ± 4.2 | 0.000 |
| 4.3 ± 1.4 | 9.1 ± 2.7 | 0.000 | 4.3 ± 1.4 | 9.2 ± 2.3 | 0.000 | 4.3 ± 1.4 | 9.2 ± 2.5 | 0.000 |
| 28.4 ± 7.0 | 44.2 ± 5.6 | 0.000 | 28.6 ± 7.0 | 43.6 ± 5.1 | 0.000 | 28.5 ± 7.0 | 43.9 ± 5.3 | 0.000 |
| 24.4 ± 7.2 | 43.1 ± 5.5 | 0.000 | 24.3 ± 7.1 | 42.6 ± 4.9 | 0.000 | 24.3 ± 7.1 | 42.9 ± 5.2 | 0.000 |
| Contralateral lung | |||||||||
| 1.9 ± 1.6 | 1.4 ± 1.4 | n.s. | 1.4 ± 1.2 | 1.5 ± 1.6 | n.s. | 1.6 ± 1.4 | 1.4 ± 1.5 | n.s. |
| 0.6 ± 2.2 | 1.2 ± 6.1 | n.s. | 0.6 ± 3.4 | 1.6 ± 7.7 | n.s. | 0.6 ± 2.9 | 1.4 ± 6.9 | n.s. |
| 0.1 ± 0.3 | 0.1 ± 0.4 | n.s. | 0.1 ± 0.4 | 0.1 ± 0.8 | n.s. | 0.1 ± 0.4 | 0.1 ± 0.6 | n.s. |
| 0.7 ± 0.6 | 0.6 ± 0.7 | n.s. | 0.5 ± 0.6 | 0.6 ± 0.8 | n.s. | 0.6 ± 0.6 | 0.6 ± 0.7 | n.s. |
| 2.6 ± 2.4 | 3.3 ± 5.4 | n.s. | 2.1 ± 2.4 | 2.7 ± 2.9 | n.s. | 2.4 ± 2.4 | 3.0 ± 4.4 | n.s. |
| 2.3 ± 2.0 | 2.6 ± 5.1 | n.s. | 1.8 ± 1.9 | 2.2 ± 2.3 | n.s. | 2.1 ± 2.0 | 2.4 ± 4.0 | n.s. |
| Total lung | |||||||||
| 13.7 ± 5.6 | 16.7 ± 7.1 | 0.013 | 17.1 ± 7.8 | 20.5 ± 7.9 | 0.018 | 15.4 ± 7.0 | 18.5 ± 7.7 | <0.001 |
| 5.0 ± 2.6 | 11.5 ± 3.3 | 0.000 | 5.6 ± 3.1 | 13.8 ± 4.1 | 0.000 | 5.3 ± 2.8 | 12.6 ± 3.9 | 0.000 |
| 1.5 ± 1.0 | 8.2 ± 2.3 | 0.000 | 1.6 ± 1.1 | 9.8 ± 2.6 | 0.000 | 1.6 ± 1.1 | 9.0 ± 2.6 | 0.000 |
| 0.5 ± 0.5 | 6.1 ± 2.1 | 0.000 | 0.6 ± 0.6 | 7.2 ± 2.3 | 0.000 | 0.5 ± 0.6 | 6.6 ± 2.2 | 0.000 |
| 2.3 ± 0.7 | 4.5 ± 1.4 | 0.000 | 2.6 ± 0.9 | 5.3 ± 1.5 | 0.000 | 2.5 ± 0.8 | 4.9 ± 1.5 | 0.000 |
| 24.7 ± 7.2 | 43.3 ± 5.5 | 0.000 | 26.1 ± 7.2 | 43.0 ± 5.0 | 0.000 | 25.4 ± 7.3 | 43.2 ± 5.2 | 0.000 |
| 19.0 ± 6.9 | 41.4 ± 5.4 | 0.000 | 19.8 ± 6.8 | 41.5 ± 5.0 | 0.000 | 19.4 ± 6.8 | 41.4 ± 5.2 | 0.000 |
| Heart | |||||||||
| 5.9 ± 4.5 | 6.9 ± 6.8 | n.s. | 2.7 ± 2.6 | 2.4 ± 2.3 | n.s. | 4.3 ± 3.9 | 4.7 ± 5.6 | n.s. |
| 2.2 ± 1.8 | 2.3 ± 1.4 | n.s. | 1.0 ± 1.1 | 1.1 ± 1.1 | 0.029 | 1.6 ± 1.6 | 1.7 ± 1.4 | 0.007 |
| 18.6 ± 25.6 | 10.0 ± 12.2 | n.s. | 1.2 ± 8.3 | 1.6 ± 10.0 | n.s. | 9.9 ± 20.8 | 6.0 ± 11.9 | n.s. |
| 0.2 ± 0.5 | 2.6 ± 3.2 | <0.001 | - | - | - | 0.1 ± 0.4 | 1.4 ± 2.6 | <0.001 |
| 2.7 ± 2.5 | 3.3 ± 1.9 | 0.007 | 0.6 ± 0.9 | 0.7 ± 0.9 | <0.001 | 1.6 ± 2.1 | 2.0 ± 2.0 | <0.001 |
| 9.3 ± 7.8 | 24.7 ± 16.8 | <0.001 | 1.4 ± 1.8 | 1.1 ± 1.6 | n.s. | 5.3 ± 6.9 | 13.5 ± 17.0 | 0.002 |
| 8.2 ± 6.7 | 21.0 ± 16.7 | <0.001 | 1.3 ± 1.7 | 1.1 ± 1.5 | n.s. | 4.7 ± 6.0 | 11.5 ± 15.7 | 0.003 |
| 10.5 ± 20.9 | 19.1 ± 15.4 | <0.001 | 0.8 ± 5.3 | 0.5 ± 2.8 | n.s. | 5.6 ± 15.9 | 10.3 ± 14.6 | <0.001 |
