‘Earlier than Early’ Detection of Breast Cancer in Israeli BRCA Mutation Carriers Applying AI-Based Analysis to Consecutive MRI Scans
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Images, Clinical, Radiological and Pathological Data Analysis
2.3. Lesion Segmentation and Morphological/Kinetic Assessment
2.4. Convolutional Neural Network (CNN) Architecture
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoniou, A.; Pharoah, P.D.P.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average Risks of Breast and Ovarian Cancer Associated with BRCA1 or BRCA2 Mutations Detected in Case Series Unselected for Family History: A Combined Analysis of 22 Studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef]
- Chen, S.; Parmigiani, G. Meta-Analysis of BRCA1 and BRCA2 Penetrance. J. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- You, C.; Xiao, Q.; Zhu, X.; Sun, Y.; Di, G.; Liu, G.; Hou, Y.; Chen, C.; Wu, J.; Shao, Z.; et al. The Clinicopathological and MRI Features of Patients with BRCA1/2 Mutations in Familial Breast Cancer. Gland. Surg. 2021, 10, 262–272. [Google Scholar] [CrossRef]
- Tilanus-linthorst, M.M.A.; Obdeijn, I.; Hop, W.C.J.; Causer, P.A.; Leach, M.O.; Pointon, L.; Hill, K.; Klijn, J.G.M.; Warren, R.M.L.; Gilbert, F.J. BRCA1Mutation and Young Age Predict Fast Breast Cancer Growth in the Dutch, United Kingdom, and Canadian Magnetic Resonance Imaging Screening TRials. Clin. Cancer Res. 2007, 13, 7357–7362. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal Evolution in Cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Elezaby, M.; Lees, B.; Maturen, K.E.; Barroilhet, L.; Wisinski, K.B.; Schrager, S.; Wilke, L.G.; Sadowski, E. BRCA Mutation Carriers: Breast and Ovarian Cancer Screening Guidelines and Imaging Considerations. Radiology 2019, 291, 554–569. [Google Scholar] [CrossRef]
- Bernstein-molho, R.; Kaufman, B.; Ben, M.A.; Sklair-levy, M.; Madoursky, D.; Zippel, D.; Laitman, Y.; Friedman, E. Breast Cancer Surveillance for BRCA1/2 Mutation Carriers—Is “Early Detection” Early Enough? Breast 2020, 49, 81–86. [Google Scholar] [CrossRef]
- Guindalini, R.S.; Zheng, Y.; Abe, H.; Whitaker, K.; Toshio, F.; Walsh, T.; Schacht, D.; Kulkarni, K.; Sheth, D.; Verp, M.S.; et al. Intensive Surveillance with Biannual Dynamic Contrast-Enhanced Magnetic Resonance Imaging Downstages Breast Cancer in BRCA1 Mutation Carriers. Clin. Cancer Res. 2020, 25, 1786–1794. [Google Scholar] [CrossRef]