| 2.3 ± 7.9 | 13.3 ± 14.2 | <0.001 | - | - | - | 1.1 ± 5.6 | 7.0 ± 12.2 | <0.001 |
| 0.7 ± 4.9 | 10.3 ± 12.4 | <0.001 | - | - | - | 0.3 ± 3.5 | 5.4 ± 10.6 | <0.001 |
| - | 5.8 ± 11.2 | <0.001 | - | - | - | - | 3.1 ± 8.6 | <0.001 |
| 4.3 ± 4.0 | 8.3 ± 5.6 | <0.001 | 0.8 ± 1.3 | 1.0 ± 1.3 | <0.001 | 2.5 ± 3.4 | 4.8 ± 5.5 | <0.001 |
| 9.2 ± 8.9 | 29.9 ± 15.6 | <0.001 | 1.8 ± 2.5 | 1.4 ± 2.1 | n.s. | 5.5 ± 7.5 | 16.4 ± 18.3 | <0.001 |
| 8.8 ± 8.5 | 28.5 ± 16.2 | <0.001 | 1.7 ± 2.4 | 1.4 ± 2.1 | n.s. | 5.2 ± 7.2 | 15.6 ± 18.0 | <0.001 |
| Others | |||||||||
| 1.6 ± 1.2 | 4.3 ± 5.1 | n.s. | 1.3 ± 0.9 | 4.6 ± 6.3 | n.s. | 1.5 ± 1.1 | 4.5 ± 5.7 | 0.016 |
| 39.8 ± 2.2 | 49.3 ± 5.8 | 0.000 | 39.4 ± 1.3 | 48.8 ± 5.8 | 0.000 | 39.6 ± 1.8 | 49.1 ± 5.8 | 0.000 |
| 35.6 ± 3.4 | 47.4 ± 5.4 | 0.000 | 35.8 ± 2.9 | 47.0 ± 5.4 | 0.000 | 35.7 ± 3.2 | 47.2 ± 5.4 | 0.000 |
| 31.7 ± 5.2 | 46.8 ± 5.4 | 0.000 | 32.1 ± 4.8 | 46.3 ± 5.3 | 0.000 | 31.9 ± 5.0 | 46.6 ± 5.3 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ott, O.J.; Stillkrieg, W.; Lambrecht, U.; Schweizer, C.; Lamrani, A.; Sauer, T.-O.; Strnad, V.; Bert, C.; Hack, C.C.; Beckmann, M.W.; et al. External-Beam-Accelerated Partial-Breast Irradiation Reduces Organ-at-Risk Doses Compared to Whole-Breast Irradiation after Breast-Conserving Surgery. Cancers 2023, 15, 3128. https://doi.org/10.3390/cancers15123128
Ott OJ, Stillkrieg W, Lambrecht U, Schweizer C, Lamrani A, Sauer T-O, Strnad V, Bert C, Hack CC, Beckmann MW, et al. External-Beam-Accelerated Partial-Breast Irradiation Reduces Organ-at-Risk Doses Compared to Whole-Breast Irradiation after Breast-Conserving Surgery. Cancers. 2023; 15(12):3128. https://doi.org/10.3390/cancers15123128
Chicago/Turabian StyleOtt, Oliver J., Wilhelm Stillkrieg, Ulrike Lambrecht, Claudia Schweizer, Allison Lamrani, Tim-Oliver Sauer, Vratislav Strnad, Christoph Bert, Carolin C. Hack, Matthias W. Beckmann, and et al. 2023. "External-Beam-Accelerated Partial-Breast Irradiation Reduces Organ-at-Risk Doses Compared to Whole-Breast Irradiation after Breast-Conserving Surgery" Cancers 15, no. 12: 3128. https://doi.org/10.3390/cancers15123128
APA StyleOtt, O. J., Stillkrieg, W., Lambrecht, U., Schweizer, C., Lamrani, A., Sauer, T.-O., Strnad, V., Bert, C., Hack, C. C., Beckmann, M. W., & Fietkau, R. (2023). External-Beam-Accelerated Partial-Breast Irradiation Reduces Organ-at-Risk Doses Compared to Whole-Breast Irradiation after Breast-Conserving Surgery. Cancers, 15(12), 3128. https://doi.org/10.3390/cancers15123128