- Shraga, S.; Grinshpun, A.; Zick, A.; Kadouri, L.; Cohen, Y.; Maimon, O.; Adler-Levy, Y.; Zeltzer, G.; Granit, A.; Maly, B.; et al. High-Risk Breast Cancer Screening in BRCA1/2 Carriers Leads to Early Detection and Improved Survival After a Breast Cancer Diagnosis. Front. Oncol. 2021, 11, 683656. [Google Scholar] [CrossRef]
- Kriege, M.; Brekelmans, C.T.M.; Boetes, C.; Besnard, P.; Zonderland, H.M.; Obdeijn, I.; Manoliu, R.; Kok, T.; Peterse, H.L.; Tilanus-linthorst, M.M.; et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef]
- Lo, G.; Scaranelo, A.M.; Aboras, H.; Ghai, S.; Kulkarni, S.; Fleming, R.; Bukhanov, K.; Crystal, P. Evaluation of the Utility of Screening Mammography for High-Risk Women Undergoing Screening Breast MR Imaging. Radiology 2017, 285, 36–43. [Google Scholar] [CrossRef]
- Warner, E.; Hill, K.; Causer, P.; Plewes, D.; Jong, R.; Yaffe, M.; Foulkes, W.D.; Ghadirian, P.; Lynch, H.; Couch, F.; et al. Prospective Study of Breast Cancer Incidence in Women With a BRCA1 or BRCA2 Mutation Under Surveillance With and Without Magnetic Resonance Imaging. J. Clin. Oncol. 2011, 29, 1664. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Lim, Y.Y.; Hurley, E.; Evans, D.G.; Howell, A.; Gadde, S. False-Negative MRI Breast Screening in High-Risk Women. Clin. Radiol. 2017, 72, 207–216. [Google Scholar] [CrossRef]
- Korhonen, K.E.; Samantha, P.; Susan, P.; Tobey, J.; Birnbaum, J.A.; Mcdonald, E.S. Breast MRI: False-Negative Results and Missed Opportunities. RadioGraphics 2021, 41, 10–15. [Google Scholar] [CrossRef]
- Gao, Y.; Reig, B.; Heacock, L.; Bennett, D.L.; Heller, S.L.; Moy, L.; Louis, S. Magnetic Resonance Imaging in Screening of Breast Cancer. Radiol. Clin. N. Am. 2021, 59, 85–98. [Google Scholar] [CrossRef]
- Bilocq-Lacoste, J.; Ferre, R.; Kuling, G.; Martel, A.L.; Tyrrell, P.N.; Li, S.; Wang, G.; Curpen, B. Missed Breast Cancers on MRI in High-Risk Patients: A Retrospective Case–Control Study. Tomography 2022, 8, 27. [Google Scholar] [CrossRef]
- Pages, E.B.; Millet, I.; Hoa, D.; Doyon, F.C. Undiagnosed Breast Cancer at MR imaging: Analysis of causes. Radiology 2012, 264, 40–50. [Google Scholar] [CrossRef]
- Seo, M.; Cho, N.; Bae, M.S.; Koo, H.R.; Kim, W.H.; Lee, S.H.; Chu, A. Features of Undiagnosed Breast Cancers at Screening Breast MR Imaging and Potential Utility of Computer-Aided Evaluation. Korean J. Radiol. 2016, 17, 59–68. [Google Scholar] [CrossRef]
- Gubern-mérida, A.; Vreemann, S.; Martí, R.; Melendez, J.; Lardenoije, S.; Mann, R.M.; Karssemeijer, N.; Platel, B. Automated Detection of Breast Cancer in False-Negative Screening MRI Studies from Women at Increased Risk. Eur. J. Radiol. 2016, 85, 472–479. [Google Scholar] [CrossRef]
- Meissnitzer, M.; Dershaw, D.D.; Feigin, K.; Bernard-davila, B.; Barra, F.; Morris, E.A. MRI Appearance of Invasive Subcentimetre Breast Carcinoma: Benign Characteristics Are Common. Br. J. Radiol. 2017, 90, 20170102. [Google Scholar] [CrossRef]
- Lång, K.; Dustler, M.; Dahlblom, V.; Åkesson, A.; Andersson, I.; Zackrisson, S. Identifying Normal Mammograms in a Large Screening Population Using Artificial Intelligence. Eur. Radiol. 2021, 31, 1687–1692. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Krupinski, E.; Mordang, J.; Schilling, K.; Heywand-Kobrunner, S.H.; Sechopoulos, I.; Mann, R.M. Detection of Breast Cancer with Mammography: Effect of an Artificial Intelligence Support System. Radiology 2019, 290, 305–314. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A.; Broeders, M.; Gennaro, G.; Clauser, P.; Helbich, T.H.; Chevalier, M.; Tan, T.; Mertelmeier, T.; et al. Stand-Alone Artificial Intelligence for Breast Cancer Detection in Mammography: Comparison With 101 Radiologists. J. Natl. Cancer Inst. 2019, 111, 916–922. [Google Scholar] [CrossRef]
- Dembrower, K.; Wåhlin, E.; Liu, Y.; Salim, M.; Smith, K.; Lindholm, P.; Eklund, M.; Strand, F. Effect of Artificial Intelligence-Based Triaging of Breast Cancer Screening Mammograms on Cancer Detection and Radiologist Workload: A Retrospective Simulation Study. Lancet Digit. Health 2020, 2, e468–e474. [Google Scholar] [CrossRef]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H.; Back, T.; Chesus, M.; Corrado, G.C.; Darzi, A.; et al. International Evaluation of an AI System for Breast Cancer Screening. Nature 2020, 577, 89–94. [Google Scholar] [CrossRef]
- Lotter, W.; Diab, A.R.; Haslam, B.; Kim, J.G.; Grisot, G.; Wu, E.; Wu, K.; Onieva, J.O.; Boyer, Y.; Boxerman, J.L.; et al. Robust Breast Cancer Detection in Mammography and Digital Breast Tomosynthesis Using an Annotation-Efficient Deep Learning Approach. Nat. Med. 2021, 27, 244–249. [Google Scholar] [CrossRef]
- Raya-Povedano, J.L.; Romero-Martín, S.; Elías-Cabot, E.; Gubern-Mérida, A.; Rodríguez-Ruiz, A.; Álvarez-Benito, M. AI-Based Strategies to Reduce Workload in Breast Cancer Screening with Mammography and Tomosynthesis: A Retrospective Evaluation. Radiology 2021, 300, 57–65. [Google Scholar] [CrossRef]
- Larsen, M.; Aglen, C.F.; Lee, C.I.; Hoff, S.R.; Lund-Hanssen, H.; Lång, K.; Nygård, J.F.; Ursin, G.; Hofvind, S. Artificial Intelligence Evaluation of 122969 Mammography Examinations from a Population-Based Screening Program. Radiology 2022, 303, 502–511. [Google Scholar] [CrossRef]
- Xu, X.; Fu, L.; Chen, Y.; Larsson, R.; Zhang, D.; Suo, S.; Hua, J.; Member, Z. Breast Region Segmentation Using Convolutional Neural Network in Dynamic Contrast Enhanced MRI. In Proceedings of the 40th Annual International Conference of the EMBC, Honolulu, HI, USA, 18–21 July 2018; pp. 750–753. [Google Scholar]
- Ha, R.; Chang, P.; Mema, E.; Mutasa, S.; Karcich, J.; Wynn, R.T.; Liu, M.Z. Fully Automated Convolutional Neural Network Method for Quantification of Breast MRI Fibroglandular Tissue and Background Parenchymal Enhancement. J. Digit. Imaging 2019, 32, 141–147. [Google Scholar] [CrossRef]
- Comes, M.C.; Fanizzi, A.; Bove, S.; Didonna, V.; Diotaiuti, S.; La Forgia, D.; Latorre, A.; Martinelli, E.; Mencattini, A.; Nardone, A.; et al. Early Prediction of Neoadjuvant Chemotherapy Response by Exploiting a Transfer Learning Approach on Breast DCE-MRIs. Sci. Rep. 2021, 11, 14123. [Google Scholar] [CrossRef] [PubMed]
- Verburg, E.; Van Gils, C.H.; Van Der Velden, B.H.M.; Bakker, M.F. Deep Learning for Automated Triaging of 4581 Breast MRI Examinations from the DENSE Trial. Radiology 2022, 302, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Giger, M.L. Use of Clinical MRI Maximum Intensity Projections for Improved Breast Lesion Classification with Deep Convolutional Neural Networks. J. Med. Imaging 2023, 5, 014503. [Google Scholar] [CrossRef]
- Kim, H.; Ko, E.Y.; Kim, K.E.; Kim, M.K.; Choi, J.S.; Ko, E.S.; Han, B.K. Assessment of Enhancement Kinetics Improves the Specificity of Abbreviated Breast MRI: Performance in an Enriched Cohort. Diagnostics 2023, 13, 136. [Google Scholar] [CrossRef]
- Frid-Adar, M.; Diamant, I.; Klang, E.; Amitai, M.; Goldberger, J.; Greenspan, H. Modeling the Intra-Class Variability for Liver Lesion Detection Using a Multi-Class Patch-Based CNN. arXiv 2017, arXiv:1707.06053v2. [Google Scholar]
- Alahmer, H.; Ahmed, A. Hierarchical Classification of Liver Tumor from CT Images Based on Difference-of-Features (DOF). In Proceedings of the International Conference of Signal and Engineering, London, UK, 29 June–1 July 2016. [Google Scholar]
- Clauser, P.; Cassano, E.; De Nicolò, A.; Rotili, A.; Bonanni, B.; Bazzocchi, M.; Zuiani, C. Foci on Breast Magnetic Resonance Imaging in High-Risk Women: Cancer or Not ? Radiol. Med. 2016, 121, 611–617. [Google Scholar] [CrossRef]
- Gibbs, P.; Onishi, N.; Sadinski, M.; Gallagher, K.M.; Hughes, M.; Martinez, D.F.; Morris, E.A.; Sutton, E.J. Characterization of Sub-1 Cm Breast Lesions Using Radiomics Analysis. J. Magn. Reson. Imaging 2019, 50, 1468–1477. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Saccarelli, C.R.; Bitencourt, A.; Gibbs, P.; Fox, M.J.; Thakur, S.B.; Martinez, D.F.; Jochelson, M.S.; Morris, E.A.; et al. Improved Characterization of Sub-Centimeter Enhancing Breast Masses on MRI with Radiomics and Machine Learning in BRCA Mutation Carriers. Eur. Radiol. 2020, 30, 6721–6731. [Google Scholar] [CrossRef]
- Mulita, F.; Verras, G.; Anagnostopoulos, C.; Kotis, K. A Smarter Health through the Internet of Surgical Things. Sensors 2022, 22, 4577. [Google Scholar] [CrossRef]
- Peta, J.; Koppu, S. An IoT-Based Framework and Ensemble Optimized Deep Maxout Network Model for Breast Cancer Classification. Electronics 2022, 11, 4137. [Google Scholar] [CrossRef]
- Majji, R.P.G.; Prakash, O.; Rajeswari, R.; Cristin, R. Smart IoT in Breast Cancer Detection Using Optimal Deep Learning. J. Digit. Imaging 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, X. Research and Progress in Magnetic Resonance Imaging of Triple-Negative Breast Cancer. Magn. Reson. Imaging 2014, 32, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Moffa, G.; Galati, F.; Collalunga, E.; Rizzo, V.; Amati, G.D.; Pediconi, F.; Kripa, E. Can MRI Biomarkers Predict Triple-Negative Breast Cancer? Diagnostics 2020, 15, 1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.E.; Lee-Felker, S. Triple-Negative Breast Cancer: Multimodality Appearance. Curr. Radiol. Rep. 2022, 11, 53–59. [Google Scholar] [CrossRef]

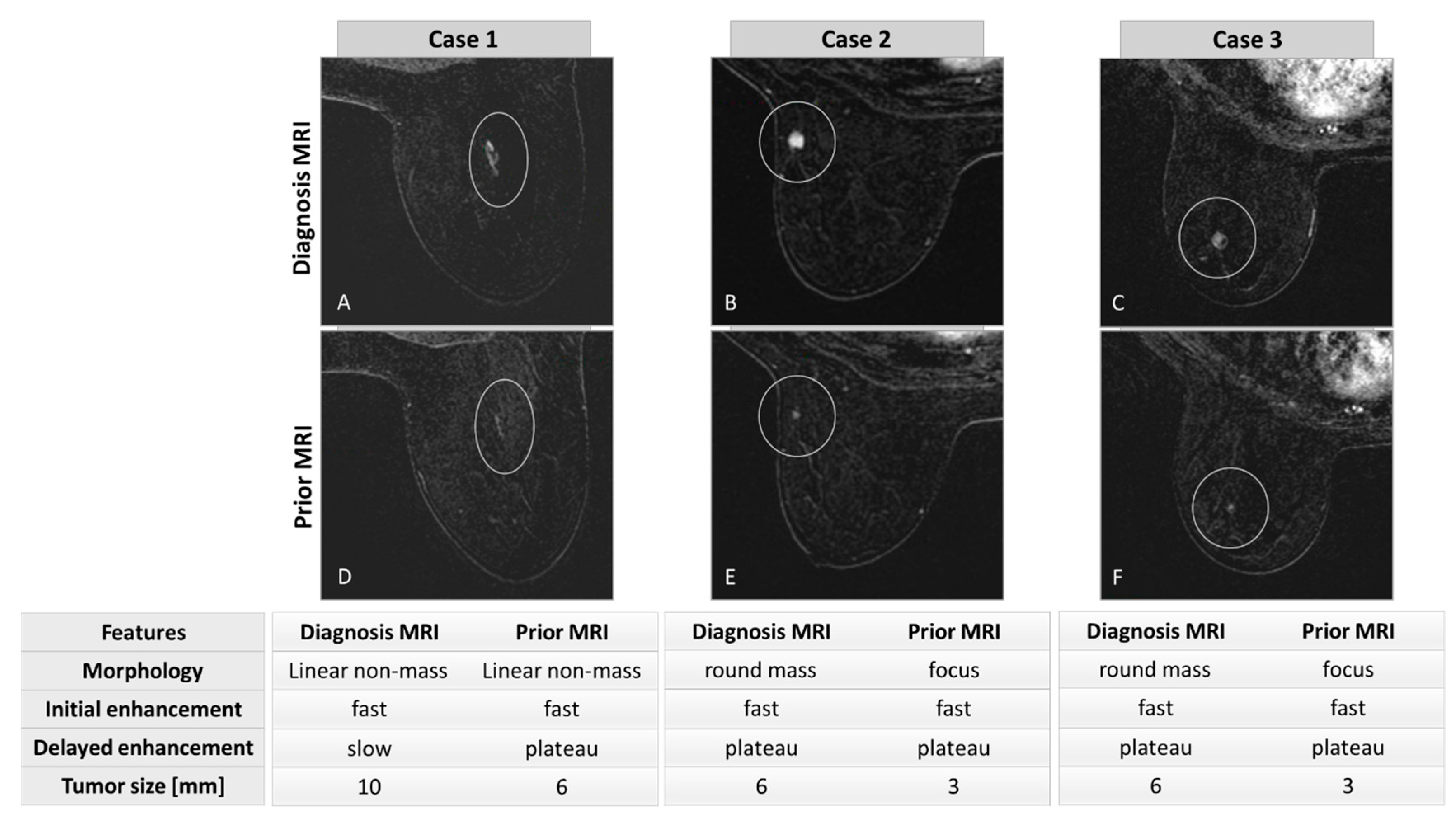

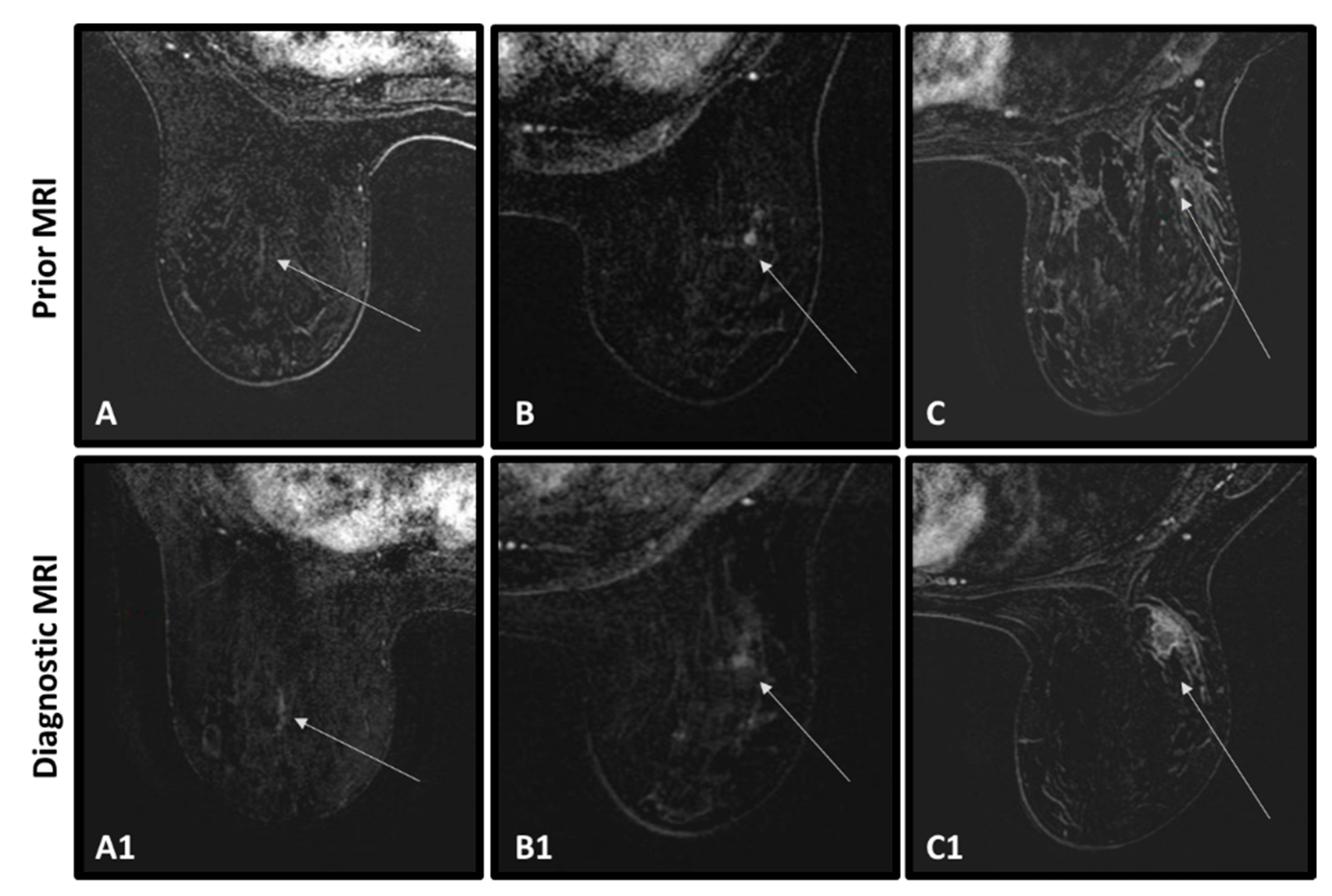
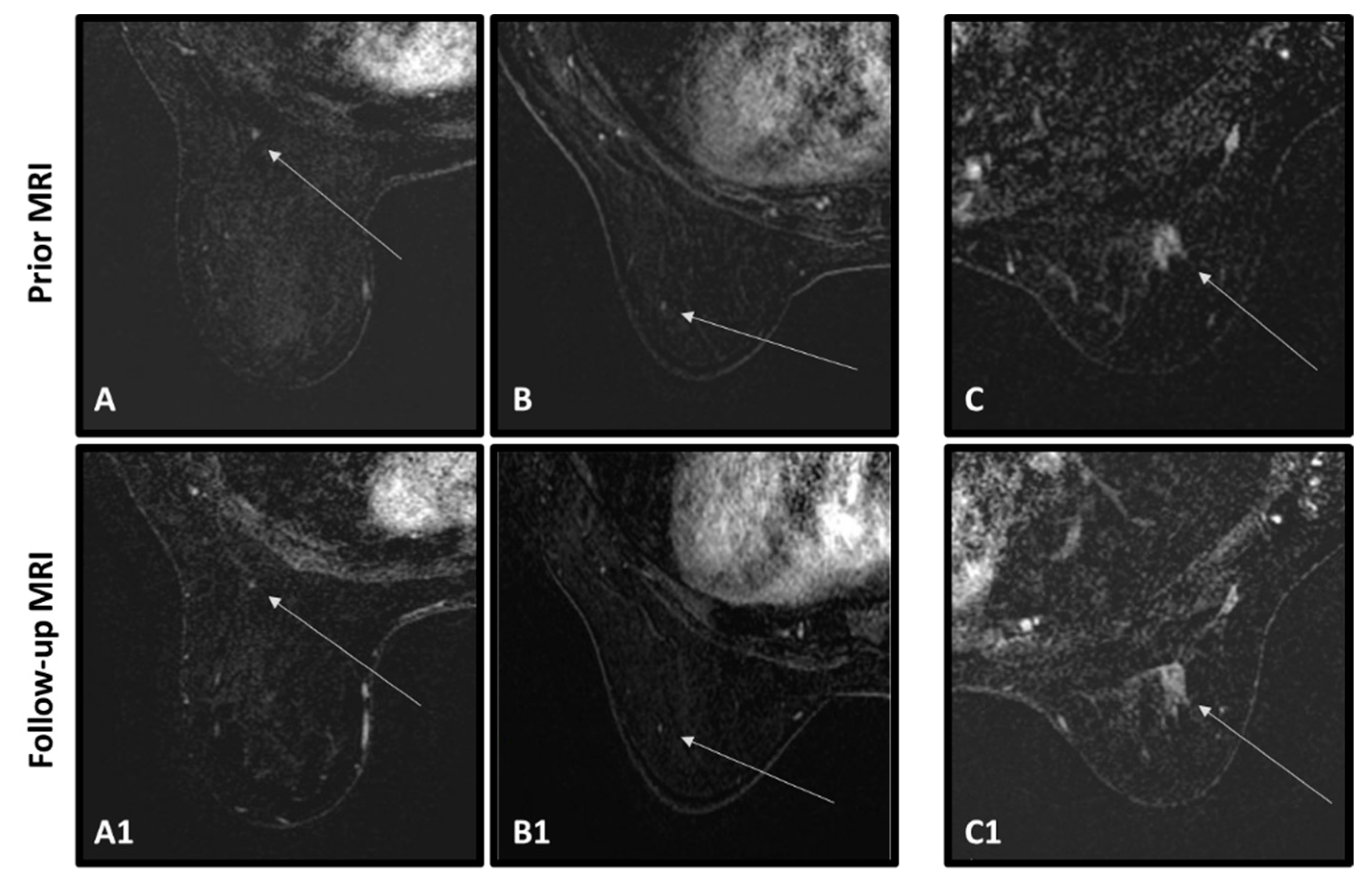
| Characteristics | All Cancer Patients | Cancer Patients with Abnormality | Cancer Patients no Abnormality | p Value Cancer with/without Abnormality | All Cancer-Free Patients | p Value Cancer/Cancer-Free |
|---|---|---|---|---|---|---|
| Number of patients | 53 | 32 (60.4) | 21 (39.6) | 53 | ||
| Age at diagnosis (range-years) | 52 ± 14 (32–80) | 51.4 ± 13.6 (33–78) | 51.9 ± 14.4 (32–80) | 0.96 | 50 ± 15.9 (23–78) | 0.71 |
| Mutated gene | 0.70 | 0.015 | ||||
| BRCA1 | 39 (73.6) | 25 (78.1) | 14 (66.7) | 25 (47.2) | ||
| BRCA2 | 14 (26.4) | 7 (21.9) | 7 (33.3) | 26 (49.1) | ||
| unknown | 2 (3.8) | |||||
| Days between scans (range-days) | 367.6 ± 130.2 (177–938) | 361 ± 118.6 (177–779) | 380 ± 148.2 (182–938) | 0.68 | 373 ± 78.5 (177–604) | 0.78 |
| BIRADS on prior scan | 0.44 | 0.28 | ||||
| 0 | 7 (13.2) | 5 (15.6) | 2 (9.5) | 4 (7.6) | ||
| 1 | 2 (3.8) | 2 (6.3) | -- | 5 (9.4) | ||
| 2 | 33 (62.3) | 17 (53.1) | 16 (76.2) | 33 (62.3) | ||
| 3 | 11 (2.8) | 8 (25) | 3 (14.3) | 8 (15.1) | ||
| 4 | -- | -- | -- | 3 (5.7) | ||
| BPE on prior scan | 0.08 | 0.09 | ||||
| minimal-mild | 31 (58.5) | 16 (50) | 15 (71.4) | 40 (75.5) | ||
| moderate-marked | 22 (41.5) | 16 (50) | 6 (28.6) | 13 (24.5) | ||
| Tumor size at diagnosis [mm] | 10.8 ± 7.3 (2–35) | 12.8 ± 8.4 (2–35) | 7.8 ± 4 (3–16) | 0.01 | ||
| Tumor type | 0.77 | |||||
| IDC | 33 (62.3) | 18 (56.3) | 15 (71.4) | |||
| DCIS | 17 (32.1) | 12 (37.5) | 5 (23.8) | |||
| IDC+DCIS | 2 (3.8) | 1 (3.1) | 1 (4.8) | |||
| unknown | 1 (1.9) | 1 (3.1) | -- | |||
| Histological grade | 0.36 | |||||
| Low | 2 (3.8) | 1 (3.1) | 2 (9.5) | |||
| Intermediate | 14 (26.4) | 8 (25) | 6 (28.6) | |||
| High | 33 (62.3) | 19 (59.4) | 14 (66.7) | |||
| unknown | 4 (7.6) | 4 (12.5) | -- | |||
| Luminal type | 0.35 | |||||
| HR+/HER2− | 18 (33.9) | 10 (31.3) | 8 (38.1) | |||
| HR+/HER2+ | 3 (5.7) | 2 (6.3) | 1 (4.8) | |||
| HR−/HER2+ | 4 (7.6) | 1 (3.1) | 1 (4.8) | |||
| Triple negative | 30 (56.6) | 19 (59.4) | 11 (52.4) |
| Cancerous Lesions | Non-Cancerous Lesions/BPE | Time | Interaction Time × Group | Paired t-Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior Scan (N = 32) | At Diagnosis (N = 32) | Prior Scan (N = 53) | Follow-Up Scan (N = 33) | F | p | F | p | Cancerous Lesions p | Non-Cancerous Lesions p | ||
| Lesion size [mm] | 6.1 ± 4.2 (1.5–17) | 10.8 ± 7.3 (2–35) | 7.4 ± 4.2 (1.7–27.3) | 7.1 ± 5.1 (2.3–28.9) | F(1,59) = 23.74 | <0.0001 | F(1,59) = 25.99 | <0.001 | <0.0001 | 0.69 | |
| Morphology | F(1,63) = 5.96 | 0.02 | F(1,63) = 13.87 | <0.001 | 0.0001 | 0.37 | |||||
| Focus | 19 (59.3) | 6 (18.8) | 12 (22.6) | 12 (36.4) | |||||||
| Mass | 6 (18.8) | 18 (56.3) | 17 (32.1) | 10 (30.3) | |||||||
| Non-mass | 7 (21.8) | 8 (25) | 25 (47.2) | 11 (33.3) | |||||||
| Kinetics | |||||||||||
| CAD | 9 (28.1) | 14 (43.8) | 4 (7.6) | 1 (3) | F(1,61) = 0.93 | 0.34 | F(1,61) = 4.78 | 0.03 | 0.09 | 0.16 | |
| Initial phase | F(1,61) = 0.41 | 0.53 | F(1,61) = 1.55 | 0.22 | |||||||
| Slow | 8 (25) | 5 (15.6) | 24 (45.3) | 16 (48.5) | |||||||
| Medium | 13 (40.6) | 13 (40.6) | 15 (28.3) | 8 (24.2) | |||||||
| Fast | 11 (34.4) | 14 (43.8) | 14 (26.4) | 9 (27.3) | |||||||
| Delayed phase | F(1,62) = 2.11 | 0.15 | F(1,62) = 0.24 | 0.75 | |||||||
| Persistent | 23 (71.9) | 21 (65.6) | 45 (84.9) | 24 (72.7) | |||||||
| Plateau | 8 (25) | 10 (31.3) | 7 (13.2) | 7 (21.2) | |||||||
| Washout | 1 (3.1) | 1 (3.1) | 1 (1.9) | 2 (6.1) | |||||||
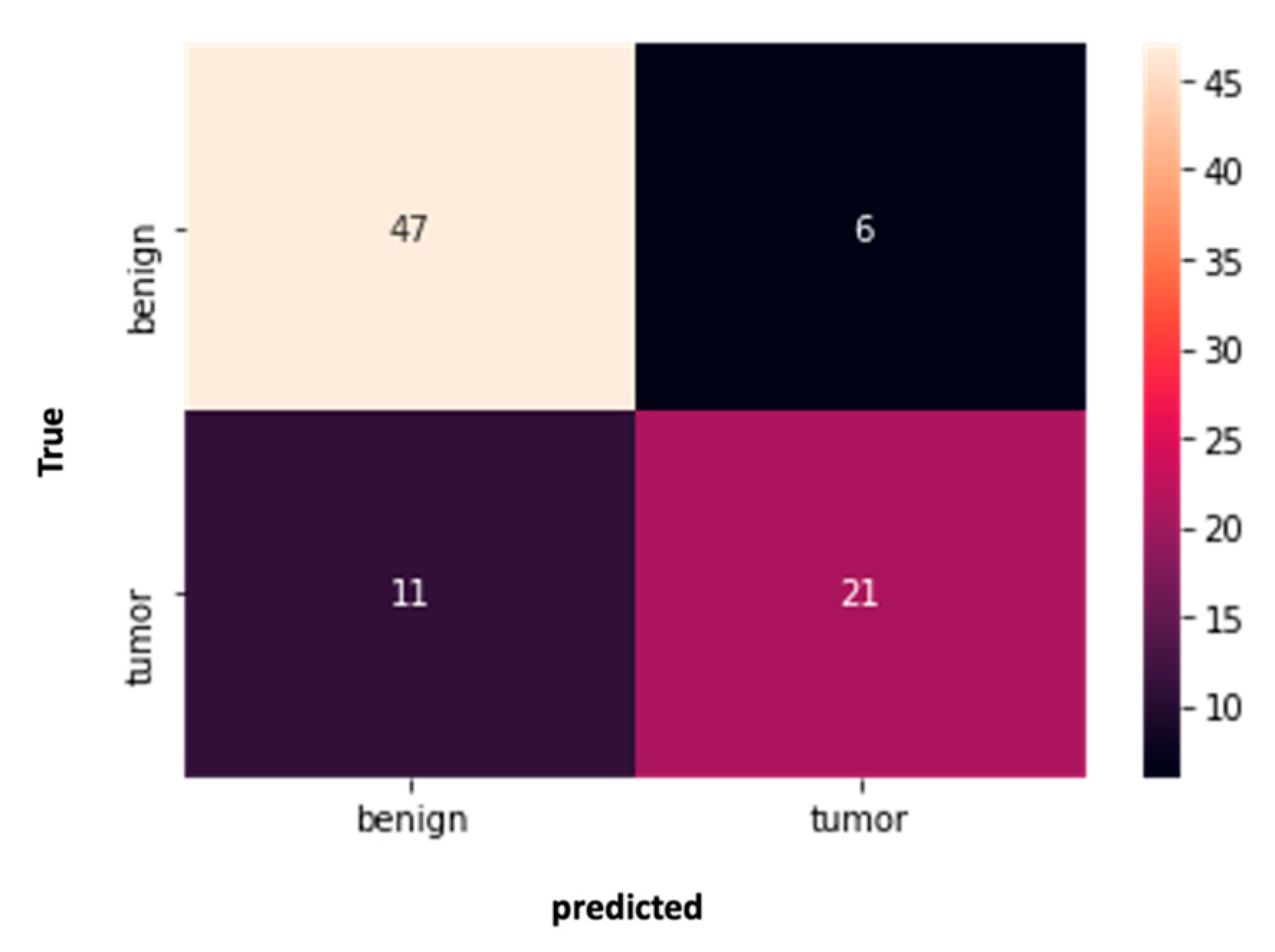
| Characteristics | AI Success (21) | AI Failure (11) | p-Value | |
|---|---|---|---|---|
| Age at diagnosis (range-years) | 52.5 ± 13.4 (34–77) | 49.3 ± 14.3 (33–78) | 0.53 | |
| Mutated gene | 0.39 | |||
| BRCA1 | 18 (85.7) | 7 (63.6) | ||
| BRCA2 | 3 (14.3) | 4 (36.4) | ||
| Days between scans (range-days) | 362.7 ± 89.3 (196–511) | 357.7 ± 166.1 (177–779) | 0.85 | |
| BIRADS on prior scan | 1 | |||
| 0 | 4 (19.1) | 1 (10) | ||
| 1 | 1 (4.8) | 1 (10) | ||
| 2 | 11 (52.4) | 6 (60) | ||
| 3 | 5 (23.8) | 2 (20) | ||
| BPE on previous scan | 0.57 | |||
| Minimal to mild | 12 (57.1) | 5 (45.5) | ||
| Moderate to marked | 9 (42.9) | 6 (54.5) | ||
| Tumor size [mm] | 10.6 ± 8 (2–28) | 16.7 ± 7.8 (7–35) | 0.05 | |
| Tumor type | 0.42 | |||
| IDC | 13 (61.9) | 5 (45.5) | ||
| DCIS | 6 (28.6) | 6 (54.5) | ||
| IDC+DCIS | 1 (4.8) | -- | ||
| unknown | 1 (4.8) | -- | ||
| Histological grade | 0.68 | |||
| Low | 0 | -- | ||
| Intermediate | 4 (19.1) | 4(40) | ||
| High | 14 (66.7) | 5(50) | ||
| unknown | 3 (14.3) | 1(10) | ||
| Molecular subtype | 0.016 | |||
| HR+/HER2- | 5 (23.8) | 5 (50) | ||
| HR+/HER2+ | 1 (4.8) | -- | ||
| HR-/HER2+ | 2 (9.5) | 2 (20) | ||
| Triple negative | 13 (61.9) | 3 (30) |
| Characteristics of Early Scan | AI Success (21) | AI Failure (11) | p-Value | |
|---|---|---|---|---|
| Morphology | 1 | |||
| focus | 12 (57.1) | 7 (63.6) | ||
| mass | 4 (19.1) | 2 (18.2) | ||
| non-mass | 5 (23.8) | 2 (18.2) | ||
| Initial enhancement | 0.71 | |||
| Slow | 5 (23.8) | 2 (18.2) | ||
| Medium | 8 (38.1) | 6 (54.5) | ||
| fast | 8 (38.1) | 3 (27.3) | ||
| Delayed phase | 0.47 | |||
| Persistent | 15 (71.4) | 8 (72.7) | ||
| Plateau | 6 (28.6) | 2 (18.2) | ||
| washout | -- | 1 (9) | ||
| CAD | 1 | |||
| Positive | 6 (28.6) | 3 (27.3) | ||
| negative | 13 (61.9) | 8 (72.7) | ||
| unknown | 1 (4.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaby, D.; Shavin, D.; Zimmerman-Moreno, G.; Nissan, N.; Friedman, E.; Sklair-Levy, M. ‘Earlier than Early’ Detection of Breast Cancer in Israeli BRCA Mutation Carriers Applying AI-Based Analysis to Consecutive MRI Scans. Cancers 2023, 15, 3120. https://doi.org/10.3390/cancers15123120
Anaby D, Shavin D, Zimmerman-Moreno G, Nissan N, Friedman E, Sklair-Levy M. ‘Earlier than Early’ Detection of Breast Cancer in Israeli BRCA Mutation Carriers Applying AI-Based Analysis to Consecutive MRI Scans. Cancers. 2023; 15(12):3120. https://doi.org/10.3390/cancers15123120
Chicago/Turabian StyleAnaby, Debbie, David Shavin, Gali Zimmerman-Moreno, Noam Nissan, Eitan Friedman, and Miri Sklair-Levy. 2023. "‘Earlier than Early’ Detection of Breast Cancer in Israeli BRCA Mutation Carriers Applying AI-Based Analysis to Consecutive MRI Scans" Cancers 15, no. 12: 3120. https://doi.org/10.3390/cancers15123120
APA StyleAnaby, D., Shavin, D., Zimmerman-Moreno, G., Nissan, N., Friedman, E., & Sklair-Levy, M. (2023). ‘Earlier than Early’ Detection of Breast Cancer in Israeli BRCA Mutation Carriers Applying AI-Based Analysis to Consecutive MRI Scans. Cancers, 15(12), 3120. https://doi.org/10.3390/cancers15123120








